Introduction
Ossifying fibromyxoid tumor (OFMT) is a rare,
recently identified soft tissue tumor of uncertain histogenesis
(1). OFMT predominantly arises in
the subcutaneous tissue of extremities, and the median age of OFMT
patients is ∼50 years (2–4). Clinically, the tumor often presents as
a small, slow-growing, painless, well-defined mass that displays a
peripheral incomplete ring of ossification on radiography.
Histopathologically, OFMT consists of uniform round, ovoid or
spindle-shaped cells arranged in nests and cords, and deposited in
a variably fibromyxoid stroma. The biological behavior of this
tumor varies. Recently, Graham et al(4) demonstrated that histopathologically
malignant OFMTs exist. OFMT may be mistaken for a number of benign
and malignant conditions, including myositis ossificans, ossifying
hematoma, tumoral calcinosis, extraskeletal chondroma, low-grade
fibromyxoid sarcoma, synovial sarcoma and extraskeletal or
parosteal osteosarcoma (5). In this
study, we describe the imaging findings of an OFMT occurring in the
subcutaneous tissue of the thigh. We also review the cytogenetic
and molecular cytogenetic features of OFMT, as well as its
clinicopathological characteristics.
Case report
A 36-year-old male presented with a 10-year history
of a slow-growing, painless mass in the left proximal thigh. The
patient’s medical history was non-contributory. Written informed
consent for publication was obtained from the patient. Physical
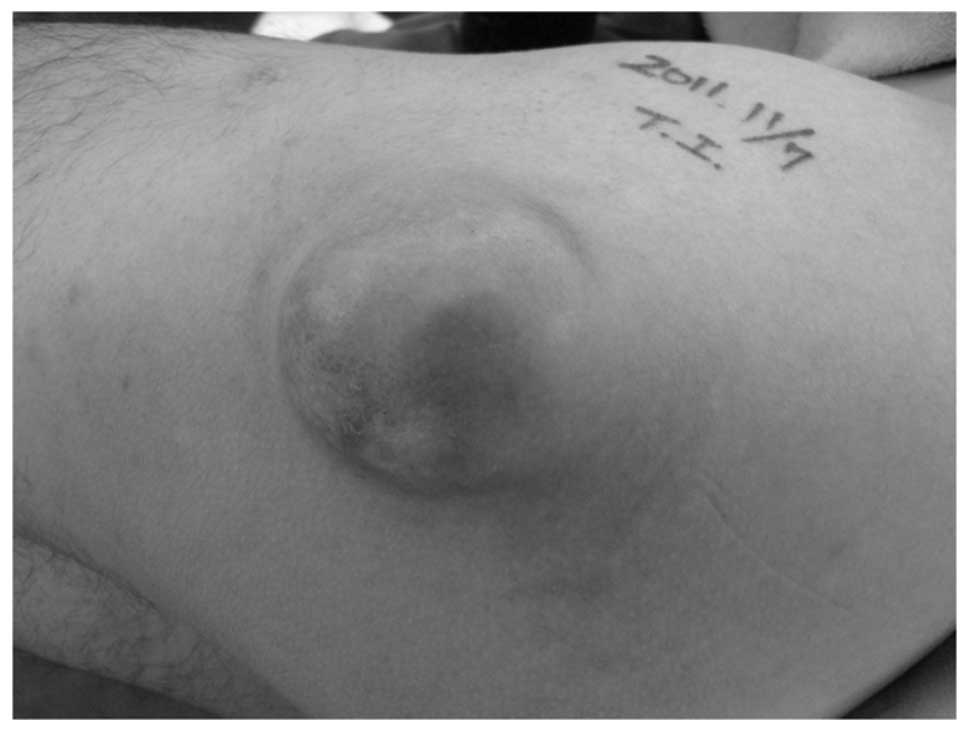
examination revealed a ∼6×5-cm superficial, firm, non-tender mass
in the posterolateral aspect of the left proximal thigh (Fig. 1). Neurological and vascular
examinations were unremarkable, while the laboratory findings were
within normal limits.
