Introduction
It was anticipated that by 2010, breast cancer would
be newly diagnosed in >1.5 million females each year, and that
500,000 females worldwide would succumb to this disease (1). Therapies that target the drivers of
individual types of breast cancer have substantially improved the
outcome of females with breast cancer (2,3).
One such pathway is the hedgehog (Hh) signaling
pathway, which specifies patterns of cell growth and
differentiation during embryogenesis in a wide range of tissues
(4). In addition to its role in
developmental patterning, this pathway plays a critical role in
mature tissue homeostasis and the maintenance of somatic cell
numbers in various organs (5).
Aberrant activation of the Hh pathway is common in breast carcinoma
(6). The Hh pathway represents an
attractive target for drug development and has demonstrated
potential in clinical trials of cancer treatments. The specificity
of cyclopamine for the Hh pathway is demonstrated by an absence of
cytotoxicity in cells that lack Hh signaling (6).
In the present study, using estrogen-responsive
(MCF-7) and estrogen-independent (MDA-MB-231) human breast cancer
cell lines, the effects of cyclopamine on breast cancer
proliferation and invasion are investigated in vitro. The
possible pathways involving its regulation of breast cancer
tumorigenesis and metastasis are explored.
Materials and methods
Cell culture and reagents
The MCF-7 and MDA-MB-231 human breast cancer cell
lines were purchased from the American Type Culture Collection
(Manassas, VA, USA). Cells were maintained in Dulbecco’s modified
Eagle’s medium supplemented with 10% fetal calf serum, L-glutamine
(5 mmol/l), non-essential amino acids (5 mmol/l), penicillin (100
U/ml) and streptomycin (100 U/ml) (Invitrogen Life Technologies,
Carlsbad, CA, USA), at 37°C in a humidified atmosphere at 5%
CO2. Cyclopamine and the MEK1/2 inhibitor, U0126, were
obtained from Cell Signaling Technology, Inc. (Beverly, MA,
USA).
The study was approved by the Ethics Committee of
Soochow University, Suzhou, Jiangsu, China.
Cell viability assay
Cell proliferation was determined using an MTT
viability assay; the most commonly used assay for determining cell
growth and death. The MTT survival assay has been described in
detail previously (7).
Exponentially growing cells were recultured (5,000 cells/well)
overnight in 96-well tissue culture plates. Up to 20 μl MTT
(Sigma-Aldrich, St Louis, MO, USA) was directly added to the media
in each well, at a final concentration of 2 mg/ml. Following 4 h of
incubation, the medium containing MTT was discarded, and 120
μl dimethyl sulfoxide was added for 10 min. The absorbance
was measured using an enzyme-linked immunosorbent assay reader at
570 nm, with the absorbance at 630 nm as the background correction.
The cell viability was expressed as the percentage of untreated
controls. All experiments were performed at least three times.
Proliferation assay
Cells were counted and plated at the same initial
density on 6-well plates. They were then treated with 10 or 20
μmol/l cyclopamine or the vehicle only, and incubated for
time periods ranging from 0–10 days. At each time point, cells were
trypsinized and counted using a Neubauer hemocytometer under trypan
blue exclusion.
Cell cycle assays
The cells were removed with trypsin and collected in
centrifuge tubes together with the culture medium. The contents
were centrifuged for 5 min at 1,800 × g. The supernatant was poured
out, washed once with 1X phosphate-buffered saline (PBS) and
centrifuged for a further 5 min. The cells were fixed with 5 ml of
pre-cooled 70% ethanol for ≥4 h. The fixed cells were centrifuged
and washed with 1X PBS. Following centrifugation, the cell pellets
were resuspended in 500 μl propidium iodide (10
μg/ml) containing 300 μg/ml RNase (Sigma-Aldrich).
Subsequently, the cells were incubated on ice for 30 min, and then
filtered with a 53-μm nylon mesh. The cell cycle
distribution was calculated from 10,000 cells with ModFit LT
software (Becton Dickinson, San José, CA, USA) using FACSCalibur
(Becton Dickinson).
