Introduction
Renal cell cancer accounts for ∼3% of adult
malignancies. Approximately one-third of patients exhibit
metastatic disease at the time of diagnosis. According to results
from Motzer et al, the overall median survival time for
advanced cases was 10 months and only 45% of patients who had a
good prognosis survived for a median follow-up time of two years
(1). The rates of response to
chemotherapy and hormonotherapy are low (∼10%). For the past 20
years, cytokines have been the main treatment for metastatic renal
cell cancer. Therefore, new methods which utilize
molecular-targeted therapies, including anti-angiogenic drugs
(2) and mammalian target of
rapamycin (mTOR) inhibitors, for example everolimus and
temsirolimus (3), have emerged.
Temsirolimus was approved by the FDA in March 2008, based on
a phase III randomized trial for advanced renal cell carcinoma that
demonstrated a statistically significant improvement in overall
survival; the median overall survival was 10.9 months in
temsirolimus-treated patients compared with 7.3 months in
interferon (IFN)-treated patients (4). Based on these results, patients with a
poor prognosis should receive temsirolimus as a first-line
treatment. Recommendations for second or successive treatment lines
are not currently provided by the main practice guidelines.
Following clinical progression, patients who had previously been
treated with a vascular endothelial growth factor-targeted agent
may benefit from a change of therapy to mTOR inhibitors (5).
Case report
Clinical presentation and diagnosis
A 57-year-old male with no relevant pre-existing
medical conditions was admitted to the Hospital Universitario
Clínico San Cecilio, Avenida, Granada, Spain in February 2000, due
