Introduction
Hypoxia, a frequent characteristic of solid tumour
growth in head and neck cancer and other cancers, stimulates a
cascade of molecular pathways resulting in angiogenesis, glycolysis
and changes in the cell cycle. The dimeric transcription factor
hypoxia-inducible factor (HIF-1) is a key regulator of the cellular
response to hypoxia (1,2). HIF-1 functions as a master regulator
of oxygen homeostasis and undergoes conformational changes in
response to different oxygen concentrations (3). HIF-1 consists of two subunits, α and
β, which are both helix-loop-helix transcription factors. The
HIF-1α subunit mediates HIF-1 function as a transcription factor in
response to cellular hypoxia (4).
Alteration and overexpression of HIF-1α has been detected in a
variety of solid tumours, including breast, lung, ovarian, and head
and neck cancers, with varying (diffuse and perinecrotic) staining
patterns. The expression of HIF-1α was determined to be of
prognostic relevance in different tumours (5–8).
However, based on a review of the literature (9), the prognostic relevance of HIF-1α in
tumours derived from squamous epithelium remains controversial. In
clinical specimens, elevated HIF-1α expression correlates with poor
outcome in colorectal, pancreatic, breast, cervical, endome-trial,
ovarian, bladder, gastric, and head and neck carcinomas. There is a
growing body of evidence suggesting that HIF-1α is involved in the
progression of oral cancers (10–13).
In clinical studies, elevated expression of HIF-1α was found to
correlate with lymph node involvement, tumour-node-metastasis (TNM)
classification, poor survival and resistance to chemo- and
radiotherapy in patients with oral squamous cell carcinomas
(12,14,15).
Contradictory results relating to the role of HIF-1α in oral
carcinomas have also been reported, however (16). Carcinoma of the tongue represents a
significant proportion of oral cancers. The tongue has a rich blood
supply, so it was of interest to examine whether hypoxic conditions
existed in this tissue. The aim of this study was, therefore, to
verify the role of HIF-1α expression in carcinoma of the tongue, to
evaluate its correlation with clinicopathological features and to
determine its value as a prognostic marker.
Materials and methods
Patients and specimens
The cohort was assembled from patients who were
histologically diagnosed with carcinoma of the tongue and who
underwent radical surgery at the Department of Oral and
Maxillofacial Surgery, Stomatological Hospital Affiliated Medical
School, Nanjing University, between 2000 and 2008. Exclusion
criteria included recurrence at presentation, pre-operative
radiotherapy, chemotherapy or hormone therapy, residual tumour at
the surgical margin or incomplete medical records. All medical
records were reviewed retrospectively, according to the inclusion
and exclusion criteria. Table I
summarises the characteristics of patients (n=120) with carcinoma
of the tongue that were recruited to this study. The median age of
the patients at the time of diagnosis was 57 years (range 33–81
years). The formalin-fixed, paraffin-embedded specimens from these
patients were used for immunohistochemical analysis. The follow-up
period was calculated from the date of surgery to the date of
death, loss of follow-up, or the 60th month, whichever came first
(median follow-up period, 47 months).
 | Table ISummary of demographic and clinical
parameters. |
Table I
Summary of demographic and clinical
parameters.
| Variable | No. | % |
|---|
| Age (years, mean ±
SD) | 57±10.1 | |
| Gender | | |
| Female | 54 | 45.0 |
| Male | 66 | 55.0 |
| Alcohol and
tobacco | | |
| Yes | 36 | 30.0 |
| No | 84 | 70.0 |
| Lymph node
metastasis | | |
| Yes | 48 | 40.0 |
| No | 72 | 60.0 |
| Clinical stage | | |
| Early (I/II) | 54 | 45.0 |
| Advanced
(III/IV) | 66 | 55.0 |
| Pathological
grade | | |
| I | 45 | 37.5 |
| II | 70 | 58.3 |
| III | 5 | 4.2 |
The study was approved by the Ethics Committee of
Stomatological Hospital Affiliated Medical School, Nanjing
University, Nanjing, China. Written informed consent was obtained
from the patients.