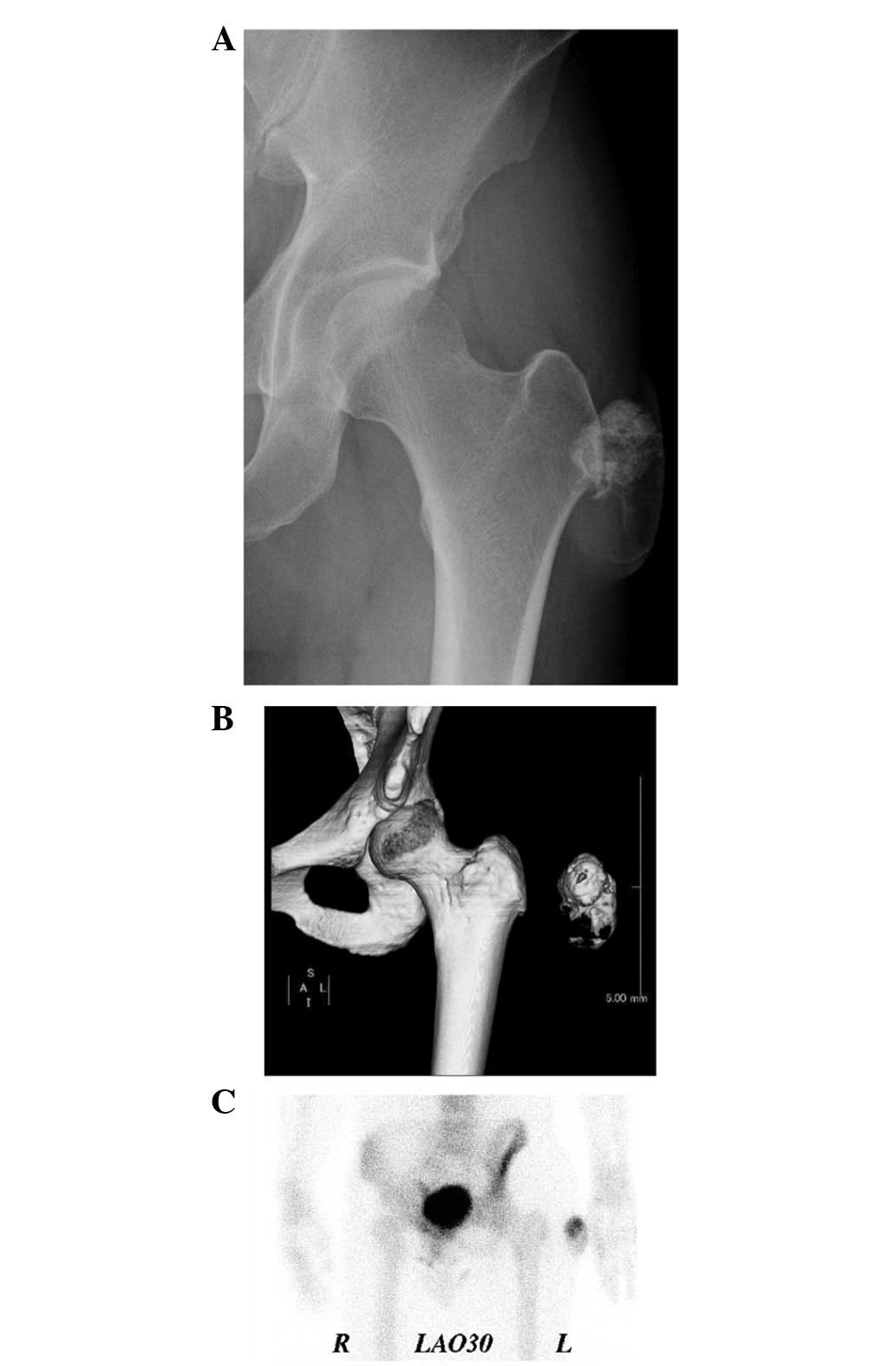
Plain radiographs showed a soft tissue mass with
amorphous calcification and extensive foci of ossification
(Fig. 2A). Computed tomography (CT)
images demonstrated and confirmed an incompletely ossified shell in
the lesion (Fig. 2B).