to an episode of macroscopic hematuria. The physical examination of
the patient at admission was normal. The patient underwent a
thyroid function test and this was also normal; the serum thyroid
stimulation hormone (TSH) level was 3.9 IU/ml (lower and uppet
limit, 0.27–4.20). Routine laboratory data revealed no abnormal
findings. During the evaluation, an abdominopelvic computed
tomography (CT) scan revealed a solid mass at the inferior pole of
the left kidney. A left nephrectomy was performed and the pathology
study reported a stage III (pT4N0M0) G2 clear cell carcinoma and a
papillary carcinoma involving either the renal pelvis or the
extracapsular region.
The study was approved by the Ethics Committee of
the Hospital Universitario Cliínico San Cecilio, Granada, Spain.
Written informed consent was obtained from the patient.
Treatment and clinical course
The patient received adjuvant radiotherapy in the
left renal fossa during the phase II trial. Two years later, a CT
scan revealed multiple predominant lower lobe metastases. The
largest tumor was 2 cm in size and located in the lower left lobe.
In December 2002, a first-line treatment with three cycles of
intravenous interleukin plus IFN was started; however, there was no
response to the therapy. Disease stabilization was achieved
following the initiation of cycles of vinblastine and IFN. After 12
cycles of this combination treatment, IFN was administered as a
monotherapy. In June 2005, novel progression of the disease to the
lungs was revealed. Subsequently, the patient received inhaled
interleukin therapy, resulting in the stabilization of the disease
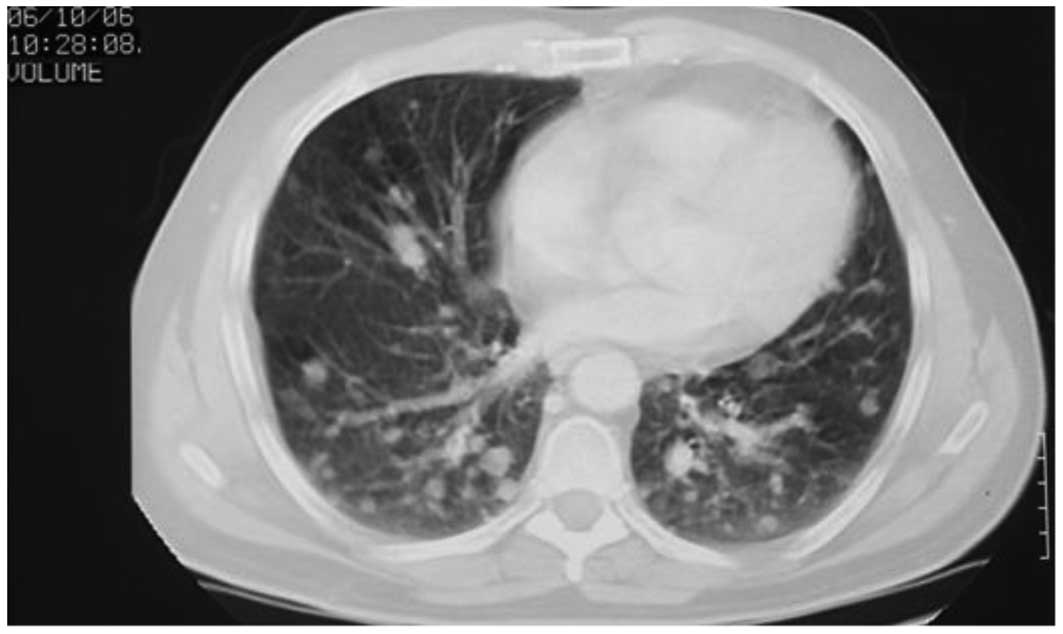
over the following 16 months. In October 2006, the CT scan
demonstrated an increase in the number and size of bilateral
