Introduction
The typical characteristics of pancreatic
adenocarcinoma are its early local recurrence, systemic
dissemination and poor response to chemotherapy and/or
radiotherapy, thus rendering a poor prognosis with currently used
treatment methods. The lack of traditional therapeutic options has
motivated the search for novel immunotherapeutic approaches to
pancreatic cancer, including cellular immunotherapy (1,2). At
present, despite the development of chemotherapy drugs, research
concerned with advanced pancreatic adenocarcinoma has only made
modest progress, and the prognosis of advanced pancreatic
adenocarcinoma remains extremely poor (3–5).
Gemcitabine-based regimens are typically offered as standard
approaches of care, and the majority of patients succumb within 6
months (6). The majority of elderly
patients who undergo surgical resection are not able to tolerate
the side-effects of chemotherapy and/or radiotherapy following
disease relapse. Celluar immunotherapy has become the fourth
treatment method for malignant tumors (7–10),
which may be a promising approach for these patients.
Cytokine-induced killer (CIK) cells are considered to be antitumor
effector cells that are capable of rapid proliferation in
vitro, with stronger antitumor activity and a broader spectrum
of tumor targets than other described antitumor effector cells
(10). Moreover, CIK cells are able
to regulate and generally enhance immune function in cancer
patients (11). There are limited
data concerning adoptive immunotherapy in pancreatic
adenocarcinoma. In the present study, we describe a patient with
advanced pancreatic adenocarcinoma, who experienced a longer
progression-free survival time (PFS) of >19 months, following
administration of CIK cell immunotherapy. The study was approved by
the Ethics Committee of Zhengzhou University, Zhengzhou, China.
Written informed consent was obtained from the patient.
Case report
A 77-year-old female was admitted to Henan Cancer
Hospital, China, with intermittent abdominal pain for one month.
Following investigation, pancreatic cancer was suspected by the
surgeons. On October 14, 2010, the patient underwent resection of
the pancreatic body and tail and the spleen. Analysis of the
pancreatic mass revealed poorly differentiated adenocarcinoma,
including squamous cell carcinoma differentiation, and the
bilateral margins were positive. Abdominal contrast-enhanced
computed tomography (CT) reexamination after one month revealed
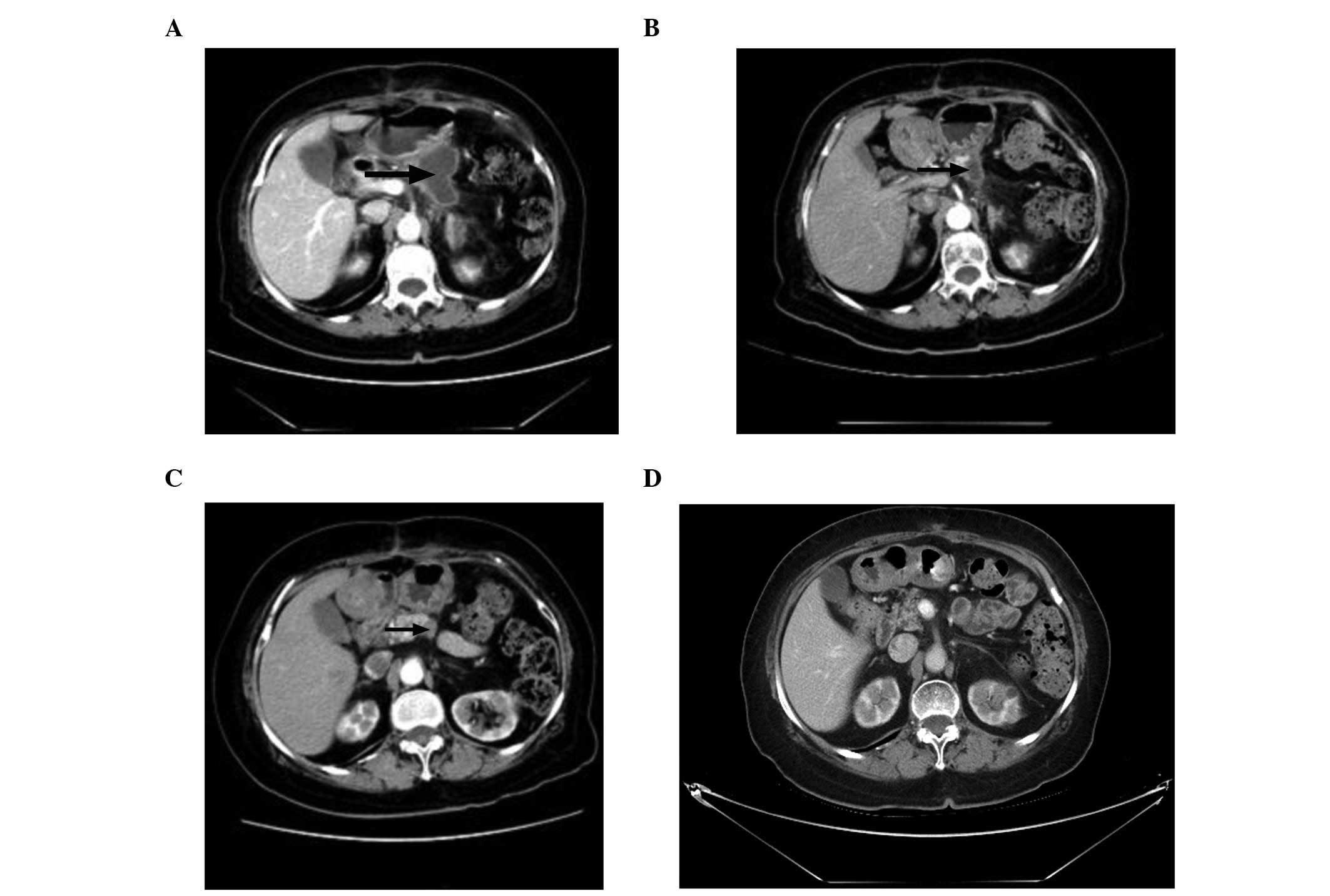
that a low-density nodule had emerged at the resection margin
(Fig. 1A). Cancer- and
tissue-specific markers tested, including carbohydrate antigen-199
(CA-199), carbohydrate antigen-724 (CA-724) and carcinoembryonic
antigen (CEA), were observed to be elevated. Notably, the CA-199
level had risen to 1,000 U/ml (reference range, 0–35 U/ml).