Immunohistochemical staining
Sections (4 μm) were deparaffinised in xylene and
rehydrated. Antigen retrieval was performed using the heat-induced
epitope retrieval method. Slides were boiled in antigen retrieval
buffer (0.001 mol/l EDTA solution, adjusted to pH 8.0) in a
pressure cooker until full pressure was reached, and maintained for
another 90 sec. After the slides were cooled to room temperature,
they were incubated with a mouse monoclonal antibody to human
HIF-1α (1:1200) for 4 h at room temperature and at 4°C overnight.
Instead of the primary antibody, the negative control was incubated
with phosphate-buffered saline (PBS; pH 7.4). The slides were
washed twice with PBS for 5 min and tissues were incubated with a
specific biotinylated secondary antibody, the
streptavidin-biotin-peroxidase complex system (Novolink Max Polymer
Detection System, Novocastra, Newcastle-upon-Tyne, UK) at 37°C in a
thermostatically-controlled container for 40 min. The slides were
washed twice for 5 min with PBS and the colour was developed with
3,3′-diaminobenzidine. The slides were counterstained with Mayer’s
haematoxylin, washed, dehydrated and cleared. Coverslips were
sealed with neutral balsam. The percentage of positive cells was
estimated using an image analysis system.
The level of expression of HIF-1α was determined
independently by three pathologists. Each pathologist determined
the percentage of positive cell nuclei in each field. Tissues were
scored according to the percentage of positive immunostaining (P)
as follows: 0 (<1%), 1 (1–5%), 2 (5–10%), and 3 (>10%).
RNA preparation and real-time
quantitative RT-PCR
Total RNA was extracted from 45 fresh, paired
samples of tongue carcinoma and corresponding adjacent normal
tissues using TRIzol reagent (Invitrogen, Life Technologies,
Gaithersburg, MD, USA) following the manufacturer’s protocol.
Real-time quantitative RT-PCR (qRT-PCR) was performed using the
Thermal Cycler Dice™ Real-Time System TP800 (Takara) according to
the standard protocol of the SYBR® Premix Ex Taq™
Perfect Real-Time system (Takara). Primers for HIF-1α were as
follows: sense 5′-TGTGAACCCATTCCTCAC CCATCA-3′, antisense
5′-CAGTTTCTGTGTCGTTGCTGC CAA-3′ and for β-actin: sense
5′-TCACCCACACTGTGCC CATCTACGA-3′ and antisense 5′-CAGCGGAACCGCTCA
TTGCCAATGG-3′. Thermal cycling conditions were 95°C for 1 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The
level of expression of HIF-1α in the tumour and adjacent normal
samples was quantified by measuring the fractional cycle number at
which the level of expression reached a fixed threshold (Ct) and
this was directly related to the amount of product. The
housekeeping gene, β-actin, was used as an internal control to
quantify the products of HIF-1α. The relative quantification was
given by the Ct values, determined for triplicate reactions for
tongue carcinoma and adjacent normal samples for HIF-1α and
β-actin. The average of triplicate Ct values for HIF-1α was
determined and the average β-actin Ct value was subtracted from the
mean HIF-1α Ct value, to obtain ΔCt (ΔCt = Ct(target gene in
carcinoma of tongue/adjacent normal sample) - Ct(β-actin gene in
carcinoma of tongue/adjacent normal sample). Relative expression
level was determined as
2–ΔΔCt, where
ΔΔCt=ΔCt (carcinoma sample)-ΔCt (adjacent normal sample).
Therefore, 2–ΔΔCt indicates the fold
change in HIF-1α expression in carcinoma samples relative to
adjacent normal samples.
Statistical analysis
SPSS 18.0 was used for statistical analysis. TNM
stage, histological differentiation status and expression of HIF-1α
were correlated with the duration of progression-free and overall
survival. Progression-free survival and overall survival were
calculated from the date of surgery to the date of
histologically-proven recurrent or metastatic carcinoma, or
disease-related mortality, respectively. Patients who succumbed to
intercurrent diseases were censored at the date of mortality.
Patients lost to follow-up were censored at the date of the last
examination. The progression-free survival curves and overall
survival curves were constructed according to Kaplan and Meier. The
log-rank test was used to assess differences between groups and
multivariate survival analysis was performed with multivariate Cox
regression analysis. Correlations between clinicopathological
features and expression of HIF-1α were evaluated using
χ2 test. The correlation between relative mRNA
expression and pathological characteristics was analysed using
non-parametric tests. P<0.05 was considered to indicate a
statistically significant result.
