Introduction
Cetuximab (Cmab) is an anti-epidermal growth factor
receptor (EGFR) antibody that has been shown to effectively combine
with cytotoxic chemotherapy as first-, second- or third-line
treatment against wild-type KRAS colorectal cancer (1–3).
However, it has occasionally been associated with the development
of hypersensitivity reactions (HSRs). In patients with severe HSRs,
further therapy with Cmab is not possible. Compared with Cmab, HSRs
have rarely been observed with panitumumab (Pmab), a fully human
IgG anti-EGFR antibody (4). Pmab is
considered to be ineffective in patients who experience failure
with Cmab therapy, due to evidence that the two antibodies target
the same receptor. However, a few studies have indicated that Pmab
is effective in patients with refractory metastatic colorectal
cancer (mCRC) following the failure of standard therapy, including
Cmab-based regimens, in the USA (5,6).
However, there are no studies on the efficacy of Pmab therapy after
the failure of Cmab therapy in patients from Asian countries. In
this study, we aimed to reveal the safety and efficacy of Pmab
therapy following disease progression with Cmab therapy in Japanese
mCRC patients.
Patients and methods
Patient information
We retrospectively reviewed 16 mCRC patients who
tolerated Pmab with clinical benefits after the failure of Cmab
therapy between August 2010 and September 2011 at Shiga University
of Medical Science. Patient medical records were reviewed for
previous therapy, toxicity and response assessment. KRAS
status was retrospectively assessed in patients with readily
available tumor tissues. Chemotherapeutic response was assessed
using the Response Evaluation Criteria in Solid Tumors (RECIST)
(7). The incidence and severity of
adverse events (AEs) were measured throughout the study and graded
using the National Cancer Institute Common Toxicity Criteria
version 4.0 (NCI-CTCAE v4.0).
This study protocol was in accordance with the
ethical guidelines established by the Declaration of Helsinki.
Written informed consent was obtained from all patients.
Statistical analysis
Progression-free survival (PFS) and overall survival
(OS) were calculated as the interval between the first day of Pmab
treatment and the date of proven recurrence or death from any
cause, respectively. PFS and OS were estimated using the
Kaplan-Meier method.
Results
Patient characteristics
The baseline characteristics of the patient
population are summarized in Table
I. Thirteen patients were male and three were female. The
median age was 65 years (range, 53–88 years). All patients had an
ECOG (European Clinical Oncology Group) performance status between
0 and 2. The site of the primary tumor was the colon in 5 of the 16
patients (31%) and the rectum in 11 patients (69%). Histologically,
the primary tumor was a well-differentiated adenocarcinoma in 6 and
a moderately differentiated adenocarcinoma in 10 patients. Ten of
the 16 patients (63%) had only one metastatic site (the lungs in 9
cases and liver in 1 case) and 6 of the 16 patients (38%) had two
or more metastatic sites, including the liver (5 patients), lungs
(5 patients) and lymph nodes (2 patients). Thirteen of the 16
patients (81%) had wild-type KRAS and three (19%) had
mutant-type KRAS. The median number of previous therapies
was three (range, 2–6 therapies). Seven of the 16 patients (44%)
received four or more previous therapies. All patients received
prior irinotecan therapy. Fifteen patients (94%) received prior
oxaliplatin and 14 patients (88%) received prior bevacizumab
therapy. All patients had been previously treated with Cmab and
only two of the 16 patients (13%) discontinued Cmab therapy due to
the development of HSRs. Other reasons for stopping Cmab therapy
included disease progression (n=13, 81%) and the inconvenience of a
bi-weekly schedule (n=1, 6%). Fourteen of the 16 patients (88%)
received standard Pmab monotherapy (6 mg/kg) intravenously every 2
weeks, while the remaining two (13%) received Pmab with mFOLFOX6
intravenously every 2 weeks. All patients in this study were
administered standard medications (i.e., corticosteroids and
antihistamines) prior to Pmab administration in order to prevent
HSRs.
 | Table IBaseline and clinical characteristics
of the metastatic colorectal cancer patients. |
Table I
Baseline and clinical characteristics
of the metastatic colorectal cancer patients.
