Introduction
In China, the incidence of lung cancer is increasing
every year, and the mortality rate of lung cancer has risen to the
highest among all types of cancer (1). The main type of lung cancer is
non-small cell lung cancer (NSCLC), which accounts for 85% of all
lung cancer cases.
Abnormalities in epigenetics are known to play
important roles in the development and progression of cancer.
Changes in DNA methylation patterns, common events in cancer cells,
may result in disturbance of the expression of genes that control
cell proliferation, differentiation and apoptosis, and lead to
carcinogenesis. Furthermore, abnormalities in DNA methylation are
promising diagnostic markers for the early detection of cancer
(2).
In this study, cancer tissues from 101 patients with
stage I NSCLC and lung tissues from 30 patients with non-cancerous
lung diseases, were detected for the methylation status of 13
genes: PITX2 (paired-like homeodomain 2), RARB (retinoic acid
receptor, β), OC2 (one cut homeobox 2), MYF6 (myogenic factor 6),
PDGFRA (platelet-derived growth factor receptor, α polypeptide),
SOX1 [SRY (sex determining region Y)-box 1], ALX1 (ALX homeobox 1),
SIX6 (SIX homeobox 6), PHOX2A (paired-like homeobox 2a), FOXL2
(forkhead box L2), SMPD3 (sphingomyelin phosphodiesterase 3), BCL2
(B-cell CLL/lymphoma 2) and HPP1 (hyperpigmentation, progressive,
1). The methyaltion frequencies of 7 genes: MYF6, SIX6, SOX1, RARB,
BCL2, PHOX2A and FOLX2 were significantly higher in stage I NSCLC
than in non-cancerous lung diseases.
Materials and methods
Clinical tissue samples
The clinical tissue samples from 101 stage I NSCLC
patients and 30 patients with non-cancerous lung diseases used in
this study were obtained from the Shanghai Chest Hospital
(Shanghai, China) and the First Affiliated Hospital of Guangxi
Medical University (Nanning, China). Informed consent was obtained
from the patients and the study was approved by the Medical
Institutional Review Boards of the two hospitals.
Tumor-node-metastasis (TNM) staging/classification of the patients
was performed according to the WHO classification. Table I shows the clinical patient
profiles.
 | Table IClinical profile of the stage I NSCLC
cancer patients and non-cancerous lung diseases controls. |
Table I
Clinical profile of the stage I NSCLC
cancer patients and non-cancerous lung diseases controls.
| Characteristics | Stage I NSCLC
(n=101) | Non-cancerous lung
lesions (n=30) |
|---|
| Gender | | |
| Female | 37 | 9 |
| Male | 64 | 21 |
| Age (years) | | |
| 31–40 | 1 | 0 |
| 41–50 | 9 | 7 |
| 51–60 | 32 | 12 |
| 61–70 | 39 | 6 |
| 71–80 | 20 | 5 |
| Range | 32–79 | 42–76 |
| Median | 61.28±8.92 | 60.48±7.90 |
| Histological
types | | |
| Squamous cell
carcinoma | 24 | |
| Adenocarcinoma | 48 | |
| Adenosquamous
carcinoma | 14 | |
| Others | 15 | |
| Types of
non-cancerous lung lesions | | |
| Pulmonary
tuberculosis | | 6 |
| Bronchiectasis | | 5 |
| Pulmonary
abscess | | 6 |
| Organizing
pneumonia | | 2 |
| Pulmonary
sclerosing hemangioma | | 3 |
| Pulmonary giant
lymph node hyperplasia | | 2 |
| Pulmonary
hamartoma | | 3 |
| Pulmonary
sequestration | | 1 |
| Pulmonary
inflammatory pseudotumor | | 2 |
DNA isolation and methylation-specific
PCR (MSP)
Genomic DNA of clinical tissues were isolated by a
standard phenol/chloroform purification method. The primers for MSP
were designed according to: http://www.urogene.org/methprimer/index1.html
(Table II). The bisulfate
conversion and PCR analysis were conducted as previously described
(3).
 | Table IIPrimer list for methylation-specific
PCR. |
Table II
Primer list for methylation-specific
PCR.