Technetium-99m hydroxymethylenediphosphonate bone scintigraphy
demonstrated heterogenous uptake in the lateral soft tissue of the
left proximal thigh (Fig. 2C).
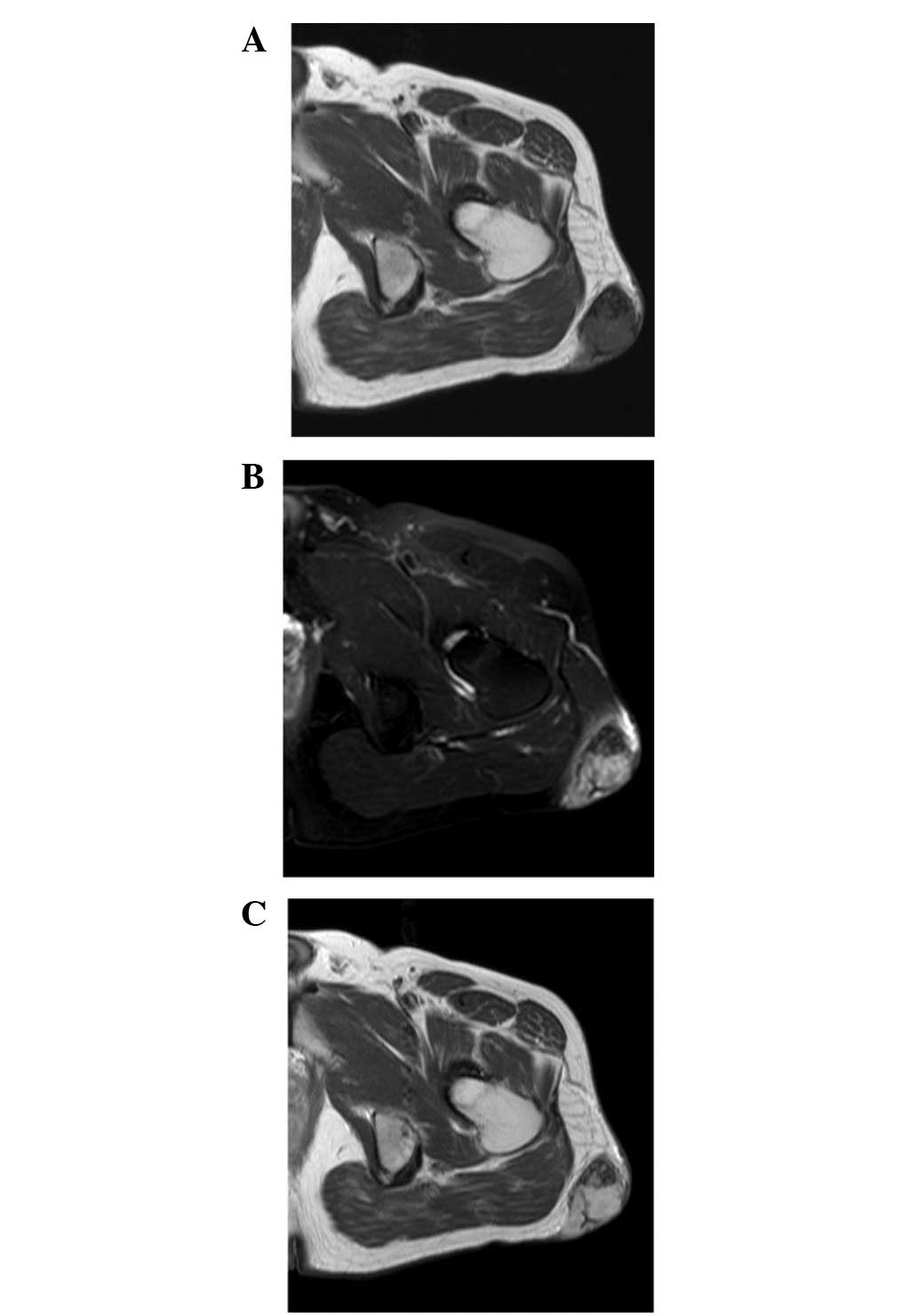
Magnetic resonance imaging (MRI) revealed a well-defined
subcutaneous mass. The mass exhibited low to intermediate signal
intensity on T1-weighted sequences (Fig. 3A) and heterogeneous high signal
intensity, with foci of low signal intensity, on T2-weighted
spectral presaturation with inversion recovery sequences (Fig. 3B). A fine linear low signal was
observed on both T1- and T2-weighted sequences. Contrast-enhanced
T1-weighted sequences demonstrated heterogenous enhancement
throughout the mass (Fig. 3C). A
benign soft tissue tumor with pronounced ossification was
clinically suggested, and the lesion was marginally excised.