multiple lung metastases (Fig. 1).
Fourth-line treatment with sunitinib was subsequently started, and
this achieved a long-term partial response over the next 20 months.
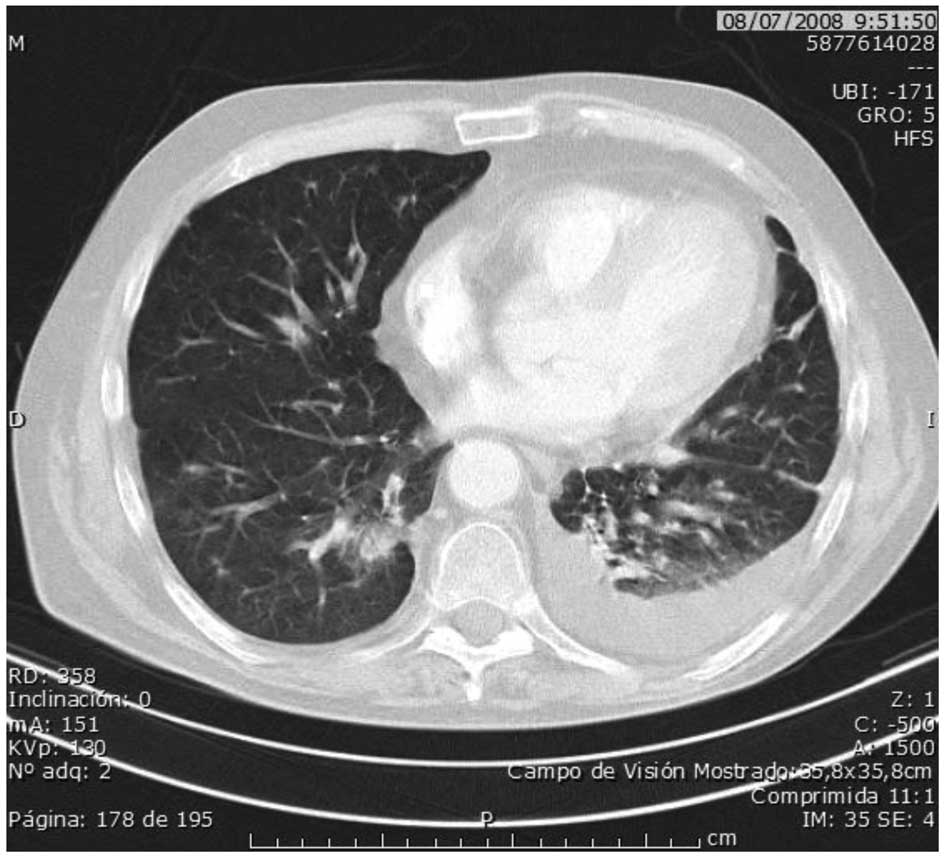
In July 2008, a new lung relapse was detected (Fig. 2), accompanied by three poor
prognostic factors (a low hemoglobin level of <10 g/dl, a serum
calcium level of >10 mg/dl and a lactate dehydrogenase level of
709 IU/l). Fifth-line treatment for metastatic disease with 25 mg
intravenous temsirolimus once a week was started and disease
stabilization was achieved in 13 months. During this period,
temsirolimus was discontinued on two occasions. The first incident
was due to toxicity (hypothyroidism, G3), which occurred after 10
months of treatment. Severe asthenia and lethargy interfered with
activities of daily living in the patient and the results of blood
tests were: TSH, 92 μIU/ml; free serum triiodothyroxine
(FT3), 0.15 pg/ml (2.60–5.10); and free serum thyroxine (FT4), 0.26
ng/dl (1.00–1.80). The patient achieved normal thyroid function
after temsirolimus therapy had been withdrawn for three weeks. In
the second instance, the treatment was ceased due to the occurence
of mumps accompanied by a high fever; this was treated with
antibiotics and anti-inflammatory drugs. Two weeks later,
temsirolimus therapy was resumed by lowering the dosage to 20 mg.
Notably, other toxicities were observed in this case, including a
G2 rash, G2 anemia, G2 leukopenia, G2 hypertriglyceridemia and G1
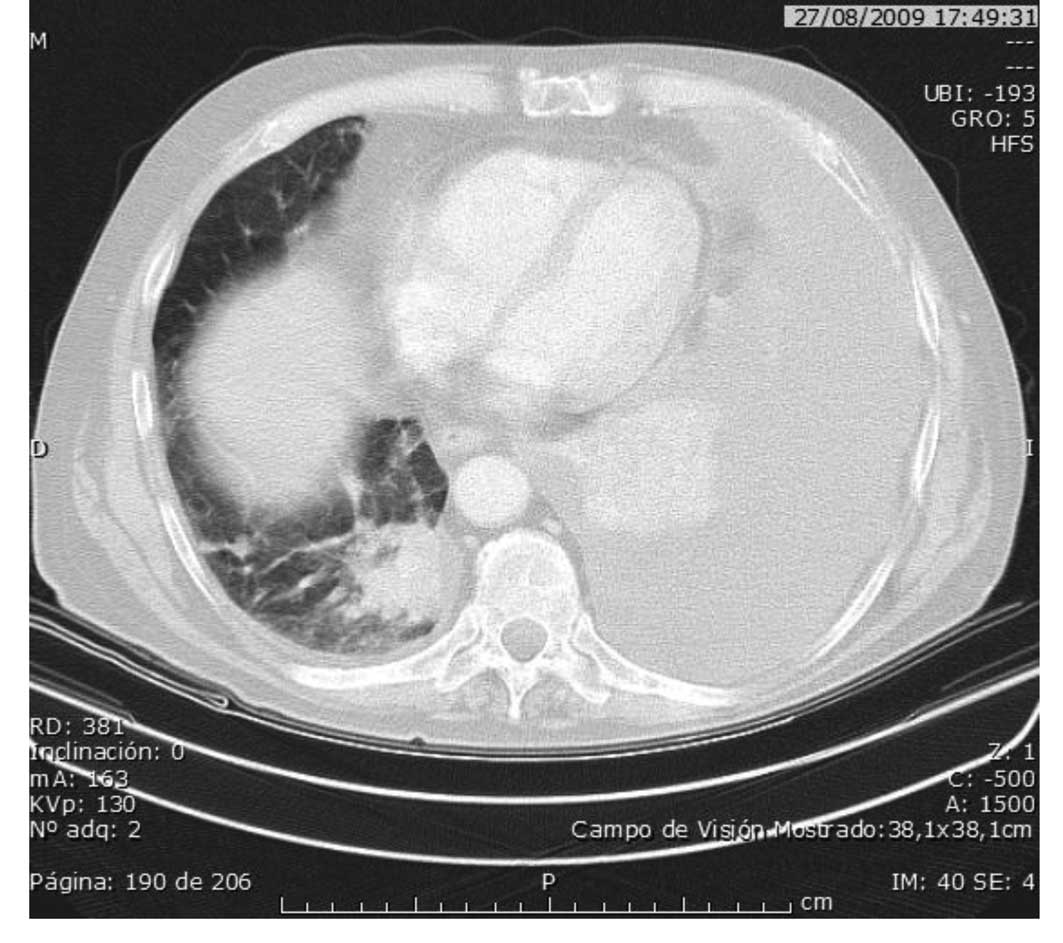
hypercholesterolemia. In August 2009, the lung disease progression
was diagnosed as a massive pleural effusion and treatment was
discontinued following evacuation by thoracentesis (Fig. 3).
