Introduction
Hepatocellular carcinoma (HCC) and intrahepatic
cholangiocarcinoma (ICC) constitute two major forms of primary
liver cancer. The two malignancies have different clinical and
pathological features and prognosis, but in some cases
histopathological overlap exists. Immunochemistry is thus required
to facilitate differential diagnosis between HCC and ICC. To date,
several antibodies, such as cytokeratin 19 (CK19), CD10, Hep Par 1,
AFP, CA19-9, MOC31, glypican-3 and CEA, have been used to
differentiate between the two malignant tumor types (1–3).
CD79α and CD79β belong to the Ig gene superfamily
and contain one extracellular Ig-like domain, a transmembrane
α-helical region and a cytoplasmic domain (3). These glycoproteins form a
disulfide-linked heterodimer in the B-cell receptor (BCR) and
pre-BCR complexex (4). The CD79α/β
complex is critical to B-cell development, mediating signal
transduction and promoting endocytosis of bound antigens for
intracellular degradation and presentation to helper T cells
(4). CD79α protein is present in
B-cell follicles in lymph nodes, plasma cells and the majority of
circulating B cells (5,6), but absent in brain, colon, kidney,
liver, muscle, pancreas and placenta tissues. CD79α expression is
not limited to B cells, as it is also detected in the normal early
myeloid precursors and megakaryocytes (7). We (unpublished data) disclosed strong
immunoreactivity of HM47/A9 with hepatocytes, but not with the bile
canaliculus or interlobular bile duct. In the present study, we
compared HM47/A9 antibody expression patterns in HCC and ICC.
Materials and methods
Cases selected for study
Normal adult livers were obtained from tissue
surrounding the liver cancer. Eight-week embryo liver was acquired
from a case of ruptured tubal pregnancy and the 20-week embryo
liver sample was obtained from a perinatal mortality. We reviewed
primary liver cancer cases between January 2002 and December 2012
in the People’s Liberation Army 152 Hospital (Henan, China),
including 82 cases of HCC, 31 cases of ICC and 11 cases of combined
HCC and cholangiocarcinoma (cHCC-CC), which were subjected to
resection or puncture. All specimens were fixed in 10%
neutral-buffered formalin, dehydrated in graded alcohol solutions,
embedded in paraffin and cut into 4-μm-thick sections for
hematoxylin and eosin staining, followed by visualization using
light microscopy.
Immunohistochemical analysis
Immunohistochemical staining was performed on
formalin-fixed, paraffin-embedded tissue sections using the
EnVision method. The primary antibodies employed included CD79α
(HM47/A9), AFP (ZSA06), CK19 (A53-B/A2.26), MOC31 (MOC31), CA19-9
(TA888), CEA (Col-1) and Hepatocyte (OCH1E5). All antibodies were
purchased from Maxin-Bio Co. (Fuzhou, China). Slides were
counterstained with hematoxylin. To observe mallory hyaline bodies
and globular hyaline bodies, slides were counterstained with eosin
after immunostaining for CD79α.
Results
Clinical features
The 82 HCC patients included 48 males and 34 females
with a median age of 46 years (range, 24–70 years). The 31 ICC
patients included 16 males and 15 females with a median age of 51
years (range, 37–74 years). The 11 cases of cHCC-CC occurred in 6
males and 5 females with a median age of 48 years (range, 26–69
years).