Transwell invasion assay
The invasion assay was carried out using Transwell
plates (Millipore, Billerica, MA, USA), as previously described
(8). The filter surfaces (pore
size, 8 μm) of the Transwell plates were uniformly coated
with 25 mg Matrigel (Becton Dickinson, San Jose, CA, USA) overnight
at 4°C, prior to the experiment. The lower chamber was filled with
culture medium containing 10% fetal calf serum. The subconfluent
proliferating cells were carefully transferred onto the coated
upper surface of the chamber. Following 24 h of incubation, the
filter was gently removed and the upper surface was wiped to remove
all attached cells. The cells that had invaded through the Matrigel
and attached to the lower surface of the filter were fixed with 4%
paraformaldehyde and stained with Giemsa (Sigma-Aldrich, St Louis,
MO, USA). Three replicates were conducted for each condition, and
15 random fields in each replicate were selected and counted using
an Olympus CKX41 inverted microscope (Olympus, Tokyo, Japan). The
results are presented as the ratio of the cells that invaded under
experimental conditions relative to those that invaded under
control conditions.
Western blot analysis
Cell lysates were prepared and western blot analysis
was performed as previously described (9). Equal aliquots of total cell protein
(50 μg/lane) were electrophoresed on sodium dodecyl sulfate
(SDS)-polyacrylamide gels, transferred onto polyvinylidene fluoride
(PVDF) membranes and then blotted using the following primary
antibodies (1:1,000 dilution; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA): β-actin (C-4), NF-κB (P65A), cyclin D1 (A-12), MMP2