| Gene name | GenBank No. | Sense 5′-3′ | Antisense 5′-3′ | Size (bp) |
|---|
| ALX1 | NC_000012.11 |
TTTTTTGGAGTACGTTATGGAGAC |
AACGCACGTAATACTCGACG | 116 |
| BCL2 | NG_009361.1 |
GAAGTCGTCGTCGGTTTG | CCCGCACCGAACATC | 183 |
| FOXL2 | NG_012454.1 |
GTTATAATATTTTTTCGGTTGTTCG |
CTAACTCCACGACCTATACTCGAT | 211 |
| HPP1 | AF242221 |
AAGAGGGGCGTTAGTTCG | CGCTCGCAAACGCTAA | 158 |
| MYF6 | NG_021392.1 |
GGAAATGCGTATTCGGTTC |
CGAACCCCCTAAAATAATCG | 182 |
| OC2 | NC_000018.9 |
CGGGTTCGTAGGTGGTTAC |
TCCACGATTTTAAATTCCGA | 177 |
| PDGFRA | NG_009250.1 |
CGTCGCGTTGTTTTATTTTC |
AATCGACCTTACGCCTATCG | 160 |
| PHOX2A | NG_008169.1 |
AGGGATAGTTATAAGCGCGG |
AAAAATACAAAATCGTATAAACCTCG | 211 |
| PITX2 | NG_007120.1 |
GATCGTTAGTCGCGTAGTCG |
TCCAACTTTCTCGCTCGAT | 177 |
| RARB | NM_016152 |
TCGAGAACGCGAGCGATTCG |
GACCAATCCAACCGAAACGA | 146 |
| SIX6 | NG_008203.1 |
TTAGTAGTTAGGCGTTGGGATC |
CCTCTCGAAATAATTACTTTACCG | 150 |
| SMPD3 | NC_000016.9 |
TCGTAGGATTTTCGAAGGATC |
CATCACCGACGAATATAATCG | 160 |
| SOX1 | NC_000013.10 |
GGTATTGGCGAATTTTAGTGTAC |
AAAAAAACGCTCCCTTAAACG | 135 |
Immunohistochemical analysis
Among the 101 cases of stage I NSCLC patients, 92
routinely underwent detection of 8 pathological protein markers by
immunohistochemistry before chemotherapy. These pathological
markers were: vascular endothelial growth factor (VEGF), human
epidermal growth factor receptor 2 (HER-2), p53, p21, epidermal
growth factor receptor (EGFR), chromogranin A (CHGA), synaptophysin
(SYN) and epithelium membrane antigen (EMA). For each sample, the
H&E-stained sections were first reviewed and marked for the
selected point. Tumor samples were embedded in paraffin and cut
into 3-μm sections. Sections were processed using the Super
Sensitive Link-Labeled Detection System (Biogenex, Menarini,
Florence, Italy). The first antibodies used in this study were
purchased from Sant Cruz Biotechnology (Santa Cruz, CA, USA). The
second antibodies were purchased from KangChen Bio-Tech Inc.
(Shanghai, China). The enzymatic activity was developed using
3-amino-9-ethylcarbazole (Dako, Milan, Italy) as a chromogenic
substrate. The result was scored by conjunction with both staining
intensity and the percentage of positive staining cells. Each
sample was given an intensity score (0–3) and a percentage of cell
positive score (0, <5%; 1, 5–25%; 2, 25–50%; 3, 50–75%; 4, >
75%). An overall immunohistochemistry score was calculated by
multiplying the intensity and percentage of cell positive scores.
Scores of 1–4 were recorded as +, 6–8 as ++, and 9–12 as +++.
Statistical analysis
All statistical calculations were performed using
the SPSS 13.0 software statistical package (SPSS Inc., Chicago, IL,
USA). The incidence of hypermethylation in NSCLC tissues vs. the
non-cancerous tissues was calculated using a 2×2 Fisher’s exact
test. The associations among the pathological variables and the
methylation status of the genes were assessed by means of
univariate and multivariate logistic-regression analysis. The area
under the receiver operating characteristic (ROC) curve (AUC) is a
measure of the ability of a continuous marker to accurately
classify tumor tissues and non-tumor tissues. Correlations between
the expression of pathological markers and gene methylation were
examined using the Chi-square test. P<0.05 was considered to
indicate a statistically significant result.
Results
Methylation frequencies of the 7 genes
differ significantly between stage I NSCLC and non-cancerous
controls
Among the 13 genes, the methylation frequencies of 7
genes (MYF6, SIX6, SOX1, RARB, BCL2, PHOX2A and FOLX2) had
significant difference between the group of stage I NSCLC and the
group of non-cancerous lung diseases (Table III). ROC curves were constructed for
each of the 7 genes to classify stage I NSCLC and non-cancerous
lung disease. The AUC of the ROC curve for MYF6 was 0.704
(P<0.0001; 95% CI, 0.613–0.795), which was the largest among the
7 genes. The sensitivity and specificity of MYF6 were 64.36 and
93.33%, respectively, in the diagnosis of stage I NSCLC. The AUC of
the ROC curves for the other 6 genes (SIX6, SOX1, RARB, BCL2,
PHOX2A and FOLX2) ranged from 0.573 to 0.667; the sensitivity of
each gene ranged from 29.70 to 51.49% and the specificity ranged
from 73.33 to 93.33%, if they were used separately to diagnose
stage I NSCLC. The methylation frequencies of the other 6 genes
(ALX1, PDGFRA, PITX2, HPP1, OC2 and SMPD3) had no significant
difference between tumors and controls, ranging from 24.75 to
59.41% in stage I NSCLC, and from 56.67 to 90.00% in non-cancerous
lung diseases (Table III).