Results
HIF-1α expression and clinical
outcome
Moderate to strong staining was observed in 81.8%
(54/66) of advanced-stage tumours and 75% (36/48) of tumours with
lymph node metastasis. In contrast, weak to moderate staining was
observed in 38.9% (21/54) of early-stage tumours and 54.2% (39/72)
of tumours with no lymph node metastasis. The correlation between
HIF-1α expression and lymph node metastasis or clinical stage was
indicated by Spearman correlative analysis (Table II, P= 0.034 and P=0.002,
respectively). HIF-1α expression did not correlate with gender,
age, histological differentiation or location of primary sites.
 | Table IICorrelation between clinical
parameters of patients with carcinoma of the tongue and
hypoxia-inducible factor-1α (HIF-1α) expression levels. |
Table II
Correlation between clinical
parameters of patients with carcinoma of the tongue and
hypoxia-inducible factor-1α (HIF-1α) expression levels.
| Categorical
variables | HIF-1α (nuclear)
|
|---|
| Negative | Positive | P-value |
|---|
| Gender | | | |
| Male | 20 | 46 | |
| Female | 25 | 29 | 0.072 |
| Alcohol and
tobacco | | | |
| Yes | 14 | 22 | |
| No | 31 | 53 | 0.837 |
| Lymph node
metastasis | | | |
| Yes | 25 | 27 | |
| No | 20 | 48 | 0.036a |
| Clinical stage (T
stage) | | | |
| T1/T2 | 27 | 27 | |
| T3/T4 | 18 | 49 | 0.009a |
| Tumour grade
(differentiation) | | | |
| Poor | 17 | 21 | |
| Moderate | 24 | 48 | |
| Well | 4 | 6 | 0.16 |
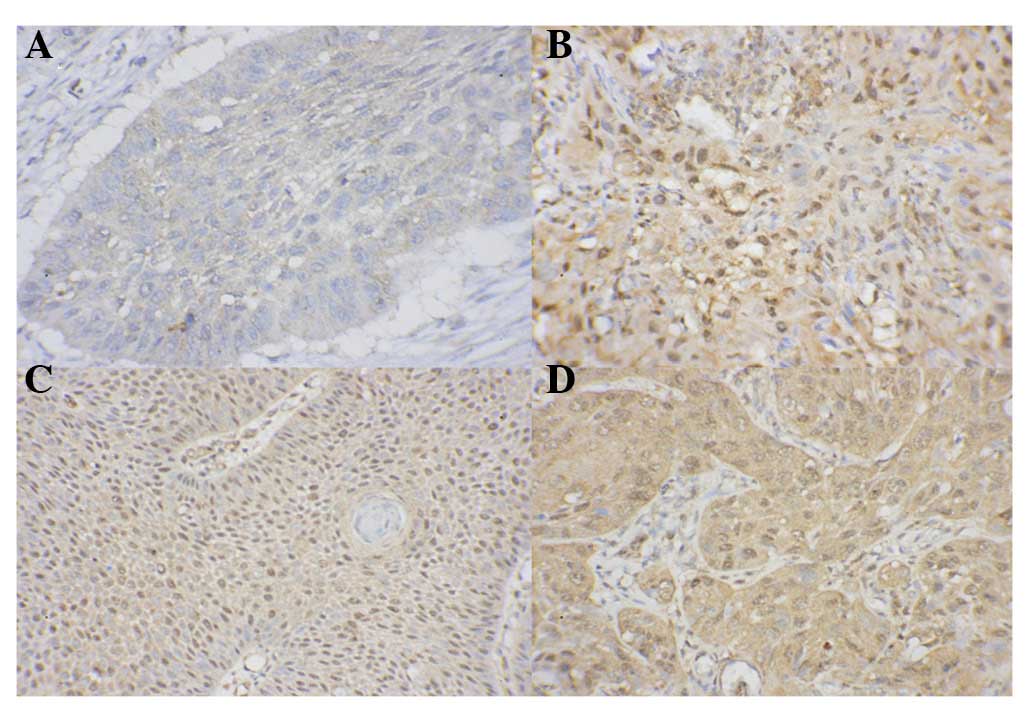
Representative immunohistochemical images are shown
in Fig. 1. Nuclear staining of
HIF-1α was detected in 75% (90/120) tongue carcinoma specimens. The
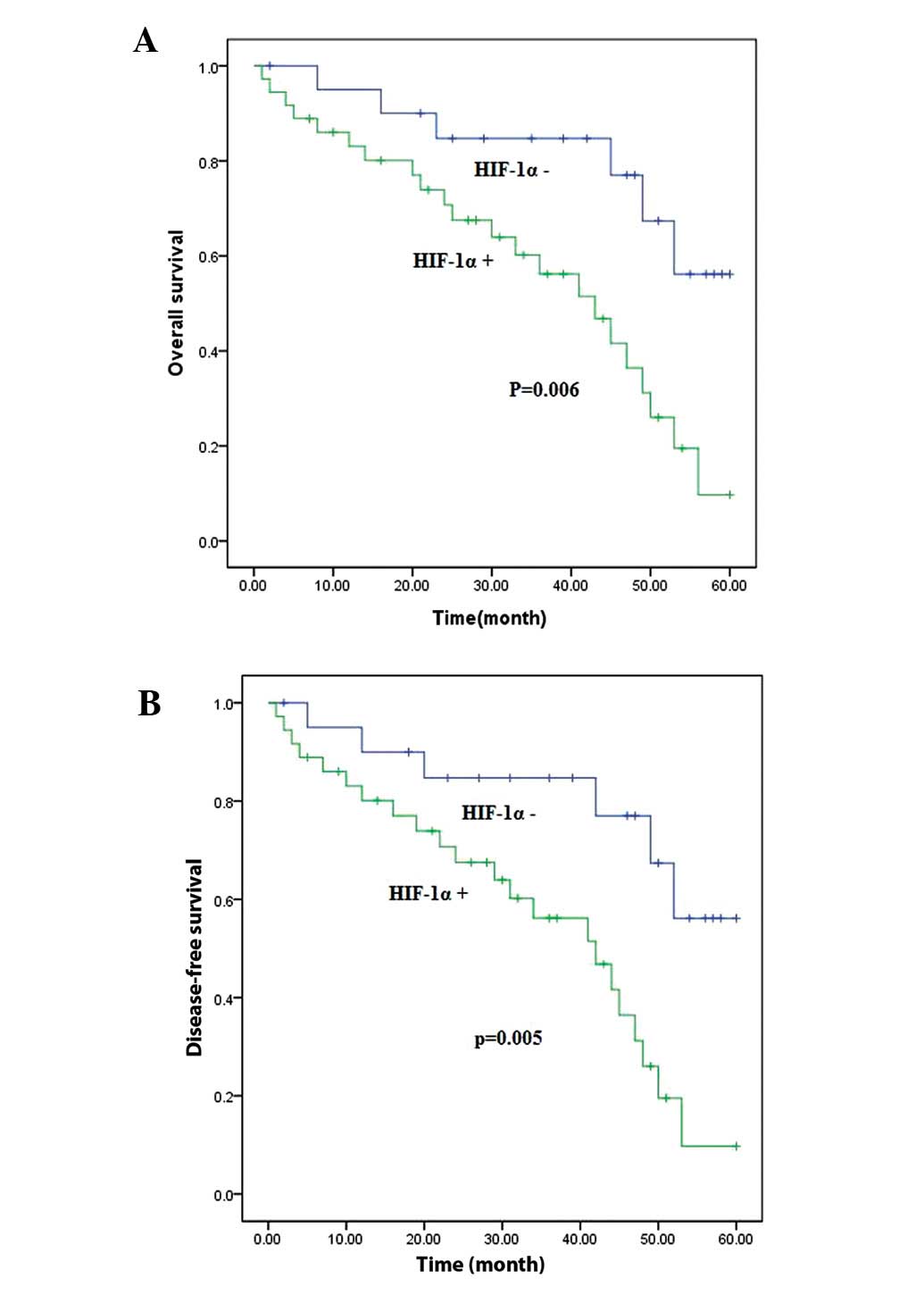