(2C1), MMP9 (6-6B); along with secondary antibody horse-radish
peroxidase-labeled goat anti-mouse (GAM-007) and goat anti-rabbit
(SC-2004) IgG. The protein bands were visualized using an enhanced
chemiluminescence system (Union Bioscience Corporation, Hangzhou,
China) with prestained markers as molecular size standards. The
densitometry of the protein bands was quantified with Quantity One
(Bio-Rad, Hercules, CA, USA), and the values were expressed
relative to β-actin (the control for loading and transfer). At
least three independent experiments were performed for each cell
type studied.
Results
Cyclopamine decreases breast cancer cell
proliferation
MTT viability assays were conducted to elucidate the
potential biological effects of cyclopamine in breast cancer cells.
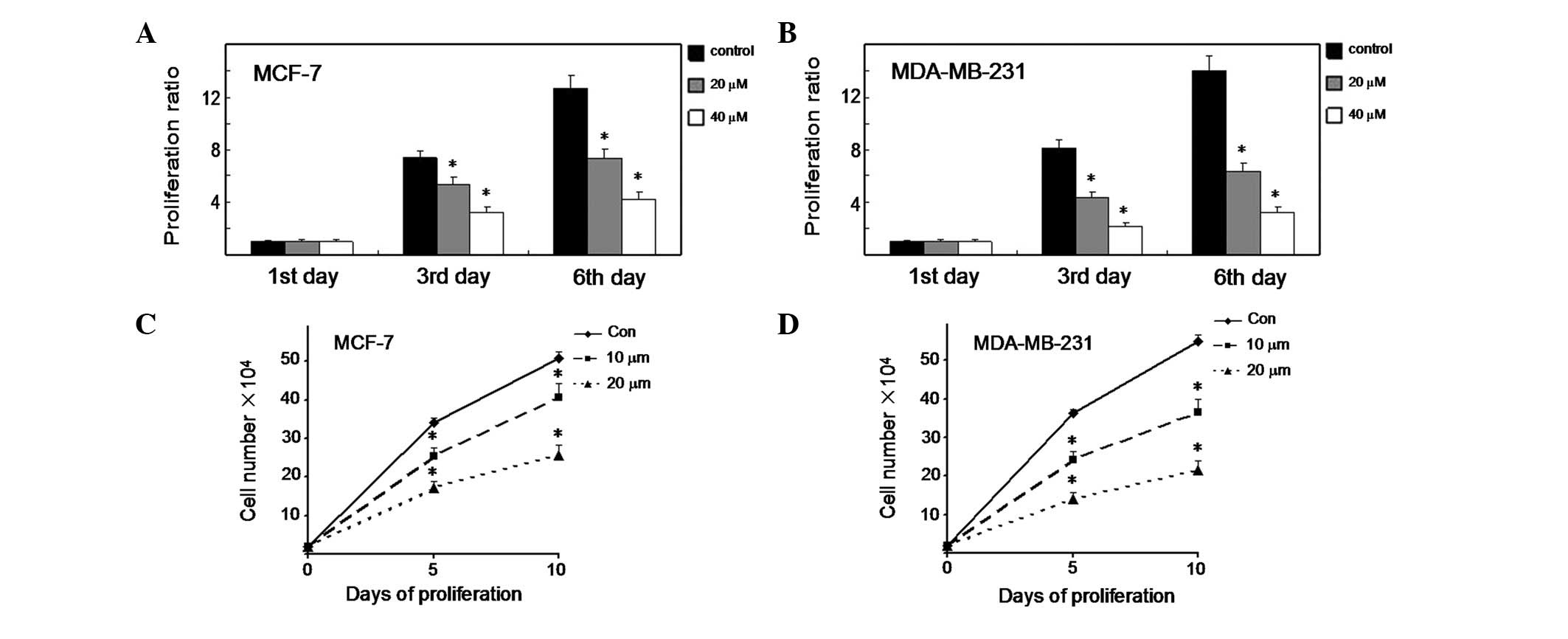
As shown in Fig. 1A, the MCF-7
cells treated with cyclopamine displayed a significant reduction in
proliferation rate compared with the control cells on days 3 and 6
(P<0.01). Significantly, cyclopamine demonstrated the same
effect in MDA-MB-231 cells (Fig.
1B) (P<0.01). We also tested the effect of cyclopamine on
cell proliferation using an alternative method. MCF-7 cells were
treated with cyclopamine (10 and 20 μM) or the vehicle only
and incubated for time periods ranging from 0–10 days (Fig. 1C). At each time point, the cells
were trypsinized and counted using a Neubauer hemocytometer under
trypan blue exclusion. Cyclopamine was observed to induce a
significant decrease in cell proliferation 5 and 10 days later
(P<0.01). Notably, cyclopamine demonstrated the same effect in
MDA-MB-231 cells (Fig. 1D)