In September 2009, the patient began a sixth line of
treatment with sorafenib and achieved a stable condition for 14
months. However, the patient had a new episode of severe pleural
effusion in December 2010 and succumbed to the disease 96 months
after the diagnosis of metastases.
Discussion
Temsirolimus was internally approved at our center,
the Hospital Clínico San Cecilio, Avenida, Granada, Spain, in the
context of a compassionate use program based on published efficacy
data from phase II-III trials (3,6).
Before this, randomized trials to support the use of temsirolimus
after failure of sunitinib in metastatic renal cancer had not been
reported. Atkins et al(6)
demonstrated the efficacy of temsirolimus treatment in 61% of
patients who received temsirolimus therapy as a third-line or later
treatment, including in cases where disease progression had
occurred following IFN, interleukin or chemotherapy schedules. A
report by Gerullis et al(7)
described a retrospective study in which sunitinib and temsirolimus
were sequentially administered for 29 and 6 weeks, respectively. In
the present case, the durations of the treatment with sunitinib and
temsirolimus were 85 weeks and 58 weeks, respectively.
In a study by Lamm et al(8), the authors showed preliminary data
with regard to the use of temsirolimus administered in pretreated
patients, whose median time to progression was 20 weeks. The
results were similar to another study that reported the efficacy
achieved with everolimus (9),
following the occurrence of disease progression during treatment
with sunitinib, sorafenib or both, where 63% disease stabilization
and a four-month median time to progression was achieved. Mackenzie
et al reported the results of 87 patients who had previously
been treated with anti-angiogenic therapy; the median time to
progression was 4 months and the median overall survival was 11
months (10).
In new-age directed therapies, questions are
continuously arising concerning the most effective sequence of drug
therapies for increased survival in metastatic renal cell cancer,
plus questions with regard to the best methods for identifying
accurate predictive markers of clinical efficacy and toxicity
(5,11). For second-line treatment, phase III
results from the INTORSECT trial on temsirolimus versus sorafenib
supports the sequence of tyrosine kinase inhibitor (TKI)-TKI rather
than TKI-mTOR (12).
In the current study, following observation of
disease progression during sunitinib therapy, we selected to
initiate temsirolimus therapy after three poor prognostic factors
were exhibited by the patient. Disease stabilization was achieved
for one year, with quality-adjusted survival without symptoms for
10 months and the occurrence of two G3 adverse events during the
last 3 months of treatment. The common G3 or G4 side-effects with
temsirolimus consisted of anemia, hyperglycemia and
fatigue/asthenia (13). No cases of
hypothyroidism have been reported from the Global ARCC trial.
Hypothyroidism is a class-type toxic effect of sunitinib and this
event is not associated with temsirolimus. Although the exact
pathophysiology of several of these off-target side-effects remains
to be determined, it may be explained by inhibition of the same
signaling pathways, however at different points (14).
Furthermore, inhibition of the mTOR pathway was
accompanied by an increase in cholesterol (326 mg/dl) and
triglyceride (426 mg/dl) levels, which may represent a set of
markers to indicate drug response efficacy (6). Based upon the rapid progression of
renal cancer, we consider this to be a noteworthy case report of
metastatic renal cancer in a 57-year-old male. The survival time
from diagnosis to lung metastasis was 8 years.