Histological, pathological and
immunochemistry findings
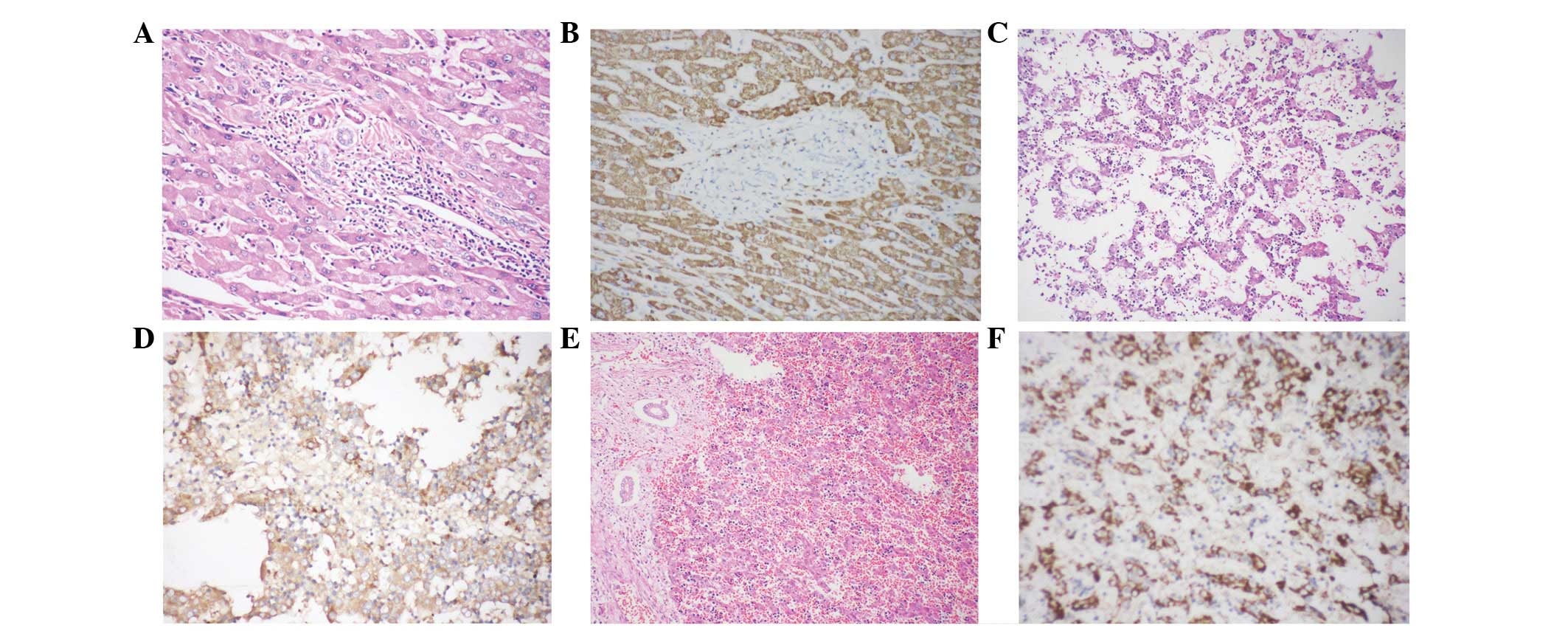
Normal hepatocytes (Fig.
1A) exhibited diffusely granular, cytoplasmic immunoreactivity
to HM47/A9 (Fig. 1B). By contrast,
no HM47/A9 positivity was observed in the bile canaliculus or
interlobular bile duct (Fig.
1B).
In the 8-week embryo liver (Fig. 1C), ∼20% of hepatocytes displayed
granular positivity for HM47/A9 (Fig.
1D). Hepatocytes of 20-week embryo liver (Fig. 1E) demonstrated diffuse
immunoreactivity to HM47/A9 with a granular pattern in the
cytoplasm (Fig. 1F), which was
retained throughout life.
HCC cells resembled hepatocytes (Fig. 2A). Some tumors had a plate-like
pattern, while other HCC cells formed a pseudo glandular pattern.
Tumor cells were round or oval-shaped with abundant granular
eosinophilic cytoplasm and single, large central nuclei. Pale
bodies and fatty changes were evident. HCC cells tested positive
for Hepatocyte (79/82, 96.3%) and AFP (24/82, 29.3%) and negative
for the CEA, CK19, CA19-9 and MOC31. All 82 HCC tumor cells
exhibited diffuse granular positivity for HM47/A9 (Fig. 2B).
