Introduction
MicroRNAs (miRNAs) are small, endogenous non-coding
RNA molecules (∼22 bases) that are capable of modulating the
post-transcriptional regulation of numerous cellular genes
(1). Regulated miRNA expression has
been demonstrated in a variety of human cancer types, including
chronic lymphocytic leukemia (2),
lung cancer (3), colorectal
neoplasia (4), and pancreatic
endocrine and acinar tumors (5).
Therefore, understanding the mechanisms that control miRNA
expression in cancer and the functional consequences of this may
provide novel insights into improvements in the classification,
prognosis prediction and treatment of cancer.
Dicer is an essential member of the RNase III
family, which controls maturation of miRNAs in the cytoplasm from
miRNA precursors (pre-miRNAs) (6).
As an upstream modulator of miRNAs, Dicer downregulation may
promote cellular transformation and tumorigenesis via a global
decrease in miRNA expression (7).
It has been demonstrated that dysregulation of miRNAs is involved
in the pathogenesis, tumor phenotype and chemosensitivity of
ovarian cancer (8). Moreover,
downregulation of Dicer is detectable in 60% of patients with
invasive epithelial ovarian cancer and is associated with poor
clinical outcomes (9). However, the
role of Dicer in the biological behavior and chemosensitivity of
ovarian cancer remains largely unclear.
Enhancer of zeste homolog 2 (EZH2), known as the
catalytic submit of polycomb repressive complex 2 (PRC2), is a
highly conserved histone methyltransferase that targets histone 3
lysine 27. EZH2 is recognized as a key epigenetic regulator
associated with transcriptional repression and gene silencing
(10). Taniguchi et al
demonstrated that epigenetic silencing of Kruppel-like factor 2,
which generally acts as a tumor suppressor in cancer through direct
transcriptional repression, is mediated by EZH2 (11). Expression of two cyclin-dependent
kinase (CDK) inhibitors CDKN1A/p21 and CDKN1C/p57 were also
demonstrated to be regulated by EZH2 in cervical and ovarian
cancer, respectively (12,13). However, whether EZH2 participates in
the regulation of Dicer expression has not yet been
investigated.
In this study, we demonstrate the effect of Dicer
downregulation on cell proliferation, cell migration ability and
response to cisplatin in ovarian cancer in vitro.
Furthermore, we reveal that EZH2 may be a key regulator of Dicer
expression.
Materials and methods
Cell lines and culture conditions
Ovarian cancer cell lines A2780 and SKOV3 were
purchased from the China Center for Type Culture Collection (CCTCC;
Wuhan, China). A2780/DDP cells and stably EZH2-short hairpin RNA
(shRNA)-transfected A2780 (shEZH2-A2780) cells were generated and
reserved by our laboratory (13,23).
All cell lines were cultured in Roswell Park Memorial Institute
(RPMI)-1640 medium (Gibco; Carlsbad, CA, USA) with 10% fetal calf
serum (FCS; Gibco), at 37°C in a humidified atmosphere of 5%
CO2. The A2780/DDP cells used in the study were cultured
in the absence of cisplatin for >1 month prior to use to exclude
the stress reaction mediated by drug treatment.
The study was approved by the Ethics Committee of
Huazhong University of Science and Technology, Wuhan, China
Transient Dicer small interfering RNA
(siRNA) transfection
Carboxyfluorescein (FAM)-labeled siRNA targeting
Dicer and negative control siRNA were chemically synthesized
(Invitrogen Life Technologies; Carlsbad, CA, USA). The siRNA
sequences were as follows: Sense: 5′-UUUGUUGCGAGGCUGAUUCTT-3′ and
anti-sense: 5′-GAAUCAGCCUCGCAACAAATT-3′ for Dicer siRNA; sense:
5′-UUCUCCGAACGUGUCACGUTT-3′ and anti-sense
5′-ACGUGACACGUUCGGAGAATT-3′ for negative control siRNA.
Lipofectamine 2000 (Invitrogen Life Technologies) was used for
transfection according to the manufacturer’s instructions. The
transfection efficiency was detected by fluorescent microscopy and
the growth medium was replaced after 6 h. Forty-eight hours after
transfection, the cells were harvested for analysis.