Kaplan-Meier survival analysis revealed a significantly worse
overall survival (P<0.001) and disease-free survival
(P<0.001) for patients with nuclear staining of HIF-1α compared
with those whose tumours were negative for HIF-1α staining
(Fig. 2). The correlations between
the relative mRNA expression of HIF-1α and pathological
characteristics of the tumour sample, such as TNM stage, clinical
stage, pathological differentiation grade and smoking and drinking
were analysed (Table II). A
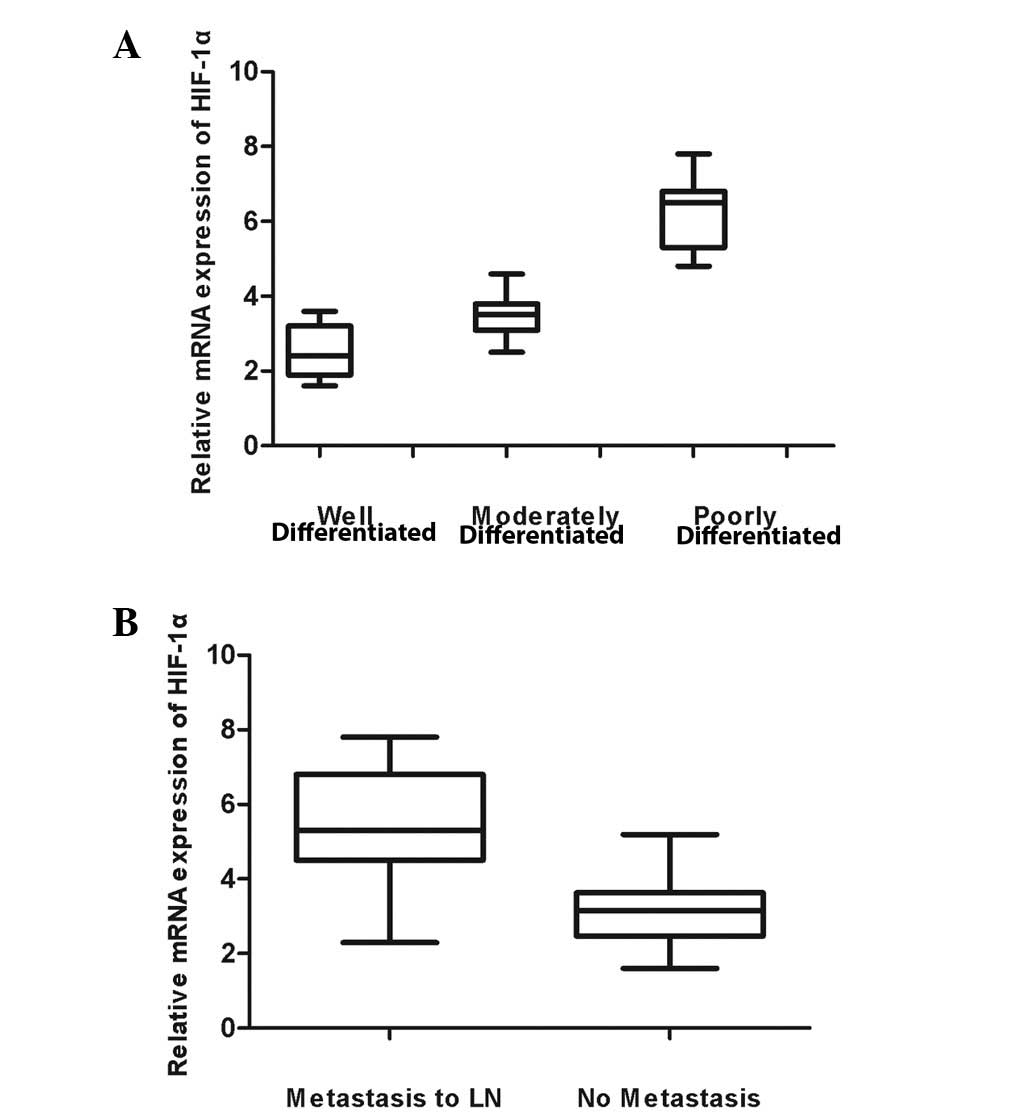
positive correlation was found between the mRNA level of HIF-1α and
the pathological differentiation grade of tongue carcinomas.
Furthermore, the expression of HIF-1α was significantly different
between groups of patients with lymph node metastasis and no
metastasis (P<0.05; Fig. 3).