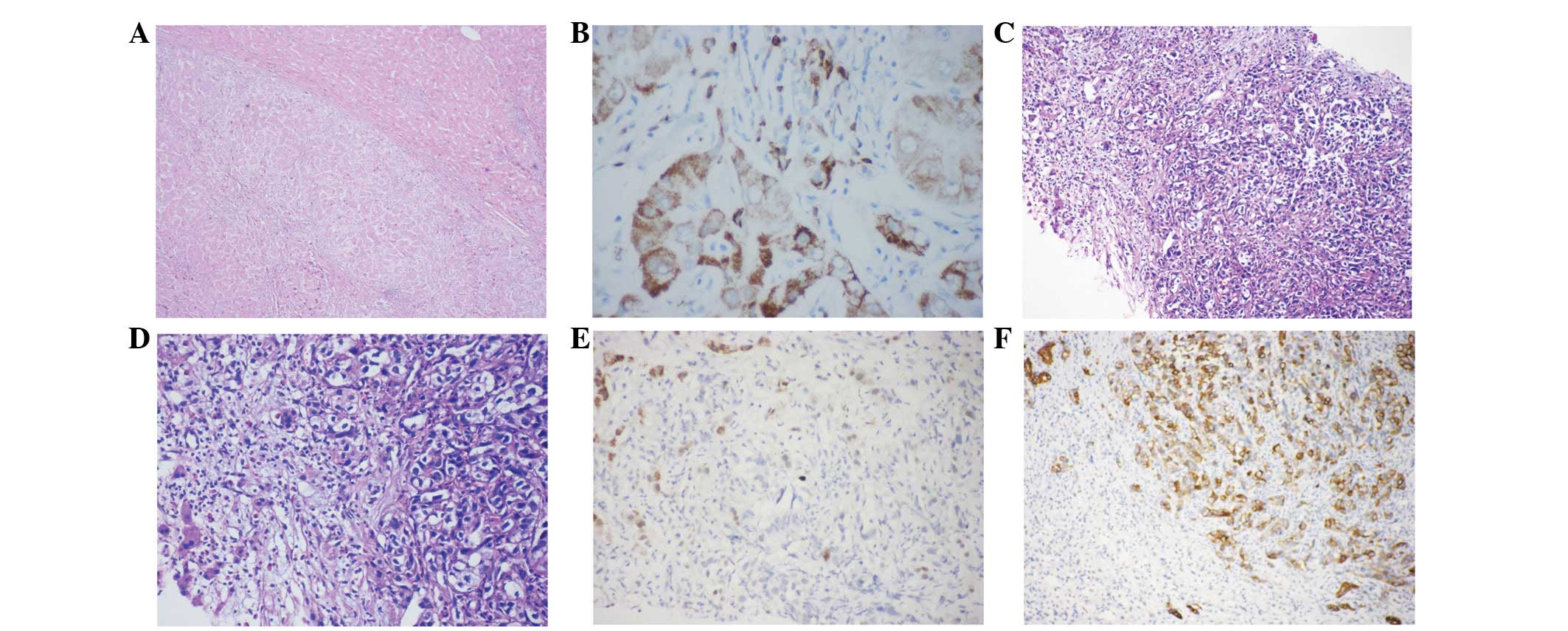 | Figure 2(A) HCC and surrounding liver tissue.
Magnification, ×24. (B) HCC exhibiting (90%) positivity for
HM47/A9. Magnification, ×240 (C and D) Low-power image depicting
ICC and residual hepatocytes. Magnification, ×60 and ×120,
respectively. (E) ICC negative for HM47/A9, hepatocytes positive
for HM47/A9. Magnification, ×120. (F) ICC positive for CK19,
hepatocytes negative for CK19. Magnification, ×120. HCC,
hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma. |
ICC cells were round or oval, and some were
pleomorphic (Fig. 2C and D). Nuclei
were small or large with more than one small nucleolus, while the
cytoplasm was eosinophilic or vacuolated. Tumor cells formed a
tubular gland or cord-like pattern, and all cases were negative for
HM47/A9 (Fig. 2E). The tumor cells
were positive for the CK19 (31/32, 96.9%) (Fig. 2F), CEA (7/10, 70%), CA19-9 (25/32,
78.1%) and MOC31 (26/32, 81.3%).
Each of the CC parts in cHCC-CC [11/11 (100%)] did
not express HM47/A9. The majority of HCC components in cHCC-CC
[10/11 (90.9%)] exhibited positive staining for HM47/A9. The
excluded case was in a 26-year-old female. These tumor cells
demonstrated a similar histology to hepatocytes, with abundant
eosinophilic or vacuolated cytoplasm and a centronucleus. Prominent
nucleoli were observed in the nucleus. Tumor cells grew as entities
and invaded the inter-lobular bile duct wall (Fig. 3A–C). Significant tumor thrombus in
the hemal tube was evident (Fig.