RNA isolation and quantitative polymerase
chain reaction (qPCR)
Total RNA was extracted from the cell lines using
TRIzol reagent (Invitrogen Life Technologies) according to the
manufacturer’s instructions. Following confirmation of the quality
and quantity of extracted total RNA by Nanodrop 2000 (Thermo
Scientific, Wilmington, DE, USA), cDNA was synthesized using a
reverse transcription kit (Toyobo; Osaka, Japan) according to the
manufacturer’s instructions. The primer sequences for Dicer mRNA
detection were as follows: Upstream: 5′-GTGGTTCGTTTTGATTTGCCC-3′
and downstream: 5′-CGTGTTGATTGTGACTCGTGGA-3′ (NM_001195573.1). The
sequences of the β-actin primers were as follows: Upstream:
5′-GTCCACCGCAAATGCTTCTA-3′ and downstream:
5′-TGCTGTCACCTTCACCGTTC-3′. All reactions were performed in
duplicate in an Applied Biosystems 7300 Real-time PCR system
(Applied Biosystems; Foster City, CA, USA). Each reaction system
contained 1 μl cDNA sample, 12.5 μl SYBR-Green
Real-time PCR Master Mix (Toyobo) and 1 μl of 10
μmol/l each primer, in a final volume of 25 μl.
Reactions were performed under the following cycling conditions:
Initial denaturation at 95°C for 1 min, followed by 40 cycles of
denaturation at 95°C for 15 sec, annealing at 58°C for 15 sec and
extension at 72°C for 45 sec. PCR products were identified by a
melting curve analysis. The relative mRNA level of the Dicer gene
was calculated using the comparative threshold cycle (Ct) method
(2−ΔΔCt) normalized by β-actin expression (24).
Protein extraction and western blot
analysis
Protein for western blot analysis was isolated from
cells by a radioimmuno-precipitation assay buffer (RIPA; Beyotime,
China) according to the manufacturer’s instructions. The protein
concentration was measured by bicinchoninic acid (BCA) assay. Fifty
micrograms of total protein was denatured by boiling for 5 min,
then separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE), transferred onto nitrocellulose
membranes and blotted with mouse anti-Dicer (1:500 dilution; Abcam,
Cambridge, MA, USA; Product No. ab14601) or mouse anti-β-actin
(1:500 dilution; Santa Cruz Biotechnology Inc.; Santa Cruz, CA,
USA). Primary antibodies were detected using horseradish
peroxidase-conjugated anti-mouse secondary antibody (1:5000; Santa
Cruz Biotechnology, Inc.) and visualized by an enhanced
chemiluminescence kit (Pierce; Rockford, IL, USA). Protein bands
were quantified following scanning by Quantity One software
(Bio-Rad; Hercules, CA, USA).
Cell proliferation assay
The cell proliferation assay was performed using a
BrdU enzyme-linked immunosorbant assay (ELISA) kit (Calbiochem; San
Diego, CA, USA) according to the manufacturer’s instructions. The
absorbance at 450–595 nm was measured with an iMark microplate
reader (Bio-Rad; Serial No. 10601).
Cell viability assays
Cells were seeded in triplicate into 96-well plates
(2000 cells/well) and then incubated with
3-(4,5-Dimethylthiazol-2-yl) -2,5-diphenyltetrazolium bromide (MTT)
for 4 h, followed by dissolution in dimethylsulfoxide (DMSO) for 10
min every 24 h for 5 days. Growth curves were generated by
calculating the mean value of the optical density measurements at
570 nm using the iMark microplate reader (Bio-Rad).
Cell cycle analysis
Cells were harvested, trypsinized, washed with
ice-cold phosphate-buffered saline (PBS) and fixed in 70% cold
ethanol at 4°C overnight. Following centrifugation (1200 g for 5
min), fixed cells were washed with PBS, and then resuspended in PBS
containing 1 mg/ml RNase A for 30 min at 37°C. Subsequently, cells
were incubated with 50 μg/ml propidium iodide for 30 min at
4°C. Cell cycle analysis was performed using a LSR flow cytometer
(Becton Dickinson; San Jose, CA, USA) with ModFit LT software
(Verity Software House; Topsham, ME, USA).
Cell migration assay
The cell migration assay was performed using a
Boyden chamber. Cells (1×105/well) were trypsinized,
resuspended in serum-free RPMI-1640 medium and then added to the
transwell inserts (6.5 mm diameter, 8 μm pore size,
polycarbonate membrane; Corning Costar; Cambridge, MA, USA).
RPMI-1640 medium (600 μl) with 10% FBS was added to the
lower chamber beneath the insert membrane. The transwell chambers
were then incubated for 24 h under culture conditions. Migrated
cells on the lower surface of the membrane were fixed with 70%
ethanol and stained with crystal violet, and were then counted in
10 randomly selected high-power fields (×400) under a microscope.
The average value was used as parameter to evaluate the migration
ability of the cells. All assays were performed in triplicate.
Drug cytotoxicity assays
Assessment of chemoresistance to cisplatin was
determined by the MTT assays. Cells were seeded in triplicate at a
density of 5000 cells/well in 96-well plates. Cells were treated
with cisplatin at various concentrations ranging from 2.5–40
μg/ml for an additional 24 h. Subsequently, 20 μl of
5 mg/ml MTT was added to each well and, after 4 h, cells were
dissolved in 150 μl of DMSO for 10 min. The absorbance at
570 nm was measured using wells without cells as blanks on an iMark
microplate reader (Bio-Rad; Serial No. 10601). The percentage of
cell survival at each dose was calculated as the absorbance ratio
of treated to untreated cells. The 50% inhibitory concentration
(IC50) values were calculated by linear interpolation.