| Characteristics | Value |
|---|
| Total number (%) | 16 (100) |
| Male/female (%) | 13/3 (81/19) |
| Age (years) | |
| Median | 65 |
| Range | 53–88 |
| Performance status
(%) | |
| 0 | 7 (44) |
| 1 | 5 (31) |
| 2 | 4 (25) |
| Primary tumor site
(%) | |
| Colon | 5 (31.25) |
| Rectum | 11 (68.75) |
| Histology (%) | |
|
Well-differentiated | 6 (37.5) |
| Moderately
differentiated | 10 (62.5) |
| Poorly
differentiated and others | 0 (0) |
| Number of metastatic
sites (%) | |
| 1 | 10 (62) |
| 2 | 3 (19) |
| ≥3 | 3 (19) |
| Sites of metastases
(%) | |
| Liver | 6 (38) |
| Lungs | 14 (88) |
| Lymph nodes | 2 (13) |
| Other | 5 (31) |
| KRAS status
(%) | |
| Wild-type | 13 (81) |
| Mutant | 3 (19) |
| Prior therapeutic
regimens (%) | |
| 2 | 6 (38) |
| 3 | 3 (19) |
| ≥4 | 7 (44) |
| Prior oxaliplatin
therapy (%) | 15 (94) |
| Prior irinotecan
therapy (%) | 16 (100) |
| Prior bevacizumab
therapy (%) | 14 (88) |
Therapeutic effect
All patients received Pmab chemotherapy until
disease progression occurred. The median number of Pmab cycles