3A). Tumor cells were immunoreactive for Hepatocyte (Fig. 3D), CK19 (Fig. 3F), CEA, CA19-9 and MOC31 (Fig. 3G), but negative for AFP and HM47/A9
(Fig. 3H). CEA (Fig. 3E) immunostaining data revealed no
bile canaliculus between carcinoma cells.
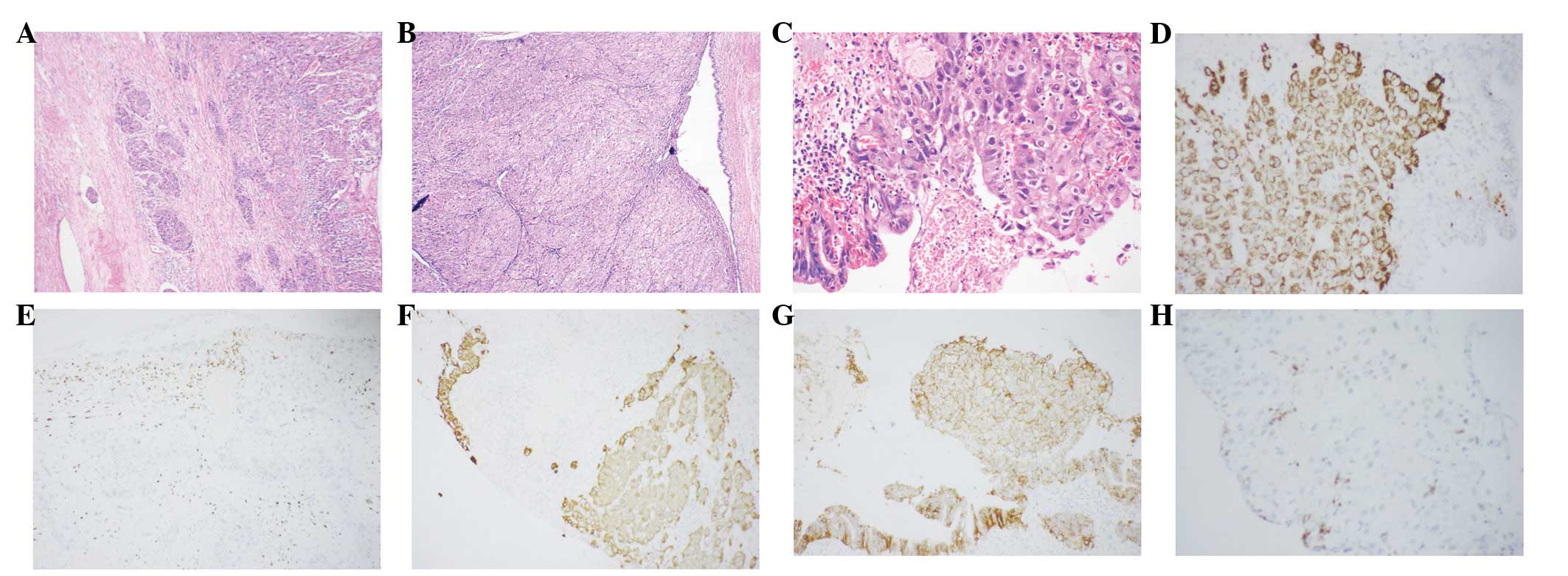 | Figure 3(A) Tumor thrombus. Magnification,
×24. (B and C) cHCC-CC invading the bile duct wall. Tumor cells
displaying features of hepatocytes. (D) Tumor cells are
immunoreactive for hepatocyte, while bile duct cells are negative
for hepatocyte. Magnification, ×24 and ×120, respectively. (E)
Inflammatory cells positive for pCEA, with no bile canaliculus
observed between tumor cells. Magnification, ×60. (F and G) Tumor
cells and bile duct cells positive for MOC31 and CK19,
respectively. Magnification, ×60 and ×120, respesctively. (H) Tumor
cells and bile duct cells negative for HM47/A9, B cells positive
for HM47/A9 Magnification, ×120. cHCC-CC, combined HCC and
cholangiocarcinoma. |
Pale bodies, mallory hyaline bodies, fatty
degeneration and globular hyaline bodies were negative for HM47/A9
(Fig. 4).