Data shown are representative of three independent experiments.
Statistical analysis
Data were expressed as mean ± standard deviation.
The statistical significance of differences were estimated by a
two-tailed Student’s t-test or a one-way analysis of variance
(ANOVA), as appropriate, using the Statistical Package for the
Social Sciences (SPSS) software, version 13 (SPSS Inc.; Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
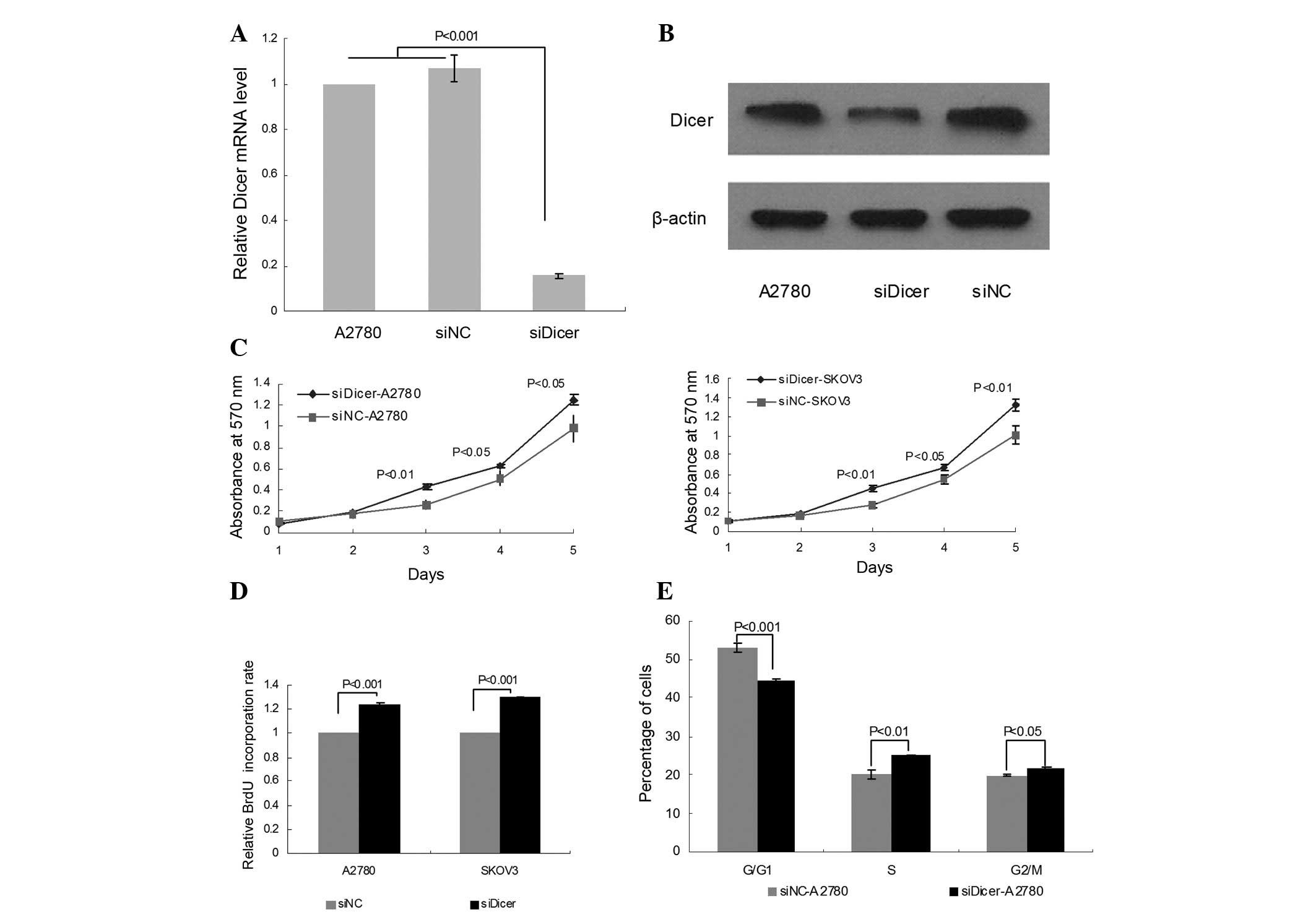
Knockdown of Dicer by siRNA
To study the function of Dicer in ovarian cancer,
transient Dicer-knockdown A2780 cells were generated using Dicer
siRNA (siDicer). Untransfected A2780 cells and negative control
siRNA (siNC)-transfected cells were used as controls. An 84.33%
decrease in the level of Dicer mRNA was observed in siDicer-A2780
cells compared with untransfected A2780 cells by qPCR (P<0.001,
Fig. 1A); whereas no significant
difference was identified between siNC-A2780 and untransfected
cells. The knockdown of Dicer was further confirmed by western blot
analysis (Fig. 1B).