(P<0.01). The results imply that cyclopamine plays a key role in
the growth control of breast cancer cells.
Cyclopamine induces G1 cell cycle arrest
and inhibits the invasive ability of both estrogen-responsive and
non-responsive human breast cancer cells
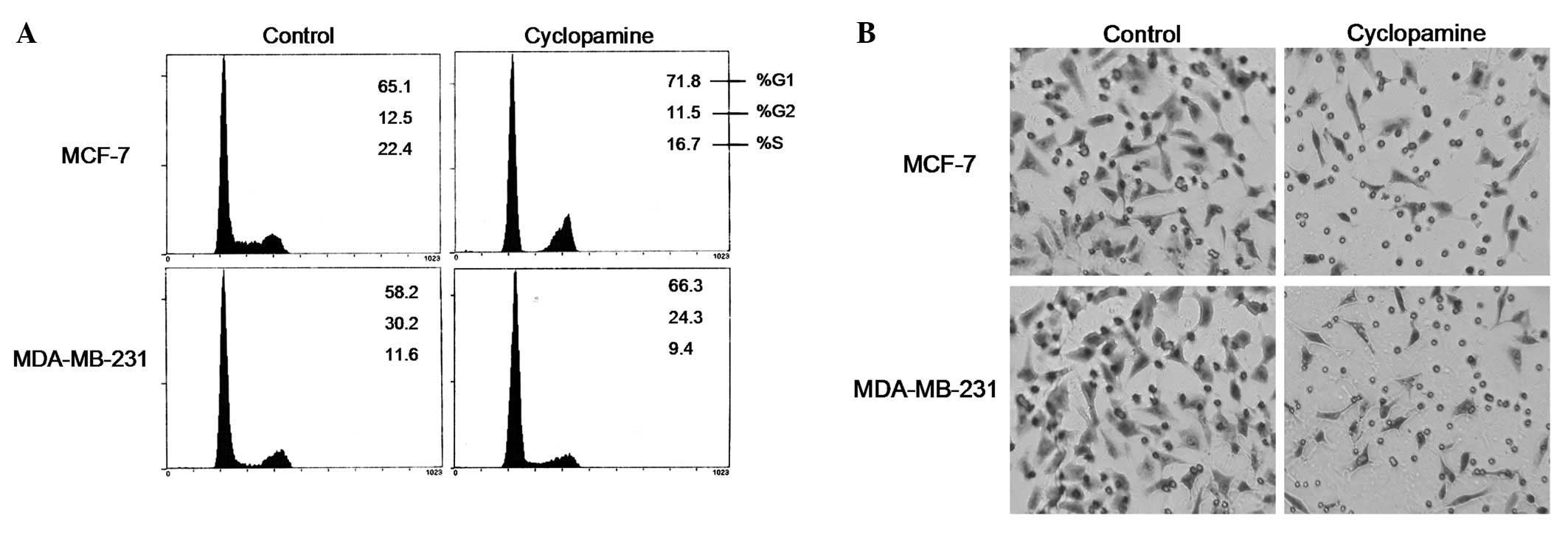
Cell cycle analysis was then performed on the
regulation of cyclopamine in breast cancer cells. The results in
Fig. 2A suggest that cyclopamine
significantly induced cell accumulation in the G1 phase (P<0.01)
and a modest decrease in the S population percentage, from 22 to
16% (P<0.01) in the MCF-7 cells. Moreover, cyclopamine also
caused a significant increase in G1 cells in the MDA-MB-231
cells.
A critical event in tumor metastasis and progression
is the ability of tumor cells to invade the extracellular matrix,
allowing the tumor cells to move beyond the restrictions of the
primary tumor environment. To examine the competency of cells to
invade through biological matrices in vitro, a Transwell
assay was conducted as described previously. The results
demonstrated that, compared with the control cells, cyclopamine
vigorously inhibited the ability of the MCF-7 and MDA-MB-231 cells
to invade through the filter coated with Matrigel. As shown in
Fig. 2B, the invasion rate of
cyclopamine-treated cells significantly decreased compared with the
control cells.
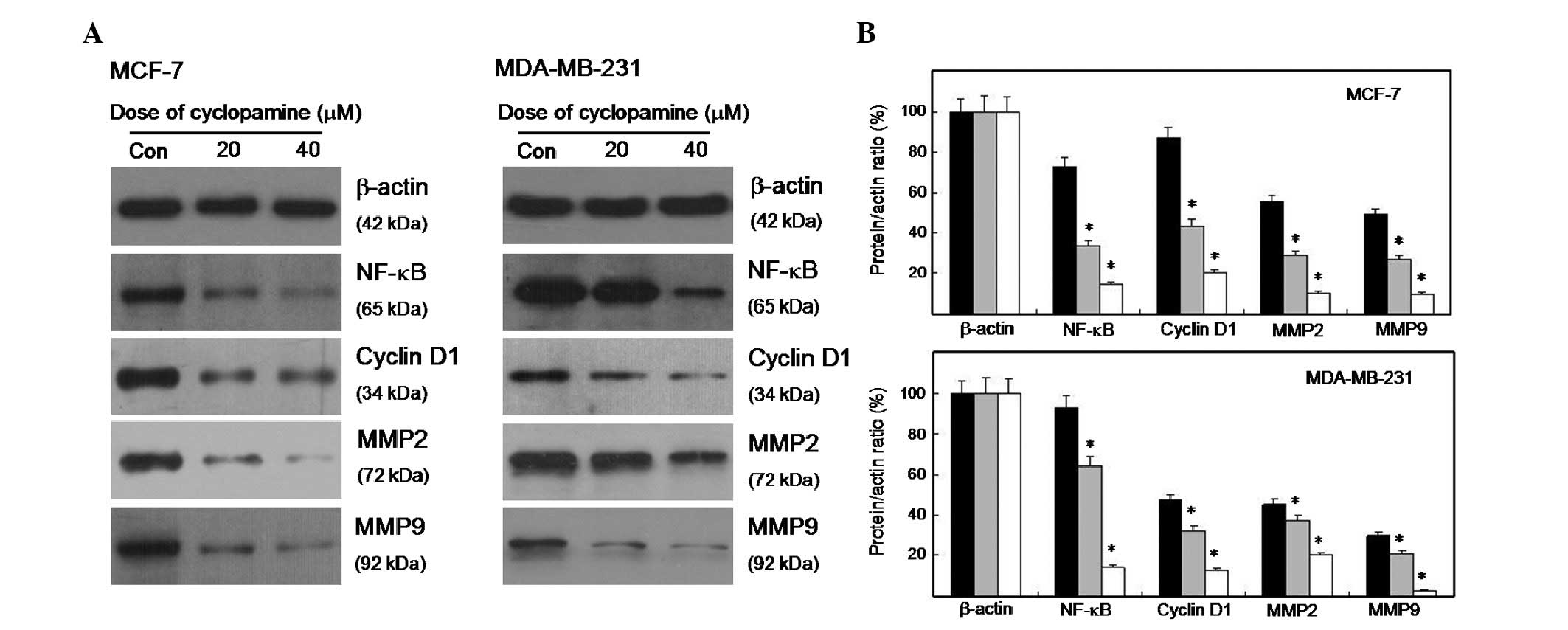
Cyclopamine affected the expression level
of cell cycle- and invasion-related proteins
The alterations in the cell cycle- and
invasion-related proteins in cyclopamine-treated cells were
evaluated and compared with the control cells using western blot