In conclusion, this case report suggests that
temsirolimus has significant activity in recurrent renal carcinoma
which had been previously treated with interleukin and sunitinib
and shows promising effects with regard to the subsequent use of
TKI-mTOR-TKI. In the future, biomarkers may allow us to
individualize second-line treatments.
References
|
1
|
Motzer RJ, Bacik J, Murphy BA, Russo P and
Mazumdar M: Interferon-alfa as a comparative treatment for clinical
trials of new therapies against advanced renal cell carcinoma. J
Clin Oncol. 20:289–296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Motzer RJ, Hutson TE, Tomczak P, et al:
Overall survival and updated results for sunitinib compared with
interferon alfa in patients with metastatic renal cell carcinoma. J
Clin Oncol. 27:3584–3590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Motzer RJ, Hudes GR, Curti BD, McDermott
DF, Escudier BJ, Negrier S, et al: Phase I/II trial of temsirolimus
combined with interferon alfa for advanced renal cell carcinoma. J
Clin Oncol. 25:3958–3964. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hudes G, Carducci M, Tomczak P, Dutcher J,
Figlin R, Kapoor A, et al: Global ARCC trial: Temsirolimus,
interferon alfa, or both for advanced renal-cell carcinoma. N Engl
J Med. 356:2271–2281. 2007. View Article : Google Scholar
|
|
5
|
González Larriba JL, Espinosa E, García
Carbonero I, García-Donas J, López M, Meana A, Puente J and
Bellmunt J: Sequential therapy in metastatic renal cell carcinoma:
pre-clinical and clinical rationale for selecting a second- or
subsequent-line therapy with a different mechanism of action.
Cancer Metastasis Rev. 31(Suppl 1): S11–S17. 2012.PubMed/NCBI
|
|
6
|
Atkins MB, Hidalgo M, Stadler WM, Logan
TF, Dutcher JP, Hudes GR, et al: Randomized phase II study of
multiple dose levels of CCI-779, a novel mammalian target of
rapamycin kinase inhibitor, in patients with advanced refractory
renal cell carcinoma. J Clin Oncol. 22:909–918. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gerullis H, Bergmann L, Maute L, Ecke TH,
Eimer C, Bagner JW and Otto T: Feasibility of sequential use of
sunitinib and temsirolimus in advanced renal cell carcinoma. Med
Oncol. 27:373–378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lamm W, Vogl UM, Bojic M, Zielinski C,
Klingler C, Kramer G and Schmidinger M: Safety and efficacy of
temsirolimus in heavily pretreated patients with metastatic renal
cell carcinoma. Acta Oncol. 51:101–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Motzer RJ, Escudier B, Oudard S, Hutson
TE, Porta C, Bracarda S, et al: Efficacy of everolimus in advanced
renal cell carcinoma: a double-blind, randomised,
placebo-controlled phase III trial. Lancet. 372:449–456. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mackenzie MJ, Rini BI, Elson P, Schwandt
A, Wood L, Trinkhaus M, Bjarnason G and Knox J: Temsirolimus in
VEGF-refractory metastatic renal cell carcinoma. Ann Oncol.
22:145–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Escudier B, Goupil MG, Massard C and
Fizazi K: Sequential therapy in renal cell carcinoma. Cancer.
115:2321–2326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hutson TE, Escudier B, Esteban E,
Bjarnason GA, Lim HY, Pittman K, et al: Temsirolimus vs Sorafenib
as Second Line Therapy in Metastatic Renal Cell Carcinoma: Phase 3
Results from the INTORSECT Trial. ESMO: Abstract LBA22,. 2012
|
|
13
|
Rodriguez-Pascual J, Cheng E, Maroto P and
Duran I: Emergent toxicities associated with the use of mTOR
inhibitors in patients with advanced renal carcinoma. Anticancer
Drugs. 21:478–486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmidinger M and Bellmunt J: Plethora of
agents, plethora of targets, plethora of side effects in metastatic
renal cell carcinoma. Cancer Treat Rev. 36:416–424. 2010.
View Article : Google Scholar : PubMed/NCBI
|