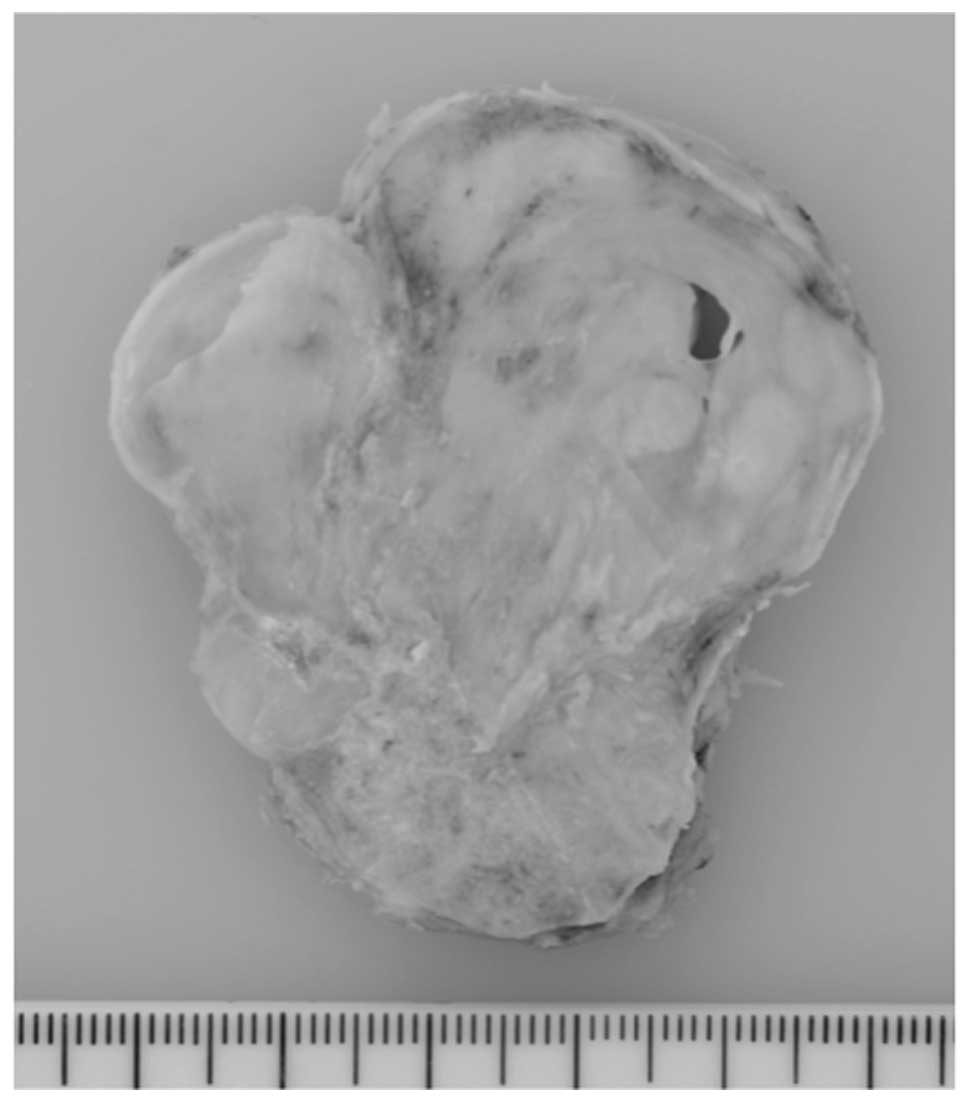
Grossly, the tumor was well circumscribed and
covered by a thin fibrous capsule. A cut section revealed that the
tumor was gray-white, solid, multinodular and rubbery to firm in
consistency (Fig. 4).