analysis. Fig. 3A shows that the
expression level of cyclin D1 decreased in cyclopamine-treated
cells. The expression levels of NF-κB, MMP2 and MMP9 were also
suppressed. The densitometry of the protein bands was quantified
with Quantity One, and the values were expressed relative to
β-actin. As shown in Fig. 3B, the
expression level of the four proteins significantly decreased
(P<0.01).
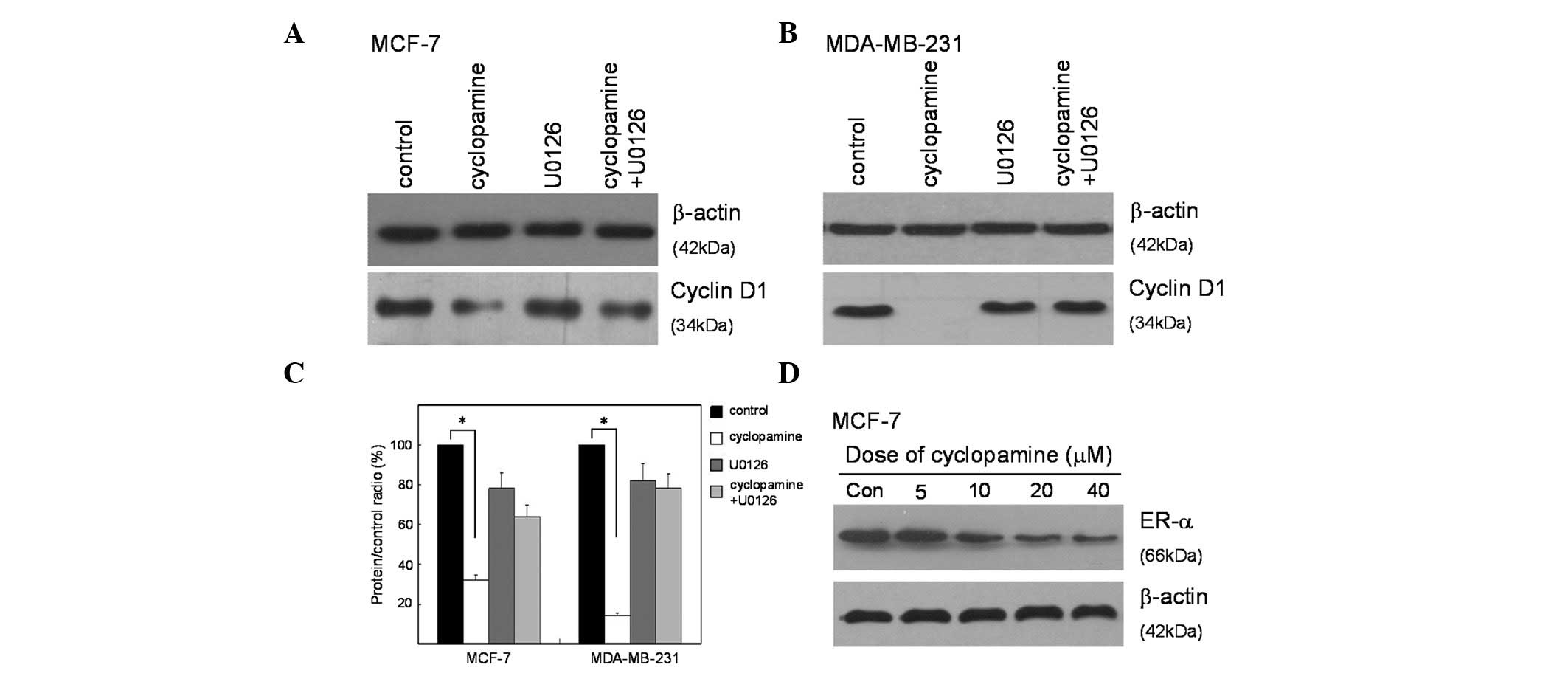
Cyclopamine modulates the expression of
cyclin D1, via the MAPK/ERK signaling pathway, and downregulates
the expression of ER-α
Western blot analysis was utilized to identify the
targets of the Hedgehog signal pathway that may be involved in
suppressing proliferation. The expression of cyclin D1 is a key
initial response to cell cycle distribution and proliferation. The
specific signaling cascade involved in this response was explored
using a MEK1/2 inhibitor (U0126) specific to the MAPK/ERK pathway.
The results showed that cyclopamine significantly inhibited the
expression of cyclin D1 in both MCF-7 and MDA-MB-231 cells. To
certify the potential effect of MAPK/ERK in response to cyclopamine
treatment, the two cell lines were treated with U0126 prior to
cyclopamine treatment. The results demonstrated that U0126 could
partially prevent cells from cyclopamine-induced cyclin D1
inhibition (Fig.4A and B). The
reduced cyclin D1 expression in cyclopamine-treated cells was
inhibited by the MEK1/2 inhibitor (Fig.
4C), which suggested that cyclopamine mediated the expression
of cyclin D1 through modulating the MAPK/ERK pathway.
The underlying mechanism for the ability of the