administered was 7 (range, 3–15). Partial radiographic responses
(PR) were noted in 2 of the 16 patients (12.5%) and stable disease
(SD) in 5 patients (31.3%). Nine patients (56.3%) had evidence of
progressive disease (PD). In terms of KRAS status, all 3
patients with mutant-type KRAS had evidence of PD and 7 of
the 13 patients with wild-type KRAS (53.8%) achieved a high
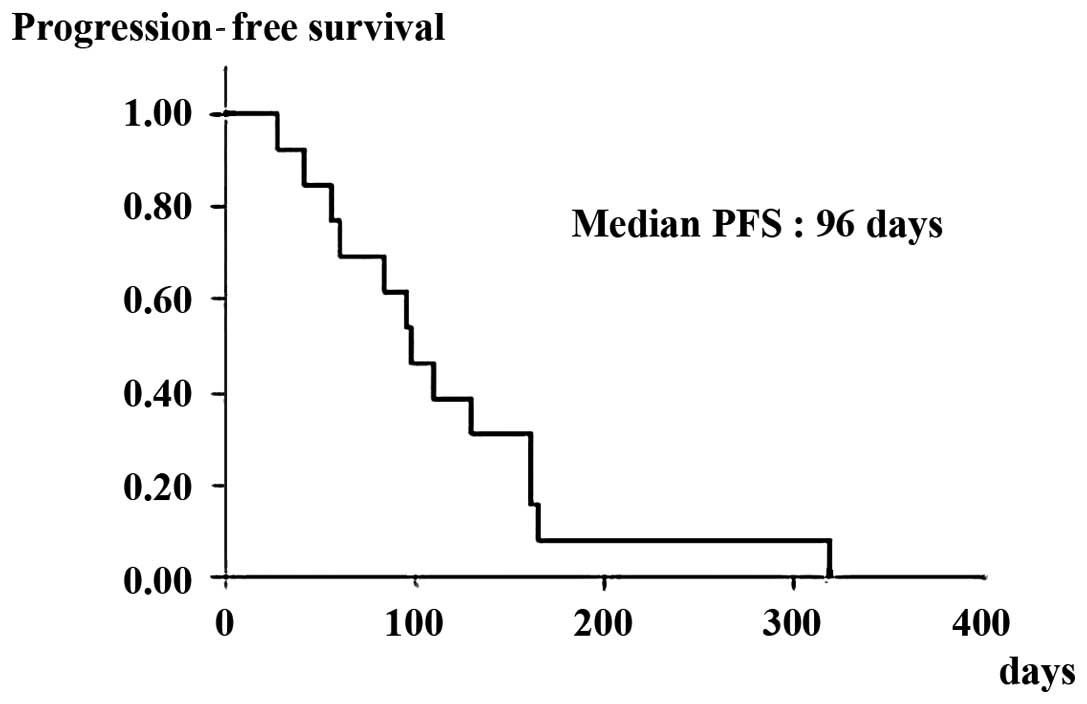
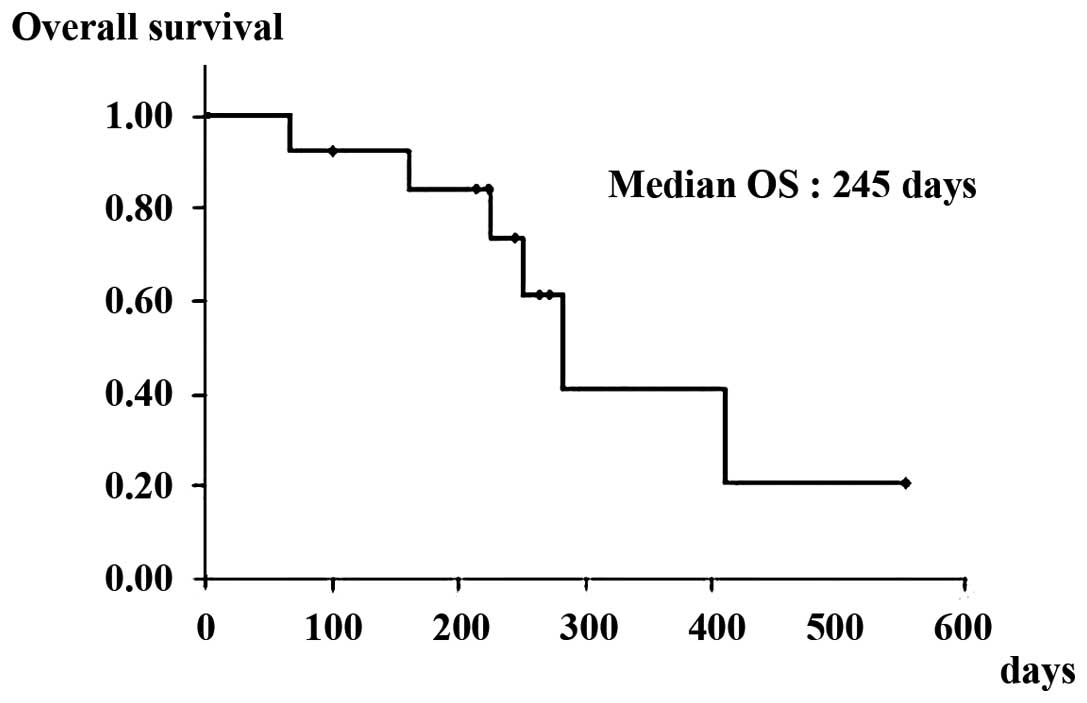
disease control rate (PR + SD). The median PFS and OS in patients
with wild-type KRAS was 96 (Fig.
1) and 245 days (Fig. 2),
respectively.
Carcinoembryonic antigen (CEA)
levels
One patient did not exhibit a change in CEA level,
regardless of tumor status. Four patients achieved a >50%
reduction (4934.5 to 793 U/l, 266.4 to 58.3 U/l, 218.2 to 55 U/l
and 31.8 to 14.5 U/l), 2 patients had a 25% reduction (459.5 to
296.5 U/l and 34.7 to 22.7 U/l) and 1 patient had a minor reduction
(442.8 to 380 U/l) in CEA levels and 8 patients had increased CEA
levels. Therefore, approximately half of the patients achieved a
reduction in CEA levels.