 | Table IIIDiagnosis performance of the 13
methylation targets in stage I NSCLC versus non-cancerous lung
diseases. |
Table III
Diagnosis performance of the 13
methylation targets in stage I NSCLC versus non-cancerous lung
diseases.
| Stage I NSCLC
| Non-cancerous lung
diseases
|
|---|
| Target | Sensitivity
(%) | pos./total | Specificity
(%) | pos./total | AUC | (95% CI) | PPV | NPV | P-value |
|---|
| MYF6 | 64.36 | 65/101 | 93.33 | 2/30 | 0.704 | 0.613–0.795 | 0.64 | 0.93 | <0.0001 |
| SIX6 | 51.49 | 52/101 | 90.00 | 3/30 | 0.650 | 0.557–0.743 | 0.51 | 0.90 | 0.0007 |
| SOX1 | 50.50 | 51/101 | 73.33 | 8/30 | 0.573 | 0.475–0.671 | 0.51 | 0.70 | 0.0213 |
| RARB | 47.52 | 48/101 | 96.67 | 1/30 | 0.667 | 0.576–0.757 | 0.48 | 0.97 | <0.0001 |
| BCL2 | 37.62 | 38/101 | 90.00 | 3/30 | 0.613 | 0.515–0.712 | 0.38 | 0.90 | 0.0035 |
| PHOX2A | 35.64 | 36/101 | 93.33 | 2/30 | 0.624 | 0.526–0.722 | 0.36 | 0.93 | 0.0013 |
| FOLX2 | 29.70 | 30/101 | 93.33 | 2/30 | 0.610 | 0.507–0.714 | 0.30 | 0.93 | 0.0082 |
| ALX1 | 59.41 | 60/101 | 56.67 | 13/30 | N | N | 0.59 | 0.57 | 0.1447 |
| PDGFRA | 37.62 | 38/101 | 70.00 | 9/30 | N | N | 0.38 | 0.70 | 0.5195 |
| PITX2 | 34.65 | 35/101 | 83.33 | 5/30 | N | N | 0.35 | 0.83 | 0.0724 |
| HPP1 | 32.67 | 33/101 | 56.67 | 13/30 | N | N | 0.33 | 0.57 | 0.2864 |
| OC2 | 24.75 | 25/101 | 86.67 | 4/30 | N | N | 0.25 | 0.87 | 0.2197 |
| SMPD3 | 24.75 | 25/101 | 90.00 | 3/30 | N | N | 0.26 | 0.90 | 0.0819 |
Expression of 8 pathological markers in
stage I NSCLC
The positive expression rates of CHGA and SYN were
the lowest among the 8 protein (both 3.26%, 3/92), while that of
EMA was the highest (100%, 92/92). The positive rates of the other
5 pathological markers were: p21 (8.70%, 8/92), VEGF (28.26%,
26/92), EGFR (29.35%, 27/92), HER-2 (39.13%, 36/92) and p53
(42.39%, 39/92).