Downregulation of Dicer promotes cell
proliferation in ovarian cancer cells
To investigate the effect of Dicer knockdown on cell
growth and proliferation in cancer cells, MTT and BrdU assays were
performed for siDicer-transfected A2780 and SKOV3 cells. The MTT
assay revealed that siDicer transfection significantly increased
the cell viability of A2780 and SKOV3 on days 3, 4 and 5 compared
with siNC (Fig. 1C). Additionally,
the BrdU incorporation assay demonstrated that the proliferation of
A2780 and SKOV3 cells was markedly stimulated by Dicer knockdown
compared with siNC-transfected cells 96 h post-transfection
(Fig. 1D).
To study whether the growth elevation upon Dicer
depletion in ovarian cancer cells is associated with cell cycle
regulation, cell cycle analysis was conducted by propidium iodide
(PI) staining and flow cytometry. Dicer knockdown significantly
increased the percentage of S and G2/M phase cells (P=0.002 and
P=0.022, respectively), which was accompanied by a fall in the
percentage of G0/G1 phase cells (P<0.001; Fig. 1E), suggesting that Dicer is a
regulator of the cell cycle, impacting cell proliferation in
ovarian cancer.
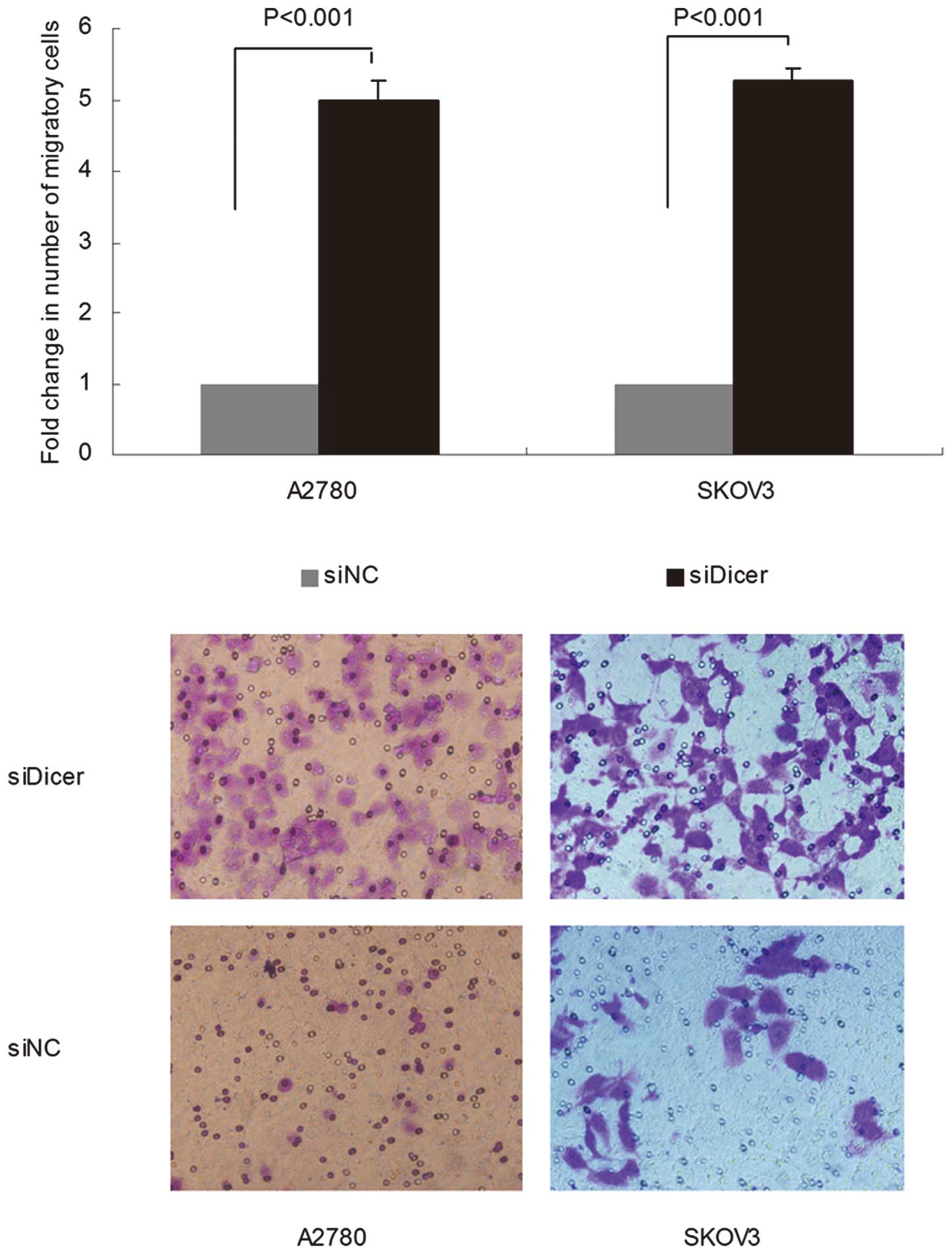
Knockdown of Dicer promotes ovarian
cancer cell migration
Transwell migration assays for siDicer-transfected
cells were subsequently performed. siDicer-A2780 and siDicer-SKOV3
cells exhibited an increased ability to migrate through an
8-μm pore size polycarbonate membrane compared with
siNC-transfected cells (P<0.001 for both cells; Fig. 2). The results thus far suggest that
downregulation of Dicer in ovarian cancer may be required for
disease progression.