Discussion
The correlation between HIF-1α expression and oral
cancers has been widely reported. The actual role of HIF-1α in
tongue carcinoma progression, however, remains unclear. The tongue
is supplied by abundant blood vessels. Regions of hypoxia are
likely to be very limited in this tissue, therefore. The aim of
this study was to examine the role of HIF-1α signalling in tongue
carcinoma progression. The results of this study reveal that,
although the blood supply in the tongue is abundant, there was
evidence of a hypoxic state in the tumours, possibly due to their
rapid growth. This suggests that HIF-1α may play an important role
in the progression of tongue carcinoma.
Several previous studies have provided evidence that
HIF-1α plays different roles in different types of tumours. Higher
levels of HIF-1α expression in this study correlated strongly with
lymph node metastasis, which indicates that HIF-1α is involved in
tongue cancer metastasis. Determining the level of expression of
HIF-1α may, therefore, provide valuable information to inform the
choice of treatment or to assess prognosis. In agreement with
previous reports (12,17,18),
this study showed that HIF-1α expression correlated with overall
survival, and disease-free survival, of patients with tongue
carcinomas. It was suggested that rapid tumour growth resulted in a
large tumour size and areas of hypoxia, which induces the
accumulation of HIF-1α (12). Once
stabilised, HIF-1α transactivates a set of downstream genes that,
in turn, facilitate tumour growth, angiogenesis and metastasis. In
this regard, the correlation between HIF-1α expression and patient
survival is easily understood. It has been reported that targeting
HIF-1α signalling in tumour cells significantly inhibits tumour
growth in mouse xenografts (19–23).
Raval et al(24) found that
HIF-1α expression was associated with improved disease-free
survival in surgically-resected head and neck squamous cell
carcinoma, however. Recent studies have revealed a tumour
suppressive role of HIF-1α, probably due to HIF-1α-induced
apoptosis or transactivation of specific genes that are targets for
negative selection in human cancers (25). In light of these discrepancies among
different tumours there may be tissue-specific differences in
response to HIF-1α regulation. In this study, increased expression
of HIF-1α was observed in more advanced stages of tongue cancer,
which indicates the critical role of HIF-1α in tongue
carcinogenesis. More importantly, the expression of HIF-1α
correlated closely with lymph node metastasis and could be a
reliable marker to predict the prognosis of tongue cancer.
Overexpression of HIF-1α could be an indicator of
poor prognosis in carcinoma of the tongue. HIF-1α overexpression
correlated with clinical stage and lymph node metastasis in
patients with carcinoma of the tongue.
Acknowledgements
This study was financially supported
by Jiangsu Provincial Natural Science Foundation (grant No.
BK2012075), National Key Disciplines Constructional Project Funding
and Nanjing Medical Science and Technique Development
Foundation.
References
|
1
|
Semenza GL and Wang GL: A nuclear factor
induced by hypoxia via de novo protein synthesis binds to the human
erythropoietin gene enhancer at a site required for transcriptional
activation. Mol Cell Biol. 12:5447–5454. 1992.
|
|
2
|
Iyer NV, Kotch LE, Agani F, et al:
Cellular and developmental control of O2 homeostasis by hypoxia
inducible factor 1 alpha. Genes Dev. 12:149–162. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruick RK and McKnight SL: A conserved
family of prolyl-4-hydroxylases that modify HIF. Science.
294:1337–1340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxiainducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci
USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakayama K, Kanzaki A, Hata K, Katabuchi
H, Okamura H, Miyazaki K, Fukumoto M and Takebayashi Y:
Hypoxia-inducible factor 1 alpha (HIF-1 alpha) gene expression in
human ovarian carcinoma. Cancer Lett. 176:215–223. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee CH, Lee MK, Kang CD, Kim YD, Park do
Y, Kim JY, Sol MY and Suh KS: Differential expression of hypoxia
inducible factor-1 alpha and tumor cell proliferation between
squamous cell carcinomas and adenocarcinomas among operable
non-small cell lung carcinomas. J Korean Med Sci. 18:196–203. 2003.