Correlation between the methylation
status of the 7 genes and the clinical characteristics of
NSCLC
The correlations between the methylation status of
the 7 genes, individually or combined, with each of the clinical
characteristics was primarily assessed by univariate analysis, and
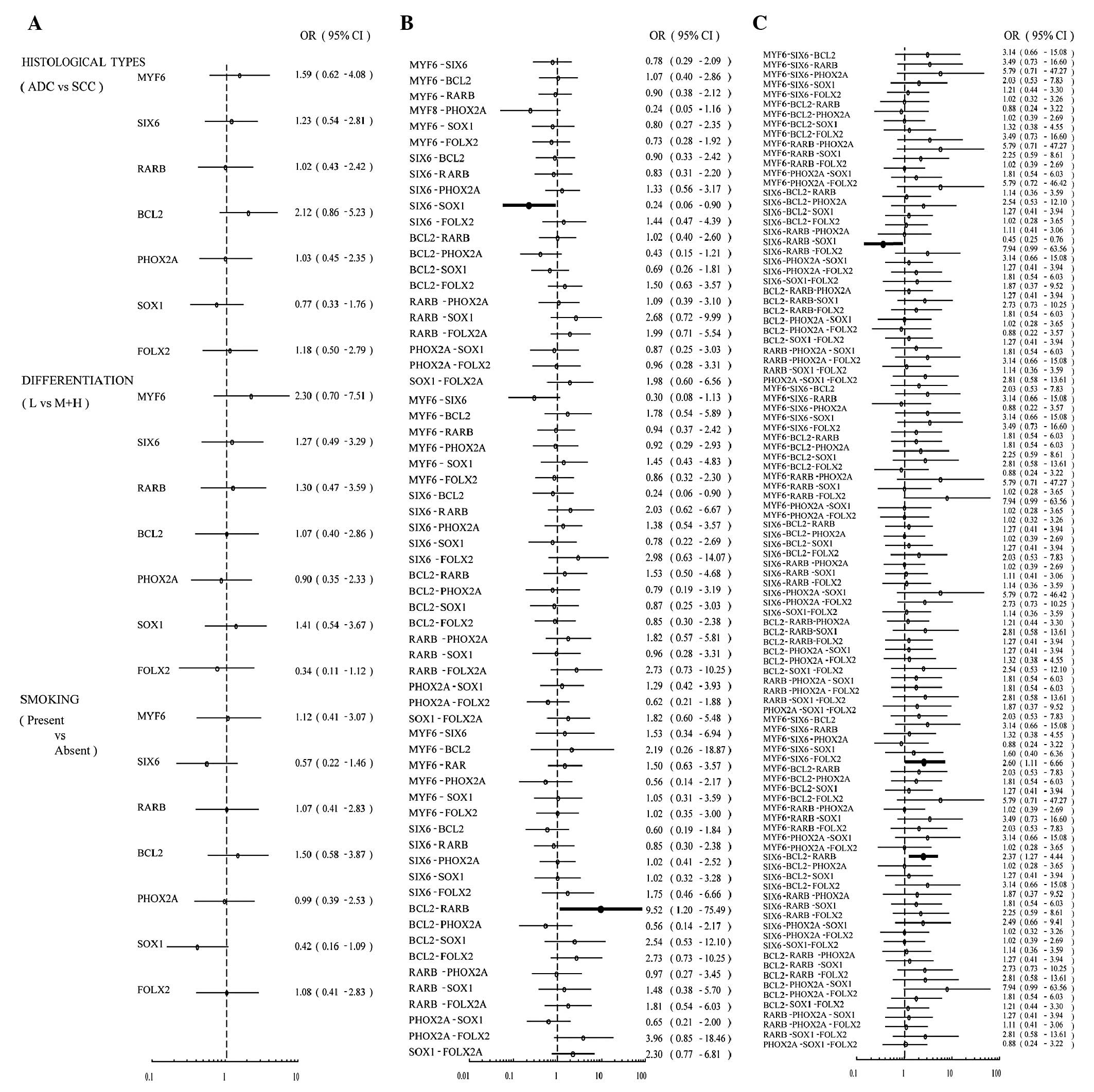
the result was displayed in the form of a forest plot (Fig. 1). The methylation status of each of
the 7 genes individually had no association with histological
types, degree of differentiation or smoking. Next, the correlation
between the co-methylation of two genes with the clinical
characteristics was assessed in 21 possible pairs of genes. The
co-methylation of SIX6 and SOX1 was negatively associated with
adenocarcinoma (ADC) with an odds ratio (OR) of 0.24 (95% CI,
0.06–0.90). The co-methylation of BCL2 and RARB was associated with
smoking (OR, 9.52; 95% CI, 1.20–75.49). We also analyzed the
correlations between co-methylation of three genes with the
clinical characteristics. The co-methylation of SIX6, RARB and SOX1
occurred less frequently in adenocarcinoma (ADC) than in squamous
cell carcinoma (OR, 0.45; 95% CI, 0.25–0.76). The co-methylation of
MYF6, SIX6 and FOLX2 (OR, 2.60; 95% CI, 1.11–6.66) or the
co-methylation of SIX6, BCL2 and RARB(OR, 2.37; 95% CI, 1.27–4.44)
were associated with smoking (Fig.
1).