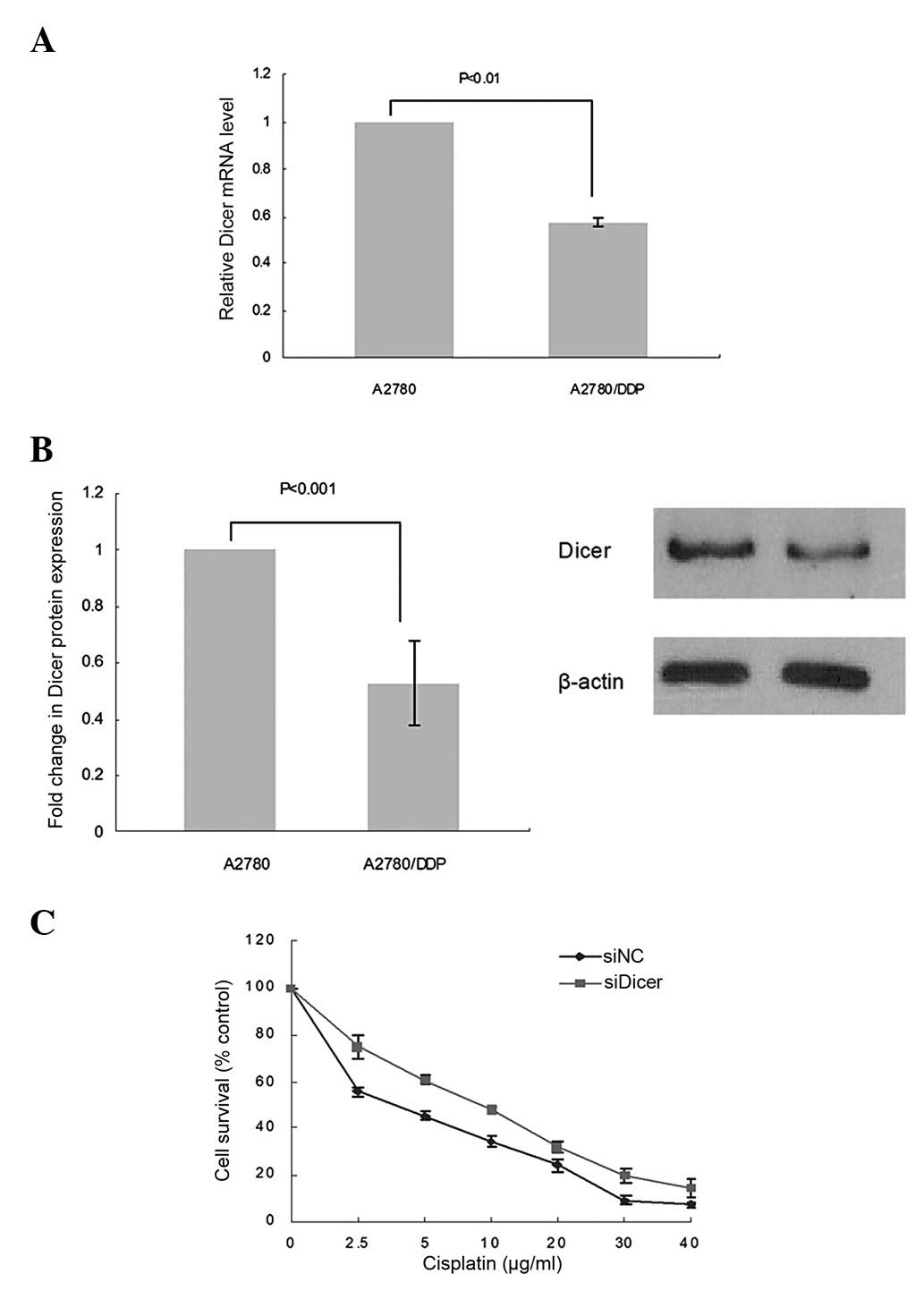
Downregulation of Dicer contributes to
cisplatin resistance in ovarian cancer cells
To delineate the role of Dicer in drug resistance,
we first compared the expression of Dicer in A2780 cells and
cisplatin-resistant cells derived from these (A2780/DDP) by qPCR
and western blot analysis. A marked downregulation of Dicer
expression was observed at both the mRNA (57.3% decrease;
P<0.01; Fig. 3A) and protein
(52.6% decrease; P<0.001; Fig.