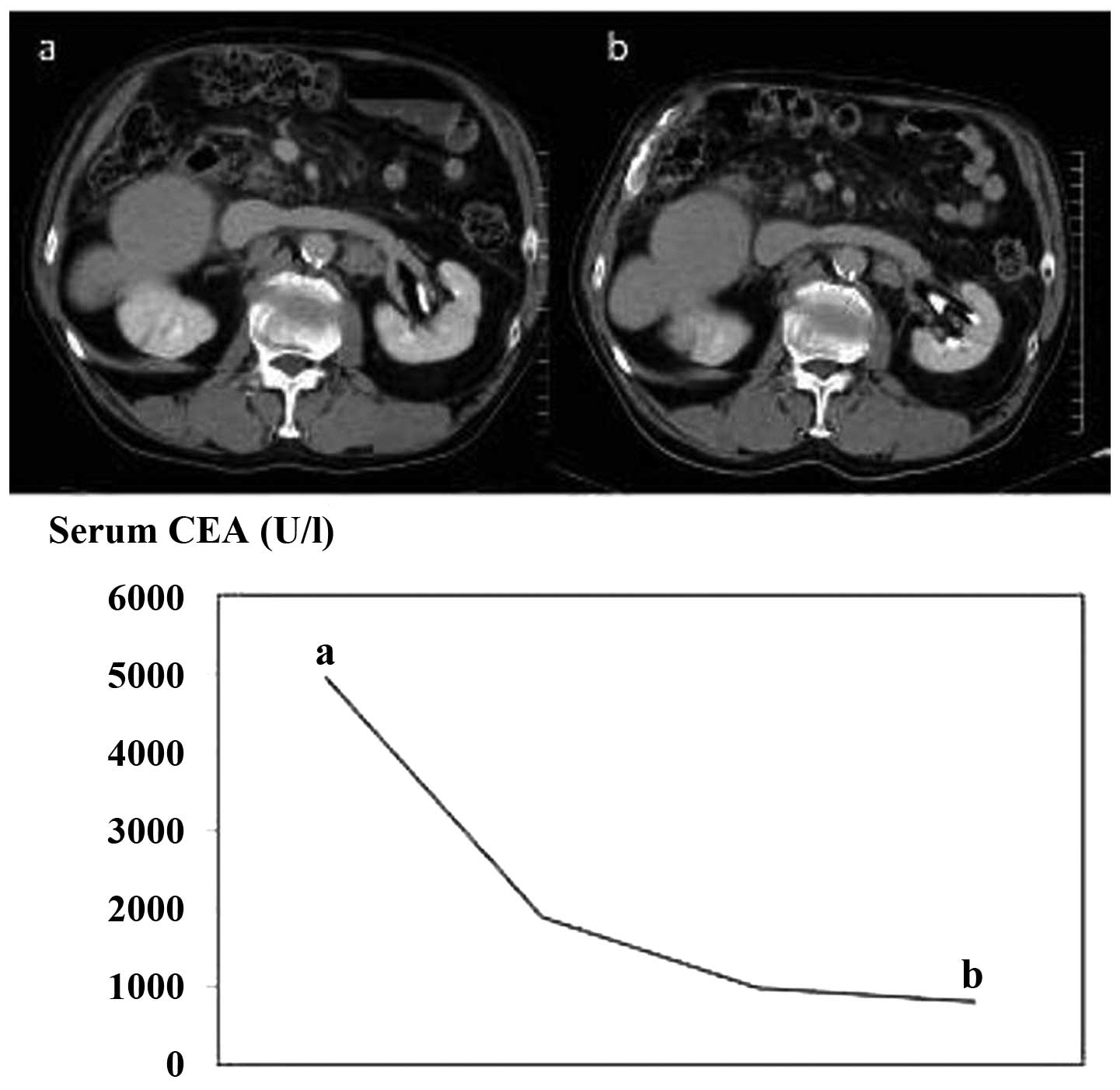
Typical case
A 73-year-old male had been diagnosed with sigmoid
colon cancer (T3N1M0 Stage IIIa) 7 years previously, for which he
underwent a sigmoidectomy with regional lymph node excision. Two
years after sigmoidectomy, the patient underwent partial
hepatectomy for solitary liver metastasis. The patient was once
again diagnosed with multiple liver metastases several months after
the partial hepatectomy, for which hepatic arterial infusion
chemotherapy (HAI) was administered. However, due to disease
progression despite the HAI, mFOLFOX6, FOLFIRI and Cmab
chemotherapy was administered. Thereafter, the patient was also
diagnosed with lung and intra-abdominal lymph node metastases and
received Pmab therapy, following which disease stability was
achieved for ∼10 months and CEA levels decreased from 4934 to 793
U/l (Fig. 3).