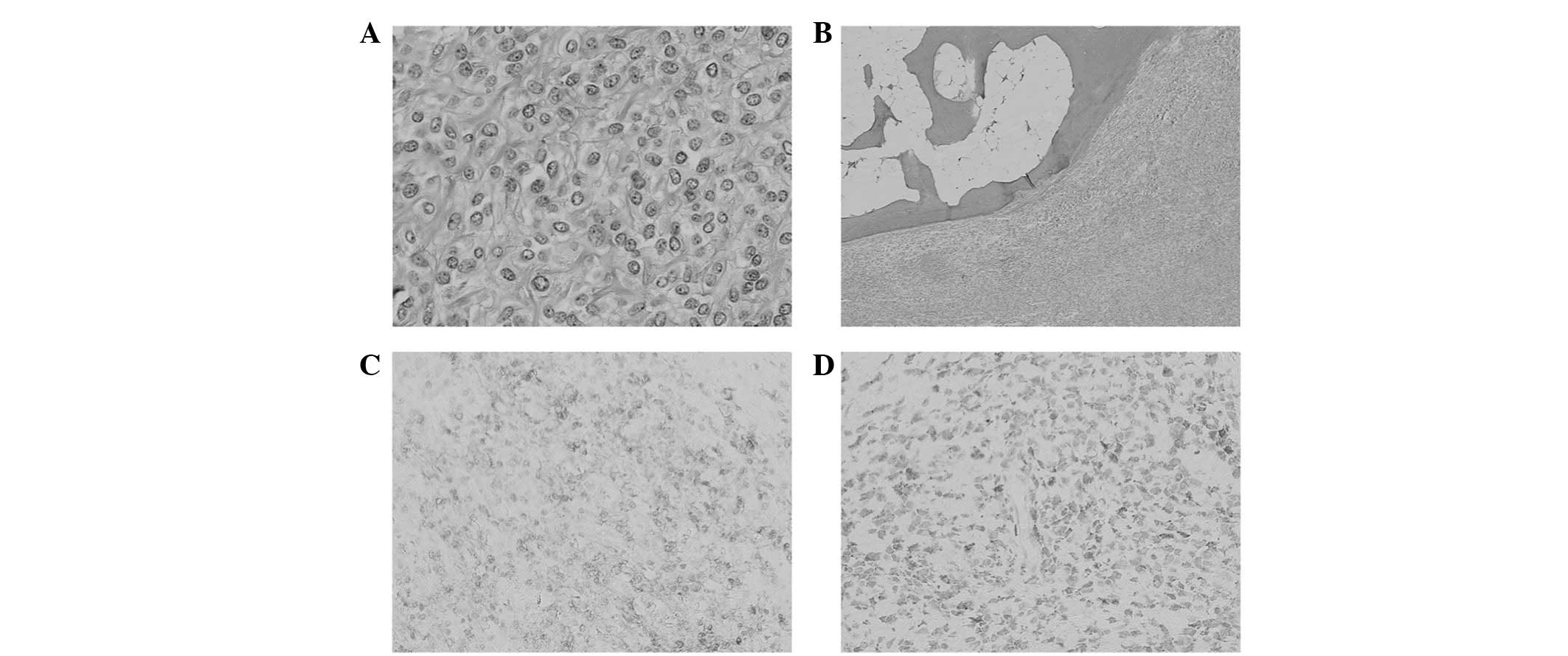
Histopathologically, the tumor was composed of uniform oval,
polygonal or spindle-shaped cells, accompanied by an abundant
fibromyxoid stroma (Fig. 5A).