To verify these correlations, multivariate
regression models were established. These indicated that the
co-methylation of SIX6 and SOX1, as well as the co-methylation of
SIX6, RARB and SOX1, was negatively associated with ADC; the latter
association being more significant (SIX6 and SOX1: OR, 0.243; 95%
CI, 0.06–0.98; P=0.045; SIX6, RARB and SOX1: OR, 0.008; 95% CI,
0.001–0.149; P=0.007). The association between the co-methylation
of SIX6, BCL2 and RARB and smoking has also been validated (OR,
3.09; 95% CI, 1.20–7.95; P=0.019). However, the association beween
smoking and the co-methylation of BCL2 and RARB, or the
co-methylation of MYF6, SIX6 and FOLX2, had no statistical
significance (P>0.05; Table
IV).
 | Table IVMultivariate analysis of the
correlation between gene methylation and clinical characteristics
of NSCLC. |
Table IV
Multivariate analysis of the
correlation between gene methylation and clinical characteristics
of NSCLC.
| Case | Genes | Status | No. of
methylations | No. of cases | OR | 95% CI | P-value |
|---|
| ADC/SCC | SIX6-SOX1 | Negative | 53 | 2 | | | |
| | Positive | 19 | 17 | 0.24 | 0.06–0.98 | 0.045 |
| SIX6-RARB-SOX1 | Negative | 60 | 0 | | | |
| | Positive | 12 | 12 | 0.01 | 0.001–0.149 | 0.007 |
| Smoking | BCL2-RARB | Negative | 85 | 1 | | | |
| | Positive | 16 | 15 | 9.16 | 0.99–84.45 | 0.051 |
| SIX6-BCL2-RARB | Negative | 93 | 1 | | | |
| | Positive | 8 | 7 | 3.09 | 1.20–7.95 | 0.019 |
|
MYF6-SIX6-FOLX2 | Negative | 87 | 1 | | | |
| | Positive | 14 | 13 | 8.11 | 0.83–78.50 | 0.071 |
A panel of 4 genes for the diagnosis of
stage I NSCLC
Combination of several markers is a common strategy
to improve diagnostic sensitivity in studies of clinical
biomarkers. In this study, the most outstanding gene for the
diagnosis of stage I NSCLC, MYF6, was found to be methylated in 65
of the 101 cases of patients with stage I NSCLC, displaying a
sensitivity of 64.36%; and the methylation of MYF6 was also found
in 2 of the 30 cases of patients with non-cancerous lung diseases,
displaying a specificity of 93.3%. In the 36 cases of stage I NSCLC
patients without MYF6 methylation, the methylation frequency of
SIX6 was 41.67% (15/36), the highest among the 6 genes other than
MYF6. Therefore, we made the first combination of MYF6 and SIX6 for
the diagnosis of stage I NSCLC. The sensitivity was improved to
79.21%, while the specificity was dropped to 90.00%. However, the
AUC of the ROC curve for the combination of MYF6 and SIX6 was 0.774
(P<0.0001; 95% CI, 0.681–0.866), higher than MYF6 alone, which
meant that the combination of MYF6 and SIX6 was superior to MYF6
alone in diagnostic power. The methylation of BCL2 was detected in
8 of the 21 cases without methylation of either MYF6 or SIX6; more
frequently than the other 4 genes, thus we made the second
combination to form a 3-gene panel (MYF6, SIX6 and BCL2). The
sensitivity, specificity and AUC were 87.13%, 86.67% and 0.812
(P<0.0001; 95% CI, 0.717–0.906), respectively. Using this method
we analyzed a total of 6 panels of genes. The AUC of the 4-gene
panel (MYF6, SIX6, BCL2 and RARB) was the largest among the them,
and thus made it the best combination of markers in this study. The
sensitivity, specificity and AUC of the 4-gene panel were 93.07%,
86.67% and 0.874 (P<0.0001; 95% CI, 0.787–0.960), respectively
(Table V).
 | Table VDiagnostic performance of different
panels of genes in stage I NSCLC, using patients with non-cancerous
lung diseases as controls. |
Table V
Diagnostic performance of different
panels of genes in stage I NSCLC, using patients with non-cancerous
lung diseases as controls.