AEs
All patients tolerated Pmab well, with no cases of
HSR. Grade 3/4 toxicities included hypomagnesemia in 1 patient and
hypocalcemia in another. Other AEs observed included grade 1–2 skin
rash in 3 patients, grade 2 hypomagnesemia in 2 patients, grade 1–2
fatigue in 3 patients and grade 1–2 appetite loss in 3 patients.
However, none of the patients required discontinuation of Pmab
therapy as a result of these AEs.
Discussion
For ∼40 years, fluoropyrimidine 5-fluorouracil was
the only drug available for the treatment of mCRC. However, over
the past 10 years, the therapeutic armamentarium for mCRC has
expanded significantly with the development and approval of three
cytotoxic agents, irinotecan, oxaliplatin and the oral
fluoropyrimidine capecitabine (8).
During this time, three new biological agents were developed,
including the anti-vascular endothelial growth factor (VEGF)
antibody bevacizumab and anti-EGFR antibodies Cmab and Pmab.
Incorporation of these biological agents into various cytotoxic
chemotherapy regimens has transformed mCRC into a chronic illness,
where the median OS is currently 24–28 months (9).
Cmab and Pmab bind with a high affinity to EGFRs and
prevent the binding of natural growth factor ligands to these
receptors (10). This inhibitory
effect leads to the repression of subsequent downstream signaling
pathways, which mediate cell growth and proliferation, survival
mechanisms against chemotherapy and/or radiation therapy,
invasion/metastasis and even angiogenesis. Cmab or Pmab therapy is
not considered to be effective following disease progression with
Pmab or Cmab therapy, respectively. However, Cmab is a chimeric
antibody that is associated with the development of HSRs. The
development of severe HSRs means premature termination of drug
therapy is necessary (11,12). Pmab is a fully human IgG2 antibody
and, in contrast to Cmab, infusion-associated reactions are usually
minor in severity and grade 3/4 reactions are rarely experienced.
As a result, premedication is not usually recommended when Pmab
therapy is administered (4). In our
study, Pmab was effective even after the failure of Cmab. In
Western countries, there have been a number of previous studies on
the safety and clinical efficacy of Pmab following disease
progression with Cmab therapy (5,6). In
the present study, >60% of patients who experienced disease
progression with Cmab therapy achieved varying levels of clinical
benefit, including disease control and a reduction in levels of the
serum tumor marker CEA, with Pmab therapy. Our results are similar
to those reported by Power et al on their clinical
experience at the Memorial Sloan-Kettering Cancer Center (6). Notably, we were able to prolong PFS by
∼3 months and the OS by ∼8 months by administering Pmab, even after
third-line chemotherapy.
At present, it is not entirely clear why patients
who had disease progression on Cmab were able to derive clinical
benefits from Pmab. Recently, Montagut et al(13) revealed that the presence of the
acquired EGFR ectodomain mutation (S492R) may provide a molecular
explanation for the clinical benefits of Pmab therapy in a subset
of patients with mCRC who did not respond to treatment with Cmab.
Another possibility is that the two antibodies may inhibit EGFR
signaling via separate mechanisms. To explore these possibilities,
Freeman et al(14) performed
epitope mapping of the two antibodies and revealed that Pmab and
Cmab bind to the same surface-exposed amino acids in domain III of
EGFRs and this inhibited the binding of all known EGFR ligands.
However, formal X-ray crystallographic studies showed that the
humanized anti-EGFR antibody matuzumab interacts with an epitope on
EGFRs that is distinct from the ligand-binding region on domain III
and the Cmab epitope (15,16). Matuzumab indirectly blocked
ligand-induced receptor activation by sterically preventing the
domain rearrangement and local conformational changes that must
occur for high-affinity ligand binding and receptor dimerization.
Structural studies of a different humanized anti-EGFR antibody,
nimotuzumab, revealed a novel mechanism in which nimotuzumab
blocked EGF binding while allowing the EGFR to adopt its active
conformation. By interfering with only ligand-dependent EGFR
activation, nimotuzumab was able to reduce EGFR signaling to a
basal, ligand-independent level. Taken together, these studies
suggest that the various anti-EGFR antibodies may inhibit
EGFR-mediated signaling through different mechanisms. As a result,
it is also conceivable that distinct mechanisms of resistance may
develop to the respective anti-EGFR antibodies.