Ossification-forming bone shells were found in the periphery of the
tumor (Fig. 5B). Necrosis and
vascular space invasion were not observed. Immunohistochemically,
the tumor cells were positive for vimentin, S-100 protein (Fig. 5C) and neuron-specific enolase
(Fig. 5D), and focally for smooth
muscle actin and CD56. Immunostaining for epithelial membrane
antigen, cytokeratin, desmin, glial fibrillary acidic protein,
chromogranin A, synaptophysin and CD57 was negative. The MIB-1
labeling index was <1%. Based on these features, the tumor was
diagnosed as an OFMT.
The postoperative course was uneventful, and the
patient was doing well with no local recurrence 11 months following
surgery.
Discussion
The majority of OFMTs are clinically and
histopathologically benign; however, it has been noted that a
subset of OFMTs have atypical histopathological features and
exhibit correspondingly more aggressive clinical behavior (2,4,6). In
2003, Folpe and Weiss (2) proposed
that OFMT may be classified as typical, atypical or malignant on
the basis of its cellularity, nuclear grade and mitotic activity.
More recently, Graham et al(4) confirmed the existence of malignant
OFMT using immunohistochemistry and gene expression profiling. In
view of the low to moderate cellularity, low nuclear grade, low
mitotic activity and the absence of necrosis or vascular space
invasion, our case was regarded as a typical OFMT.
The histogenesis of OFMT remains uncertain. Graham
et al(4) recently suggested
that this tumor exhibits a scrambled phenotype. Typically, as in
our case, the cells are positive for vimentin and express S-100
protein. Atypical or malignant areas are observed to express S-100
protein less frequently than the typical areas (2). Other useful markers are CD10 and EAAT4
(3,4).
To date, a limited number of imaging findings of
OFMT have been described in detail in the literature (5,7–10).
Plain radiographs typically reveal a non-specific soft tissue mass
with an incomplete rim of ossification. Erosion or periosteal
reaction of the underlying bone is rarely observed. CT scans
clearly demonstrate the presence of surrounding or intralesional
ossification (7). The MRI
appearances of OFMT are variable. The lesion is isointense to
muscle on T1-weighted images and shows intermediate-to-high signal
intensity on T2-weighted images. There are high signal intensity
areas on T1- and T2-weighted images, suggesting hemorrhage and
implying a high degree of vascularity (8,9). In
addition, areas of ossification demonstrate low signal intensity on
T1- and T2-weighted images. As with our case, the ossific element
of OFMT has osteoblastic activity that is detected on bone
scintigraphy (5,9,11).
Only seven cases of OFMT have been cytogenetically
described (2,12–15).
Clonal abnormalities of chromosome band 6p21 are prominent.
Notably, a balanced or unbalanced t(6;12) (p21;q24) translocation
appears to be characteristic for OFMT. A recent fluorescence in
situ hybridization (FISH) study by Graham et al(4) revealed INI-1 deletion in 71% of
cases. Most recently, Gebre-Medhin et al(15) demonstrated that PHF1 (at
6p21) is frequently rearranged in OFMT, including atypical and
malignant variants. Moreover, PHF1 was fused to EP400
(at 12q24) in one atypical case with the t(6;12) translocation.
OFMT is the second neoplasm to be identified, in addition to
endometrial stromal tumor, in which PHF1 is involved in
fusions with ectopic sequences. A FISH assay for PHF1
rearrangements would therefore be useful for the differential
diagnosis of OFMT and its histopathological mimics (16).
In summary, we have described the imaging findings
of a typical OFMT with clinical and histopathological correlation.
Although rare, OFMT ought to be considered in the differential
diagnosis of a well-circumscribed, slow-growing, painless,
subcutaneous mass with irregular ossifications and/or
calcifications.
Acknowledgements
This study was supported in part by
the Ogata Foundation and the Foundation for the Promotion of
Medical Science.