| NSCLC (n=101)
| Non-cancerous lung
diseases (n=30)
|
|---|
| Sensitivity
(%) | pos./total | Specificity
(%) | pos./total | AUC | 95% CI | PPV | NPV | P-value |
|---|
| MYF6 | 64.36 | 65/101 | 93.33 | 2/30 | 0.681 | 0.587–0.774 | 0.970 | 0.438 | <0.0001 |
| MYF6,SIX6 | 79.21 | 80/101 | 90.00 | 3/30 | 0.774 | 0.681–0.866 | 0.964 | 0.571 | <0.0001 |
| MYF6,SIX6,BCL2 | 87.13 | 88/101 | 86.67 | 4/30 | 0.812 | 0.717–0.906 | 0.957 | 0.667 | <0.0001 |
|
MYF6,SIX6,BCL2,RARB | 93.07 | 94/101 | 86.67 | 4/30 | 0.874 | 0.787–0.960 | 0.961 | 0.798 | <0.0001 |
|
MYF6,SIX6,BCL2,RARB, PHOX2A | 94.06 | 95/101 | 83.33 | 5/30 | 0.868 | 0.792–0.964 | 0.950 | 0.807 | <0.0001 |
|
MYF6,SIX6,BCL2,RARB, PHOX2A,SOX1 | 96.04 | 97/101 | 63.33 | 11/30 | 0.836 | 0.731–0.940 | 0.898 | 0.826 | <0.0003 |
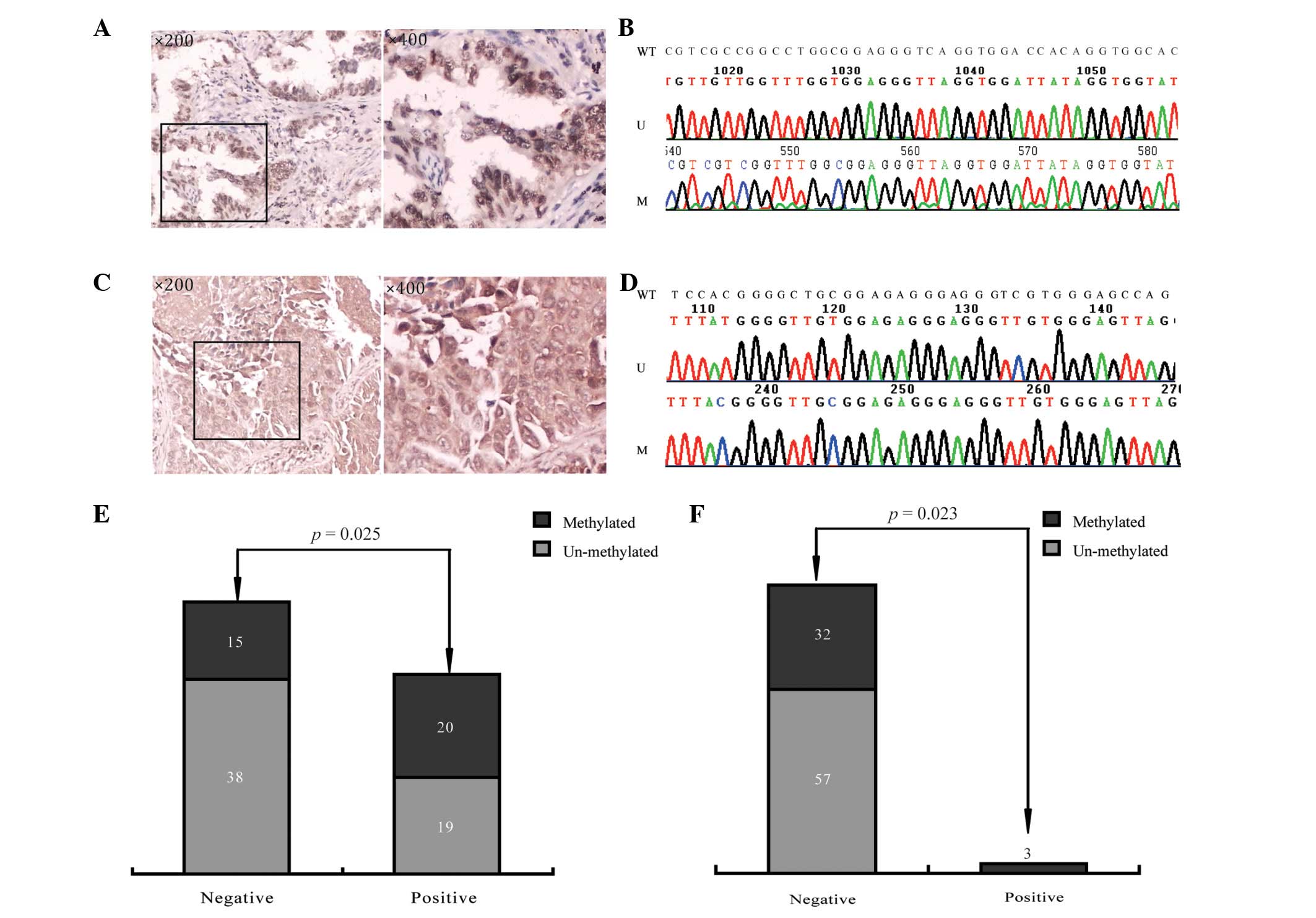
Correlations between the expression of
pathological markers and gene methylation in stage I NSCLC
We analyzed the data and aimed to explore whether
the expression of each protein was associated with the methylation
status of any of the 7 genes. We found that the expression of p53
was positively associated with the methylation of BCL2 (P=0.025)
and the expression of CHGA was positively associated with the
methylation of PHOX2A (P=0.023; Fig.