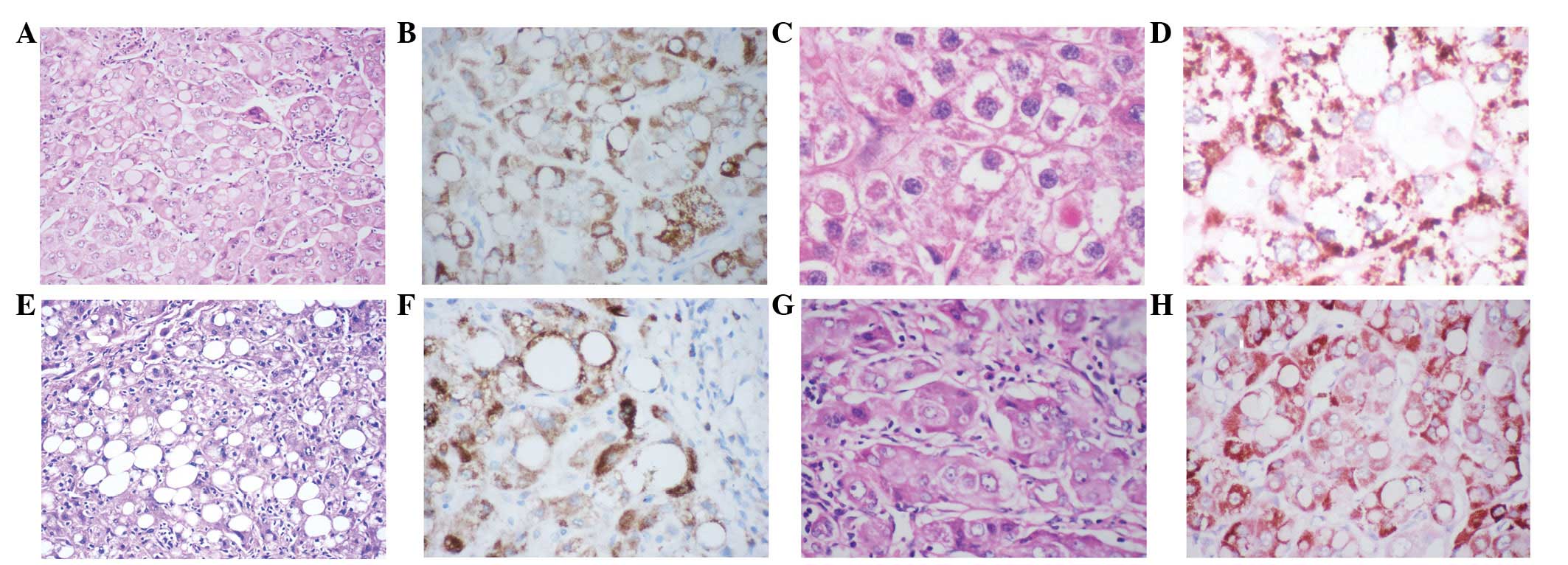 | Figure 4(A) Pale bodies, (C) mallory hyaline
bodies, (E) fatty changes, and (G) globular hyaline bodies negative
for HM47/A9 (B, D, F and H). (B and F) counterstained with
hematoxylin; (D and H) counterstained with hematoxylin and eosin.(A
and E; magnification, ×120) (B,C,D,F and G; magnification,
×240). |
Discussion
CD79α, also known as Igα, is encoded by mouse B
cell-specific gene 1 (mb-1) (3).
The gene has been identified in pre-B and B cells, as well as
thymocytes and peripheral blood T cells (3,8), but
is absent in HeLa and kidney cells (3). In addition, no CD79α antigen has been
detected in the liver, brain, colon, muscle or placenta tissue
(5). However, we showed that
hepatocytes exhibit strong cytoplasmic granular staining for
HM47/A9, while the bile canaliculus and interlobular bile duct are
negative for HM47/A9. Our results are inconsistent with previous
reports (5), which may be
attributed to our usage of the antibody clone. To date, JCM117 and
HM-57 have been widely used by researchers to study CD79α
expression (5), compared with 11E3,
11D10, SP18 and HM47/A9, which have seldom been employed. This
discrepancy may be due to the different clones of CD79α
antibodies.
CD79α expression precedes immunoglobulin heavy-chain
gene rearrangement and CD20 expression, and disappears later than
CD20 in the plasma cell (5). In our
experiments, partial CD79α expression in human hepatocytes was
observed in the 8-week embryo, and did not disappear in the adult.