3B) level in A2780/DDP cells compared with parental A2780
cells.
To verify the effect of Dicer knockdown on cisplatin
sensitivity, the cell viability was assessed by MTT assays
following treatment with various concentrations of cisplatin. Cell
survival following cisplatin treatment was significantly increased
in siDicer-A2780 compared with siNC-A2780 cells. Depletion of Dicer
in the A2780 cells caused a 0.89-fold increase in the cisplatin
IC50value (7.56 vs. 4.01 μg/ml; P<0.01;
Fig. 3C), indicating a causal
correlation between Dicer repression and cisplatin resistance in
ovarian cancer. However, further studies are required to clarify
the underlying mechanism.
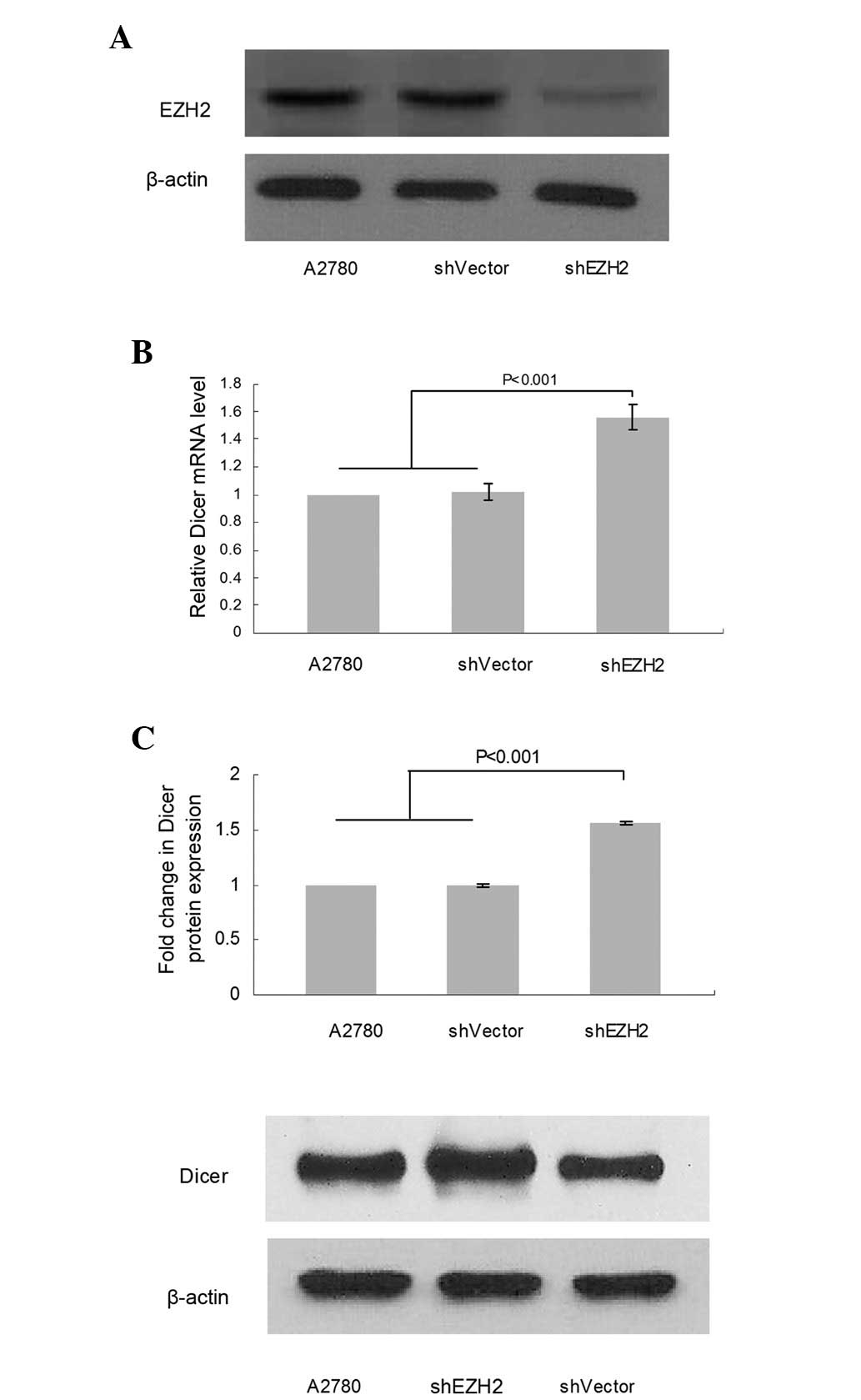
Loss of EZH2 increased Dicer expression
in ovarian cancer cell lines
To investigate whether EZH2 is involved in the
regulation of Dicer expression in ovarian cancer, we analyzed the
alteration in Dicer expression following EZH2 depletion mediated by
shRNA (Fig. 4A) using qPCR and
western blot analysis. qPCR revealed that the Dicer mRNA level
increased by 55.7% in shEZH2-A2780 cells compared with NC
(P<0.001; Fig. 4B) and a
corresponding increase (56.0%) at the protein level was revealed by
western blot analysis (P< 0.001; Fig. 4C). This result suggests that EZH2 is
involved in the regulation of Dicer expression.