References
|
1
|
Enzinger FM, Weiss SW and Liang CY:
Ossifying fibromyxoid tumor of soft parts: a clinicopathological
analysis of 59 cases. Am J Surg Pathol. 13:817–827. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Folpe AL and Weiss SW: Ossifying
fibromyxoid tumor of soft parts: a clinicopathologic study of 70
cases with emphasis on atypical and malignant variants. Am J Surg
Pathol. 27:421–431. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miettinen M, Finnell V and Fetsch JF:
Ossifying fibromyxoid tumor of soft parts-a clinicopathologic and
immunohistochemical study of 104 cases with long-term follow-up and
a critical review of the literature. Am J Surg Pathol. 32:996–1005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Graham RP, Dry S, Li X, Binder S, Bahrami
A, Raimondi SC, Dogan A, Chakraborty S, Souchek JJ and Folpe AL:
Ossifying fibromyxoid tumor of soft parts: a clinicopathologic,
proteomic, and genomic study. Am J Surg Pathol. 35:1615–1625. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ogose A, Otsuka H, Morita T, Kobayashi H
and Hirata Y: Ossifying fibromyxoid tumor resembling parosteal
osteosarcoma. Skeletal Radiol. 27:578–580. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kilpatrick SE, Ward WG, Mozes M, Miettinen
M, Fukunaga M and Fletcher CD: Atypical and malignant variants of
ossifying fibromyxoid tumor. Clinicopathologic analysis of six
cases. Am J Surg Pathol. 19:1039–1046. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schaffler G, Raith J, Ranner G, Weybora W
and Jeserschek R: Radiographic appearance of an ossifying
fibromyxoid tumor of soft parts. Skeletal Radiol. 26:615–618. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harish S, Polson A, Morris P, Malata C,
Griffiths M and Bearcroft PW: Giant atypical ossifying fibromyxoid
tumour of the calf. Skeletal Radiol. 35:248–253. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cha JH, Kwon JW, Cho EY, Lee CS, Yoon YC
and Choi SH: Ossifying fibromyxoid tumor invading the spine: a case
report and review of the literature. Skeletal Radiol. 37:1137–1140.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Green RAR, Briggs TWR and Tirabosco R:
Ossifying fibromyxoid tumour, an unusual cause of rim ossification:
imaging features and correlation with histopathology. Eur J Radiol
Extra. 70:e37–e40. 2009. View Article : Google Scholar
|
|
11
|
Raith J, Ranner G, Schaffler G, Gröll R,
Lindbichler F, Fritz K and Weybora W: Bone scan in ossifying
fibromyxoid tumor of soft parts. Clin Nucl Med. 23:262–264. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sovani V, Velagaleti GV, Filipowicz E,
Gatalica Z and Knisely AS: Ossifying fibromyxoid tumor of soft
parts: report of a case with novel cytogenetic findings. Cancer
Genet Cytogenet. 127:1–6. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishio J, Iwasaki H, Ohjimi Y, Ishiguro M,
Isayama T, Naito M, Okabayashi H, Kaneko Y and Kikuchi M: Ossifying
fibromyxoid tumor of soft parts. Cytogenetic findings. Cancer Genet
Cytogenet. 133:124–128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawashima H, Ogose A, Umezu H, Hotta T,
Tohyama T, Tsuchiya M and Endo N: Ossifying fibromyxoid tumor of
soft parts with clonal chromosomal aberrations. Cancer Genet
Cytogenet. 176:156–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gebre-Medhin S, Nord KH, Möller E, Mandahl
N, Magnusson L, Nilsson J, Jo VY, Vult von Steyern F, Brosjö O,
Larsson O, Domanski HA, Sciot R, Debiec-Rychter M, Fletcher CDM and
Mertens F: Recurrent rearrangement of the PHF1 gene in ossifying
fibromyxoid tumors. Am J Pathol. 181:1069–1077. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nishio J: Updates on the cytogenetics and
molecular cytogenetics of benign and intermediate soft tissue
tumors (Review). Oncol Lett. 5:12–18. 2013.PubMed/NCBI
|