Based on these results, we conclude that HM47/A9 expression is
activated with human hepatocyte development and maintained
constitutively throughout human life. HM47/A9 may also be
critically involved in human hepatocyte function. The liver is
important in the metabolism of fat, carbohydrate and protein,
intermediary metabolism as well as secretion. Further studies are
required to elucidate the specific correlation between HM47/A9 and
diverse liver functions.
We additionally compared HM47/A9 expression in
hepatocytes from normal and diseased livers. As mentioned
previously, HM47/A9 was expressed in hepatocytes, but not in the
bile canaliculus or interlobular bile duct. In view of these
findings, we hypothesized that HM47/A9 expression patterns differ
among HCC, ICC and hepatic metastatic carcinoma. In this study, we
observed that 82/82 HCC cases expressed HM47/A9, while no cases of
ICC were positive for HM47/A9 (0/31). These results strongly
support the utility of the HM47/A9 antibody in distinguishing HCC
from ICC.
None of the 11 CC areas in cHCC-CC expressed
HM47/A9. A majority of HCC components (10/11) in cHCC-CC showed
positive staining for HM47/A9. The excluded case was a notable case
of cHCC-CC, a rare subtype of primary liver carcinoma (9–11).
Primary liver cancer cells exhibited features of HCC and invaded
the interlobular bile duct. Tumor cells exhibited positivity for
Hepatocyte, MOC31, CEA and CK19, and negativity for CD117 and AFP.
Based on morphological and immunochemical results, diagnosis should
be HCC with bile duct differentiation. Notably, however, these
tumor cells were negative for HM47/A9. Earlier research has shown
that cHCC-CC displays morphological and clinical similarities with
cholangiocarcinoma and leads to poorer prognosis compared with pure
HCC (12–14). However, other studies have reported
similarities between cHCC-CC and HCC in terms of male/female ratio,
status of hepatitis viral infection and serum AFP level (9,14).
These two inconsistent findings may be attributed to different
etiological roles according to the geographic situation or the
existence of other carcinogenesis mechanisms (15,16).
The case in this study displayed normal serum AFP level and no
background chronic liver disease, with significant tumor thrombus.
Our data support the findings of William RJ, who reported that
cHCC-CC prognosis is similar to that of cholangiocarcinoma and
worse than for pure HCC. Immunochemistry results disclosed tumor
cell negativity for HM47/A9. As specified earlier, normal
hepatocytes are positive for HM47/A9, while the bile canaliculus
and interlobular bile duct are negative for HM47/A9. Thus, the poor
prognosis of combined HCC and cholangiocarcinoma may be related to
the loss of HM47/A9.
We additionally examined HM47/A9 expression in pale
bodies, fatty degeneration, clear cell change, mallory hyaline
bodies, intranuclear inclusion and glycogen. Notably, HM47/A9
granular expression was observed around pale bodies, fatty
degeneration, clear cell change and mallory bodies, indicating that
HM47/A9 is not a component of intermediate filaments, endoplasmic
reticulum, glycogen and fat (17–20).
In conclusion, our results strongly suggest that
HM47/A9 is an important protein component in hepatocytes that may
be effectively employed to differentiate HCC from ICC. HCC with
bile duct differentiation had poor prognosis, which may be related
to the loss of HM47/A9.
References
|
1
|
Wennerberg AE, Nalesnik MA and Coleman WB:
Hepatocyte paraffin 1: a monoclonal antibody that reacts with
hepatocytes and can be used for differential diagnosis of hepatic
tumors. Am J Pathol. 143:1050–1054. 1993.
|
|
2
|
Porcell AI, De Young BR, Proca DM and
Frankel WL: Immunohistochemical analysis of hepatocellular and
adeno-carcinoma in the liver: MOC31 compares favorably with other
putative markers. Mod Pathol. 13:773–778. 2000. View Article : Google Scholar
|
|
3
|
Shirakawa H, Kuronuma T, Nishimura Y,
Hasebe T, Nakano M, Gotohda N, Takahashi S, Nakagohri T, Konishi M,
Kobayashi N, Kinoshita T and Nakatsura T: Glypican-3 is a useful
diagnostic marker for a component of hepatocellular carcinoma in
human liver cancer. Int J Oncol. 34:649–656. 2009.PubMed/NCBI
|
|
4
|
Herren B and Burrows PD: B cell-restricted
human mb-1 gene: expression, function, and lineage infidelity.