Discussion
In the present study, reduced expression of Dicer in
ovarian cancer was demonstrated to be associated with activated
tumor cell proliferation, enhanced migration ability and increased
cisplatin resistance. A number of studies have demonstrated similar
effects of Dicer silencing in other types of cells. Dicer knockdown
substantially increased the invasion ability of breast cancer cells
(14) and the migratory capacity of
human embryonic kidney (HEK) 293T cells in vitro(15). Moreover, at the molecular level,
inhibition of Dicer in human cancer U251, MCF-7 and SCG7901 cell
lines enhanced the expression of cell cycle-associating molecules
cyclin A and PCNA, as well as invasion-promoting factors MMP-2 and
MMP-9. Adenoviral gene silencing of Dicer in subcutaneous MCF-7
xenografts significantly increased the tumor growth in
vivo(16). Kumar et al
revealed that defective miRNA maturation enhanced tumor
transformation and invasion in vitro and in vivo, and
that conditional depletion of Dicer enhanced tumor development in a
K-Ras-induced mouse model of lung cancer (17). Furthermore, Dicer was demonstrated
to be required for proliferation, viability, migration and
differentiation in corticoneurogenesis in a mouse model (18). A study on Dicer expression and
function in ovarian cancer performed by Faggad et al
indicated that decreased Dicer expression was significantly
correlated with a global downregulation of the microRNA, advanced
disease stages and reduced patient survival in serous tumors
(19).
In accordance with the results of previous studies,
the present study demonstrated that the reduced expression of Dicer
in ovarian cancer is associated with activated tumor cell
proliferation and enhanced migration ability. Additionally, for the
first time, the role of Dicer in cisplatin resistance in ovarian
cancer cells was investigated. Knockdown of Dicer in A2780 cells by
siRNA was observed to promote cell cycle progression and to
decrease sensitivity to cisplatin. A previous study had
demonstrated that ablation of Dicer in the MCF-7 breast cancer cell
line led to significant G1 arrest and increased sensitivity to
cisplatin (20), suggesting that
the role of Dicer in the regulation of the cell cycle and drug
response is tumor type-specific. However, the effect on the
invasion of Dicer silencing compared with that on cell survival and
cisplatin resistance was observed to be more significant,
suggesting that there are other factors besides Dicer affecting
these pathways.
Although there are numerous studies concerned with
Dicer, the regulation of its expression is poorly understood.
Merritt et al measured the Dicer mRNA level in specimens of
invasive epithelial ovarian cancer from 111 patients. Decreased
Dicer expression was observed in 60% of cases. Mutational analysis
in a subgroup of ovarian cancer specimens revealed rare missense
mutations (2/37) in the Dicer gene, but its presence or absence was
not correlated with the level of Dicer mRNA expression (9). Tokumaru et al demonstrated that
let-7 miRNA inhibits the expression of Dicer, representing a
negative feedback loop on overall miRNA production (21). Furthermore, Wiesen and Tomasi
revealed that Dicer is post-transcriptionally regulated by cellular
stresses and interferons (22).
Previous studies demonstrated that EZH2 is upregulated in ovarian
cancer and contributes to tumor progression and the development of
cisplatin resistance in vitro and in vivo(13,23).
To validate the regulation pathway and to explore the mechanism
whereby EZH2 regulates Dicer expression requires further
investigation.
In summary, we have demonstrated that loss of Dicer
is capable of promoting cell proliferation, increasing cell
migratory capacity and decreasing ovarian cancer sensitivity to
cisplatin. Furthermore, for the first time, we provide evidence
that implicates EZH2 in the regulation of Dicer expression. Further
investigation into the function of Dicer in carcinogenesis and its
regulation pathways in human ovarian cancer tissue, additional cell
lines and animal models will promote our exploitation of novel
anti-cancer targets.