View Article : Google Scholar
|
|
7
|
Beasley NJ, Leek R, Alam M, Turley H, Cox
GJ, Gatter K, Millard P, Fuggle S and Harris AL: Hypoxia-inducible
factors HIF-1alpha and HIF-2 alpha in head and neck cancer:
relationship to tumor biology and treatment outcome in surgically
resected patients. Cancer Res. 62:2493–2497. 2002.PubMed/NCBI
|
|
8
|
Bos R, Van Der Groep P, Greijer AE,
Shvarts A, Meijer S, Pinedo HM, Semenza GL, Van Diest PJ and Van
Der Wall E: Levels of hypoxia inducible factor-1alpha independently
predict prognosis in patients with lymph node negative breast
carcinoma. Cancer. 97:1573–1581. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar
|
|
10
|
Zhang Q, Zhang ZF, Rao JY, et al:
Treatment with siRNA and antisense oligonucleotides targeted to
HIF-1 alpha induced apoptosis in human tongue squamous cell
carcinomas. Int J Cancer. 111:849–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cohen NA, Lai SY, Ziober AF and Ziober BL:
Dysregulation of hypoxia inducible factor-1α in head and neck
squamous cell carcinoma cell lines correlates with invasive
potential. Laryngoscope. 114:418–423. 2004.
|
|
12
|
Liang X, Yang D, Hu J, Hao X, Gao J and
Mao Z: Hypoxia inducible factor-α expression correlates with
vascular endothelial growth factor-C expression and
lymphangiogenesis/angiogenesis in oral squamous cell carcinoma.
Anticancer Res. 28:1659–1666. 2008.
|
|
13
|
Yao H, Wang H, Zhang Z, Jiang BH, Luo J
and Shi X: Sulforaphane inhibited expression of hypoxia-inducible
factor-1α in human tongue squamous cancer cells and prostate cancer
cells. Int J Cancer. 123:1255–1261. 2008.
|
|
14
|
Lin PY, Yu CH, Wang JT, et al: Expression
of hypoxia-inducible factor-1 alpha is significantly associated
with the progression and prognosis of oral squamous cell carcinomas
in Taiwan. J Oral Pathol Med. 37:18–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sasabe E, Zhou X, Li D, Oku N, Yamamoto T
and Osaki T: The involvement of hypoxia-inducible factor-1α in the
susceptibility to γ-rays and chemotherapeutic drugs of oral
squamous cell carcinoma cells. Int J Cancer. 120:268–277. 2007.
|
|
16
|
Fillies T, Werkmeister R, van Diest PJ,
Brandt B, Joos U and Buerger H: HIF-1α overexpression indicates a
good prognosis in early stage squamous cell carcinomas of the oral
floor. BMC Cancer. 5:842005.
|
|
17
|
Roh JL, Cho KJ, Kwon GY, et al: The
prognostic value of hypoxia markers in T2-staged oral tongue
cancer. Oral Oncol. 45:63–68. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Winter SC, Shah KA, Han C, et al: The
relation between hypoxiainducible factor (HIF)-1α and HIF-2α
expression with anemia and outcome in surgically treated head and
neck cancer. Cancer. 107:757–766. 2006.
|
|
19
|
Carroll VA and Ashcroft M: Role of
hypoxia-inducible factor (HIF)-1α versus HIF-2α in the regulation
of HIF target genes in response to hypoxia, insulin-like growth
factor-I, or loss of von Hippel-Lindau function: implications for
targeting the HIF pathway. Cancer Res. 66:6264–6270. 2006.
|
|
20
|
Imamura T, Kikuchi H, Herraiz MT, et al:
HIF-1α and HIF-2α have divergent roles in colon cancer. Int J
Cancer. 124:763–771. 2009.
|
|
21
|
Kung AL, Zabludoff SD, France DS, et al:
Small molecule blockade of transcriptional coactivation of the
hypoxia-inducible factor pathway. Cancer Cell. 6:33–43. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li L, Lin X, Staver M, et al: Evaluating
hypoxia-inducible factor-1alpha as a cancer therapeutic target via
inducible RNA interference in vivo. Cancer Res. 65:7249–7258. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rapisarda A, Shoemaker RH and Melillo G:
Targeting topoisomerase I to inhibit hypoxia inducible factor 1.
Cell Cycle. 3:172–175. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Raval RR, Lau KW, Tran MG, et al:
Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and
HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol
Cell Biol. 25:5675–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Scortegagna M, Martin RJ, Kladney RD,
Neumann RG and Arbeit JM: Hypoxia-inducible factor-1α suppresses
squamous carcinogenic progression and epithelial-mesenchymal
transition. Cancer Res. 69:2638–2646. 2009.
|