Immunol Res. 26:35–43. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Torres RM, Flaswinkel H, Reth M and
Rajewsky K: Aberrant B cell development and immune response in mice
with a compromised BCR complex. Science. 272:1804–1808. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chu PG and Arber DA: CD79: a review. Appl
Immunohistochem Mol Morphol. 9:97–106. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mason DY, Cordell JL, Brown MH, Borst J,
Jones M, Pulford K, Jaffe E, Ralfkiaer E, Dallenbach F, Stein H,
Pileri S and Gatter KC: CD79a: a novel marker for B-cell neoplasms
in routinely processed tissue samples. Blood. 86:1453–1459.
1995.PubMed/NCBI
|
|
8
|
Bhargava P, Kallakury BV, Ross JS, Azumi N
and Bagg A: CD79a is heterogeneously expressed in neoplastic and
normal myeloid precursors and megakaryocytes in an antibody
clone-dependent manner. Am J Clin Pathol. 128:306–313. 2007.
View Article : Google Scholar
|
|
9
|
Yu LM and Chang TW: Human mb-1 gene:
complete cDNA sequence and its expression in B cells bearing
membrane Ig of various isotypes. J Immunol. 148:633–637.
1992.PubMed/NCBI
|
|
10
|
Ng IO, Shek TW, Nicholls J and Ma LT:
Combined hepatocellularcholangiocarcinoma: a clinicopathological
study. J Gastroenterol Hepatol. 13:34–40. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yano Y, Yamamoto J, Kosuge T, Sakamoto Y,
Yamasaki S, Shimada K, Ojima H, Sakamoto M, Takayama T and Makuuchi
M: Combined hepatocellular and cholangiocarcinoma: a
clinicopathologic study of 26 resected cases. Jpn J Clin Oncol.
33:283–287. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jarnagin WR, Weber S, Tickoo SK, Koea JB,
Obiekwe S, Fong Y, DeMatteo RP, Blumgart LH and Klimstra D:
Combined hepatocellular and cholangiocarcinoma: demographic,
clinical, and prognostic factors. Cancer. 94:2040–2046. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tickoo SK, Zee SY, Obiekwe S, Xiao H, Koea
J, Robiou C, Blumgart LH, Jarnagin W, Ladanyi M and Klimstra DS:
Combined hepatocellular-cholangiocarcinoma: a histopathologic,
immunohistochemical, and in situ hybridization study. Am J Surg
Pathol. 26:989–997. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim H, Park C, Han KH, Choi J, Kim YB, Kim
JK and Park YN: Primary liver carcinoma of intermediate
(hepatocyte-cholangiocyte) phenotype. J Hepatol. 40:298–304. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujii H, Zhu XG, Matsumoto T, Inagaki M,
et al: Genetic classification of combined
hepatocellular-cholangiocarcinoma. Hum Pathol. 31:1011–1017. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park HS, Bae JS, Jang KY, Lee JH, Yu HC,
Jung JH, Cho BH, Chung MJ and Moon WS: Clinicopathologic study on
combined hepatocellular carcinoma and cholangiocarcinoma: with
emphasis on the intermediate cell morphology. J Korean Med Sci.
26:1023–1030. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nzeako UC, Goodman ZD and Ishak KG:
Hepatocellular carcinoma in cirrhotic and noncirrhotic livers. A
clinico-histopathologic study of 804 North American patients. Am J
Clin Pathol. 105:65–75. 1996.
|
|
18
|
Nakanuma Y and Ohta G: Is mallory body
formation a preneo-plastic change? A study of 181 cases of liver
bearing hepatocellular carcinoma and 82 cases of cirrhosis. Cancer.
15:2400–2404. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakashima O, Sugihara S, Eguchi A, Taguchi
J, Watanabe J and Kojiro M: Pathomorphologic study of pale bodies
in hepato-cellular carcinoma. Acta Pathol Jpn. 42:414–418.
1992.PubMed/NCBI
|
|
20
|
Nayar R, Bourtsos E and DeFrias DV:
Hyaline globules in renal cell carcinoma and hepatocellular
carcinoma. A clue or a diagnostic pitfall on fine-needle
aspiration? Am J Clin Pathol. 114:576–582. 2000. View Article : Google Scholar : PubMed/NCBI
|