In conclusion, Pmab therapy may represent an
alternative treatment strategy for patients, Japanese or otherwise,
with refractory mCRC who have experienced failure with standard
therapies, including Cmab-based regimens. Our relatively small
clinical experience suggests that Cmab and Pmab may exert their
antitumor activity via different mechanisms, however, further study
is required to investigate this hypothesis.
References
|
1
|
Bokemeyer C, Bondarenko I, Hartmann JT, de
Braud F, Schuch G, Zubel A, et al: Efficacy according to biomarker
status of cetuximab plus FOLFOX-4 as first-line treatment for
metastatic colorectal cancer: the OPUS study. Ann Oncol.
22:1535–1546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sobrero AF, Maurel J, Fehrenbacher L,
Scheithauer W, Abubakr YA, Lutz MP, et al: EPIC: phase III trial of
cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin
failure in patients with metastatic colorectal cancer. J Clin
Oncol. 26:2311–2319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pfeiffer P, Nielsen D, Yilmaz M, Iversen
A, Vejlø C and Jensen BV: Cetuximab and irinotecan as third line
therapy in patients with advanced colorectal cancer after failure
of irinotecan, oxaliplatin and 5-fluorouracil. Acta Oncol.
46:697–701. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohenuram M and Saif MW: Panitumumab the
first fully human monoclonal antibody: from the bench to the
clinic. Anticancer Drugs. 18:7–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saif MW, Kaley K, Chu E and Copur MS:
Safety and efficacy of panitumumab therapy after progression with
cetuximab: experience at two institutions. Clinical Colorectal
Cancer. 9:315–318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Power DG, Shah MA, Asmis TR, Garcia JJ and
Kemeny NE: Safety and efficacy of panitumumab following cetuximab:
retrospective review of the Memorial Sloan-Kettering experience.
Invest New Drugs. 28:353–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Persijn van Meerten EL, Gelderblom H
and Bloem JL: RECIST revised: implications for the radiologist. A
review article on the modified RECIST guideline. Eur Radiol.
20:1456–1467. 2010.PubMed/NCBI
|
|
8
|
Meyerhardt JA and Mayer RJ: Systemic
therapy for colorectal cancer. N Engl J Med. 352:476–487. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van Cutsem E: First-line treatment:
approaches with cytotoxic and biologic agents. New Treatment
Strategies for Metastatic Colorectal Cancer. Chu E: CMP Healthcare
Media; Manhasset, NY: pp. 21–46. 2008
|
|
10
|
Mendelsohn J and Baselga J: Epidermal
growth factor receptor targeting in cancer. Semin Oncol.
33:369–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jean GW and Shah SR: Epidermal growth
factor receptor monoclonal antibodies for the treatment of
metastatic colorectal cancer. Pharmacotherapy. 28:742–754. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cmelak AJ, Lordick F, Borner M, Goldberg
RM and Saif MW: Management of infusion reactions in clinical trials
and beyond: the US and EU perspectives. Oncology (Williston Park).
23(Suppl 1): 18–25. 2009.PubMed/NCBI
|
|
13
|
Montagut C, Dalmases A, Bellosillo B,
Crespo M, Pairet S, Iglesias M, et al: Identification of a mutation
in the extracellular domain of the Epidermal Growth Factor Receptor
conferring cetuximab resistance in colorectal cancer. Nat Med.
18:221–223. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Freeman D, Sun J, Bass R, et al:
Panitumumab and cetuximab epitope mapping and in vitro activity. J
Clin Oncol. 26May 20;(Suppl): 145362008.
|
|
15
|
Leahy DJ: A molecular view of anti-ErbB
monoclonal antibody therapy. Cancer Cell. 13:291–293. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmiedel J, Blaukat A, Li S, Knöchel T
and Ferguson KM: Matuzumab binding to EGFR prevents the
conformational rearrangement required for dimerization. Cancer
Cell. 13:365–373. 2008. View Article : Google Scholar : PubMed/NCBI
|