2 and Table VI).
 | Table VICorrelations between the expression
of pathological markers with gene methylation in stage I NSCLC. |
Table VI
Correlations between the expression
of pathological markers with gene methylation in stage I NSCLC.
| CHGA | EGFR | EMA | HER-2 | P21 | P53 | SYN | VEGF |
|---|
| MYF6 | N | N | N | N | N | N | N | N |
| SIX6 | N | N | N | N | N | N | N | N |
| RARB | N | N | N | N | N | N | N | N |
| BCL2 | N | N | N | N | N | 0.025 | N | N |
| PHOX2A | 0.023 | N | N | N | N | N | N | N |
| SOX1 | N | N | N | N | N | N | N | N |
| FOLX2 | N | N | N | N | N | N | N | N |
Discussion
This study showed that 7 genes (MYF6, SIX6, SOX1,
RARB, BCL2, PHOX2A and FOLX2) were frequently methylated in 101
cases of patients with stage I NSCLC, while rarely methylated in 30
patients with non-cancerous lung diseases. A panel of 4 genes
(MYF6, SIX6, BCL2 and RARB) was able to diagnose stage I NSCLC from
non-cancerous lung diseases with a sensitivity of 93.07% and a
specificity of 86.67%.
RARB and BCL2 have already been found to be
hypermethylated in NSCLC (4,5). The
protein encoded by SOX1 acts as transcription factor and plays a
part in the regulation of embryonic development and in the
determination of cell fate. Although the methylation of SOX1 have
been found to be associated with genitourinary tumors, including
cervical cancer, prostate cancer and ovarian cancer, this is the
first time that methylation of SOX1 has been found in NSCLC
(6–8). It has been reported that SOX1
antibodies are common in the serum of patients with small cell lung
carcinoma (SCLC) and may be serve as specific serological markers
(9). The methylation of 4 genes,
MYF6, SIX6, PHOX2A, FOLX2, has never previously been reported in
any types of cancer. MYF6 (12q21) is involved in muscle
differentiation, SIX6 (14q23.1) is thought to be involved in eye
development, and PHOX2A (11q13.2) is vital for development of the
autonomic nervous system (10),
FOXL2, as a forkhead transcription factor, may be involved in
ovarian development and function. Further studies in the functions
of these gene may help to reveal the mechanism of malignant
transformation of non-small-cell lung cells.
We found that the co-methylation of SIX6 and SOX1
correlated with squamous cell carcinoma (SCC), while the
methylation of neither of them individually demonstrated an
association with SCC, which may imply that the methylation of these
two genes had a superimposed effect on the development of SCC. The
co-methylation of BCL2, RARB and SIX6, but not the methylation of
either single gene, was associated with smoking. We postulated that
cigarette smoking may cause the methylation of the 3 genes through
a common pathway.
To explore the possible pathway through which gene
methylation promotes the carcinogenesis and progression of NSCLC,
we investigated the expression of 8 classical components (VEGF,
HER-2, P53, P21, EGFR, CHGA, SYN and EMA) of common oncogenic
pathways in 92 cases of stage I NSCLC using immuno histochemistry.
Analyzing the expression data with the DNA methylation status in
this study, we found that the expression of P53 and CHGA were
positively associated with the methylation of BCL2 and PHOX2A,
respectively. Bcl2, encoded by the gene BCL2, as a critical
pro-survival member of Bcl2 protein family which regulates
apoptosis, is usually considered to be a downstream target of p53
and can be negatively regulated by p53 (11,12).
The overexpression of Bcl2 has been found in numerous types of
cancer, including breast cancer, prostate cancer, B-cell lymphoma
and colorectal adenocarcinoma (13). However, recently, the
hypermethylation of BCL2 gene has been reported in certain types of
cancer, such as prostate cancer (14). Furthermore, the methylation of BCL2
was found to be associated with tumor invasion in peripheral
pulmonary adenocarcinoma (5). In
this study, the methylation frequency of BCL2 was significantly
higher in lung tissues of stage I NSCLC, than in non-cancerous lung
diseases, and its methylation was positively associated with the
expression of P53 in stage I NSCLC. Wang et al reported that
wild-type p53 negatively regulated DNMT1 expression through
interaction with specificity protein 1 (Sp1) protein (6). Since DNA methylation was usually
accompanied by gene silencing, the role of BCL2 in the development
and progression of cancer was complicated and further studies are
warranted. The methylation of PHOX2A demonstrated positive
association with the expression of CHGA. CHGA protein was thought
to be a tumor marker in neuroendocrine tumors (NETs), but high
expression of CHGA was also found in several other types of solid
tumor, such as small cell lung cancer (15), and higher expression of CHGA was
associated with higher pathological stage in prostate cancer
(16). The function of PHOX2A in
carcinogenesis of NSCLC may be correlated with CHGA.