majority of anticancer drugs to cause a growth arrest of breast
cancer, is that the drugs downregulate the expression of ER-α. To
determine whether cyclopamine has a similar effect on ER-α
production, MCF-7 human breast cancer cells were treated with a
range of concentrations of cyclopamine and the level of total ER-α
protein was monitored by western blot analysis of total cell
extracts. As shown in Fig. 4D,
cyclopamine strongly downregulated ER-α levels, and β-actin was
used as a constitutive gel loading control.
Disscusion
Breast cancer is the second leading cause of
cancer-related mortality in females worldwide. Alternative
strategies are required to combat the deaths caused by this
disease. Chemotherapy, radiation, surgery and immunotherapy are
among the current treatment options for breast cancer. Chemotherapy
using synthetic compounds, although demonstrated to be effective in
cancer treatment, also induces severe side effects due to their
toxicity in non-cancerous tissues (10). In recent years, therapeutic
strategies that specifically target aberrant signaling pathways in
metastatic breast cancer greatly enhance survival, while at the
same time reducing bystander toxicity in non-tumor tissues
(11).
The MAPK pathway plays an important role in
regulating a number of downstream molecules including kinases and
scaffold proteins (12). The
balance between these molecules exerts cellular responses,
including cell proliferation, cell cycle arrest, migration and
differentiation. Extracellular signal-regulated kinases are members
of the MAPK family that transduce signals from various
environmental stresses, growth factors and steroid hormones
(13).