Acknowledgements
This study was supported by the Health
Department of Hubei Province, China (JX5A07, 2011).
References
|
1
|
Wahid F, Shehzad A, Khan T, et al:
Micrornas: Synthesis, mechanism, function, and recent clinical
trials. Biochim Biophys Acta. 1803:1231–1243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Calin GA, Liu CG, Sevignani C, et al:
MicroRNA profiling reveals distinct signatures in B cell chronic
lymphocytic leukemias. Proc Natl Acad Sci USA. 101:11755–11760.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takamizawa J, Konishi H, Yanagisawa K, et
al: Reduced expression of the let-7 microRNAs in human lung cancers
in association with shortened postoperative survival. Cancer Res.
64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Michael MZ, O’Connor SM, van Holst
Pellekaan NG, et al: Reduced accumulation of specific microRNAs in
colorectal neoplasia. Mol Cancer Res. 1:882–891. 2003.PubMed/NCBI
|
|
5
|
Roldo C, Missiaglia E, Hagan JP, et al:
MicroRNA expression abnormalities in pancreatic endocrine and
acinar tumors are associated with distinctive pathologic features
and clinical behavior. J Clin Oncol. 24:4677–4684. 2006. View Article : Google Scholar
|
|
6
|
Bernstein E, Caudy AA, Hammond SM, et al:
Role for a bidentate ribonuclease in the initiation step of RNA
interference. Nature. 409:363–366. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kumar MS, Lu J, Mercer KL, et al: Impaired
microRNA processing enhances cellular transformation and
tumorigenesis. Nat Genet. 39:673–677. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mezzanzanica D, Bagnoli M, De Cecco L, et
al: Role of microRNAs in ovarian cancer pathogenesis and potential
clinical implications. Int J Biochem Cell Biol. 42:1262–1272. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Merritt WM, Lin YG, Han LY, et al: Dicer,
drosha, and outcomes in patients with ovarian cancer. N Engl J Med.
359:2641–2650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simon JA and Lange CA: Roles of the EZH2
histone methyltransferase in cancer epigenetics. Mutat Res.
647:21–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taniguchi H, Jacinto FV, Villanueva A, et
al: Silencing of Kruppel-like factor 2 by the histone
methyltransferase EZH2 in human cancer. Oncogene. 31:1988–1994.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fang J, Zhang MX and Li QL: Enhancer of
zeste homolog 2 expression is associated with tumor cell
proliferation and invasion in cervical cancer. Am J Med Sci.
342:198–204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo JF, Cai J, Yu LL, et al: EZH2
regulates expression of p57 and contributes to progression of
ovarian cancer in vitro and in vivo. Cancer Science.
102:530–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Noh H, Hong S, Dong Z, et al: Impaired
microRNA processing facilitates breast cancer cell invasion by
upregulating urokinase-type plasminogen activator expression. Genes
Cancer. 2:140–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang KF, Song GB, Shi YS, et al: Dicer
knockdown induces fibronectin-1 expression in HEK293T cells via
induction of Egr1. Biochim Biophys Acta. 1800:380–384. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han L, Zhang A, Zhou X, et al:
Downregulation of Dicer enhances tumor cell proliferation and
invasion. Int J Oncol. 37:299–305. 2010.PubMed/NCBI
|
|
17
|
Kumar MS, Lu J, Mercer KL, et al: Impaired
microRNA processing enhances cellular transformation and
tumorigenesis. Nat Genet. 39:673–677. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McLoughlin HS, Fineberg SK, Ghosh LL, et
al: Dicer is required for proliferation, viability, migration and
differentiation in corticoneurogenesis. Neuroscience. 223:285–295.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Faggad A, Budczies J, Tchernitsa O, et al:
Prognostic significance of Dicer expression in ovarian cancer-link
to global microRNA changes and oestrogen receptor expression. J
Pathol. 220:382–391. 2010.PubMed/NCBI
|
|
20
|
Bu Y, Lu C, Bian C, et al: Knockdown of
Dicer in MCF-7 human breast carcinoma cells results in G1 arrest
and increased sensitivity to cisplatin. Oncol Rep. 21:13–17.
2009.PubMed/NCBI
|
|
21
|
Tokumaru S, Suzuki M, Yamada H, et al:
Let-7 regulates Dicer expression and constitutes a negative
feedback loop. Carcinogenesis. 29:2073–2077. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wiesen JL and Tomasi TB: Dicer is
regulated by cellular stresses and interferons. Mol Immunol.
46:1222–1228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu S, Yu L, Li Z, Shen Y, et al:
Overexpression of EZH2 contributes to acquired cisplatin resistance
in ovarian cancer cells in vitro and in vivo. Cancer
Biol Ther. 10:788–795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔ C (T)) Method. Methods. 25:402–408. 2001.
|