Tumorigenesis is an intricate process, involving a
variety of genetic and epigenetic aberrations. Even a single
tumor-related gene may simultaneously display several types of
abnormalities, and contribute to tumorigenesis through several
different ways. In this study, 5 genes were for the first time
found to be hypermethylated in NSCLC, and the function of those
genes and how they act in the carcinogenesis of NSCLC is worth
further exploration.
Acknowledgements
This study was supported in part by
the State Key Laboratory of Oncogenes and Related Genes (Grant No.
91-11-01), the Medical Guide Project from Science and Technology
Commission of Shanghai (114119a4100) and the Shanghai Science
Foundation (Grant No. 09ZR1429900; Y. Zhao). Also the Youth Fund of
the National Natural Science Foundation of China (No. 81201576, K.
Ma) and The Key Research Projects of the Shanghai Health Bureau
(No.2010010; Q. Lin), and the Shanghai Natural Science Foundation
(No.11ZR1433800; Q. Lin).
References
|
1
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rauch TA, Wang Z, Wu X, Kernstine KH,
Riggs AD and Pfeifer GP: DNA methylation biomarkers for lung
cancer. Tumour Biol. 33:287–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu J, Zhu T, Wang Z, et al: A novel set of
DNA methylation markers in urine sediments for sensitive/specific
detection of bladder cancer. Clin Cancer Res. 13:7296–7304. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feng Q, Hawes SE, Stern JE, et al: DNA
methylation in tumor and matched normal tissues from non-small cell
lung cancer patients. Cancer Epidemiol Biomarkers Prev. 17:645–654.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chung JH, Lee HJ, Kim BH, Cho NY and Kang
GH: DNA methylation profile during multistage progression of
pulmonary adenocarcinomas. Virchows Arch. 459:201–211. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin R, Wu C, Chang J, et al: Dysregulation
of p53/Sp1 control leads to DNA methyltransferase-1 overexpression
in lung cancer. Cancer Res. 70:5807–5817. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mathews LA, Hurt EM, Zhang X and Farrar
WL: Epigenetic regulation of CpG promoter methylation in invasive
prostate cancer cells. Mol Cancer. 9:2672010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Su HY, Lai HC, Lin YW, Chou YC, Liu CY and
Yu MH: An epigenetic marker panel for screening and prognostic
prediction of ovarian cancer. Int J Cancer. 124:387–393. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Titulaer MJ, Klooster R, Potman M, et al:
SOX antibodies in small-cell lung cancer and Lambert-Eaton
myasthenic syndrome: frequency and relation with survival. J Clin
Oncol. 27:4260–4267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Benfante R, Antonini RA, Kuster N, et al:
The expression of PHOX2A, PHOX2B and of their target gene
dopamine-beta-hydroxylase (DbetaH) is not modified by exposure to
extremely-low-frequency electromagnetic field (ELF-EMF) in a human
neuronal model. Toxicol In Vitro. 22:1489–1495. 2008. View Article : Google Scholar
|
|
11
|
Frenzel A, Grespi F, Chmelewskij W and
Villunger A: Bcl2 family proteins in carcinogenesis and the
treatment of cancer. Apoptosis. 14:584–596. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kelly PN and Strasser A: The role of Bcl-2
and its pro-survival relatives in tumourigenesis and cancer
therapy. Cell Death Differ. 18:1414–1424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu W, Bulgaru A, Haigentz M, Stein CA,
Perez-Soler R and Mani S: The BCL2-family of protein ligands as
cancer drugs: the next generation of therapeutics. Curr Med Chem
Anticancer Agents. 3:217–223. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vasiljevic N, Wu K, Brentnall AR, et al:
Absolute quantitation of DNA methylation of 28 candidate genes in
prostate cancer using pyrosequencing. Dis Markers. 30:151–161.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Molina R, Alvarez E, Aniel-Quiroga A, et
al: Evaluation of chromogranin A determined by three different
procedures in patients with benign diseases, neuroendocrine tumors
and other malignancies. Tumour Biol. 32:13–22. 2011. View Article : Google Scholar
|
|
16
|
Ma Z, Tsuchiya N, Yuasa T, et al: Clinical
significance of polymorphism and expression of chromogranin a and
endothelin-1 in prostate cancer. J Urol. 184:1182–1188. 2010.
View Article : Google Scholar : PubMed/NCBI
|