The present study has identified that cyclopamine
has potent antiproliferative properties as a potential therapeutic
agent for the treatment of human breast cancers by suppressing
MAPK/ERK-mediated signaling. Our results show that cyclopamine
significantly increased the potency of the cell cycle arrest in
both human estrogen-responsive and estrogen-independent human
breast cancer cells, which suggests that it may potentially be used
for treating a wide range of breast cancer types, which has been a
fundamental problem with the available therapeutic compounds.
Furthermore, cyclopamine inhibits the expression of
ER-α, which mediates the mitogenic properties of estrogens, and
acts with tamoxifen to inhibit the proliferation of
steroid-responsive MCF-7 human breast cancer cells to a greater
extent than either compound alone. This result implicates the use
of cyclopamine in combination with anti-estrogen therapies that
would allow lower doses and thereby reduced side effects or
decreased resistance to the anti-estrogens.
Acknowledgements
This study was supported by grants
from the Program for Changjiang Scholars and Innovative Research
Team in University (IRT0849) and the Priority Academic Program
Development of Jiangsu Higher Education Institutions (PAPD).
References
|
1
|
Ginsburg OM and Love RR: Breast cancer: a
neglected disease for the majority of affected women worldwide.
Breast J. 3:289–295. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McDermott SP and Wicha MS: Targeting
breast cancer stem cells. Mol Oncol. 5:404–419. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laxmi Y, Elegbedea JA and Carpera SW:
Methyl jasmonate decreases membrane fluidity and induces apoptosis
via tumor necrosis factor receptor 1 in breast cancer cells.
Anticancer Drugs. 8:766–776. 2008.PubMed/NCBI
|
|
4
|
Shibani M, Natalya F, Andrea S, et al:
Hedgehog signaling and response to cyclopamine differ in epithelial
and stromal cells in benign breast and breast cancer. Cancer Biol
Ther. 6:674–683. 2006.PubMed/NCBI
|
|
5
|
Kumara S, Indrajit R, Anchooria RK, et al:
Targeted inhibition of hedgehog signaling by cyclopamine prodrugs
for advanced prostate cancer. Bioorg Med Chem. 6:2764–2768. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bara EE, Chaudhrya A, Lina A, et al:
Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like
cancer cells in glioblastoma. Stem Cells. 10:2524–2533. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li XL, Meng QH, Fan SJ, et al:
Adenovirus-mediated expression of UHRF1 reduces the
radiosensitivity of cervical cancer HeLa cells to
gamma-irradiation. Acta Pharmacol Sin. 4:458–466. 2009.PubMed/NCBI
|
|
8
|
Jiao Y, Ge CM, Meng QH, et al:
Adenovirus-mediated expression of Tob1 sensitizes breast cancer
cells to ionizing radiation. Acta Pharmacol Sin. 10:1628–1636.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiao Y, Ge CM, Meng QH, et al:
Dihydroartemisinin is an inhibitor of ovarian cancer cell growth.
Acta Pharmacol Sin. 7:1045–1056. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Somesh B and Alahari SK: miRNA control of
tumor cell invasion and metastasis. Int J Cancer. 6:1283–1290.
2010.
|
|
11
|
Nguyena HH, Lavrenovc SN, Sundara SN, et
al: 1-Benzyl-indole-3-carbinol is a novel indole-3-carbinol
derivative with significantly enhanced potency of
anti-proliferative and anti-estrogenic properties in human breast
cancer cells. Chem Biol Interact. 3:255–266. 2010. View Article : Google Scholar
|
|
12
|
Chen L, Mayer JA, Krisko TI, et al:
Inhibition of the p38 kinase suppresses the proliferation of human
ER-negative breast cancer cells. Cancer Res. 23:8853–8861. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li MW, Mruk DD, Cheng CY, et al:
Mitogen-activated protein kinases in male reproductive function.
Trends Mol Med. 15:159–168. 2009. View Article : Google Scholar : PubMed/NCBI
|