Considering that the patient demonstrated poor health and low
Karnofsky performance status (KPS) scores (50 scores), as well as
being unable to tolerate the side-effects of chemotherapy and/or
radiotherapy, CIK cell immunotherapy was administered. Mononuclear
cells were collected from the patient’s 50 ml peripheral blood and
cultured in GT-T551 medium containing anti-CD3 antibody,
recombinant human interleukin-1α (IL-1α) and interferon-γ (IFN-γ),
at 37°C with 5% CO2 for 24 h. Subsequently, recombinant
human IL-2 (rhIL-2) was added to the medium. The medium was
replaced by fresh IL-2- and IFN-γ-containing medium every 5 days.
At day 10, CIK cells were harvested and their phenotypes were
analyzed. All products were free of bacterial, mycoplasma and
fungal contamination. The endotoxin level was <5 EU. Phenotypic
analysis of autologous CIK cells in the patient prior to culture
and following 10 days of cultivation demonstrated that the
percentages of CD3+, CD3+CD4+,
CD3+CD8+, CD3+CD56+ and
CD25+ cell subsets increased from 45.40±5.21,
29.08±4.86, 18.80±5.45, 3.77±1.665 and 12.51±4.01% to 90.06±9.22,
44.50±8.18, 38.40±4.19, 15.21±4.53 and 31.75±5.87%, respectively
(P<0.01). However, the percentages of
CD3+/16+/56+, CD14+ and
CD20+ cell subsets decreased from 14.73±3.54, 16.46±6.06
and 11.19±3.18% to 6.78±1.91, 5.87±2.09 and 7.84±2.53%,
respectively (P<0.05). The total number of CIK cells in one
cycle is ∼ 5×109. From November 10, 2010 to June 27,
2011, the patient recieved 4 cycles of CIK cell immunotherapy and 2
million units of IL-2 from day 1–5 per infusion of CIK cells. In
the period of CIK cell therapy, no adverse reactions were observed.
Following 4 cycles of CIK cell immunotherapy, the abdominal
contrast-enhanced CT reexamination demonstrated that the
low-density nodule reduced significantly (Fig. 1B). The cancer- and tissue-specific
markers (CA-199, CA-724 and CEA) returned to their normal levels.
The patient then received another 12 cycles of CIK cell
immunotherapy. Abdominal contrast-enhanced CT examination indicated
that the low-density nodule had slightly decreased (Fig. 1C) compared with the result
demonstrated in Fig. 1B. The
cancer- and tissue-specific marker levels (CA-199, CA-724 and CEA)
remained at their normal levels. Following 16 cycles of CIK cell
immunotherapy, it was recommended that the patient undergo
abdominal contrast-enhanced CT examination every 3 months. The
abdominal CT scan on February 29, 2012 demonstrated that the
low-density nodule had almost disappeared (Fig. 1D). From March 7, 2012 to April 29,
2012, the patient received a further 4 cycles of CIK cell infusion
to ensure treatment efficacy. However, on May 28, 2012, the
abdominal contrast-enhanced CT scan revealed that the low-density
nodule had emerged in the same place as previously, and the CA-199
level had elevated to 1,000 U/ml once more. At present, the
progression-free survival time (PFS) of the patient is >19
months and the patient has not succumbed. During immunotherapy, the
patient had a good quality of life and their KPS score increased
from 50 to 80.
Discussion
In the field of oncology, pancreatic cancer remains
a fatal disease with a poor prognosis, thus presenting a challenge
for oncologists (12,13). In patients with advanced pancreatic
cancer, the mean overall survival time is <6 months (6). Chemotherapy and radiotherapy are the
most common approaches for treating locally relapsed and metastatic
pancreatic cancer (14); however,
the overall survival time is only modestly prolonged. Additionally,
adverse reactions to chemotherapy/radiotherapy may be not tolerable
in elderly patients. However, the lack of conventional therapeutic
options is also important in motivating the search for novel
therapeutic approaches to pancreatic cancer. In the present case,
the patient was diagnosed with advanced pancreatic cancer, and had
a poor prognosis following surgery. Additionally, the patient was
not able to tolerate the side-effects of traditional chemotherapy
and radiotherapy. Subsequently, CIK cell immunotherapy alone was
administered to the patient, achieving a beneficial effect.
Presently, immunotherapy has become the fourth
treatment modality for malignant tumors, and may be a promising
approach for pancreatic cancer (7–10).
Certain studies have demonstrated that CIK cells are heterogeneous
cell populations that express CD3 and CD56 as well as the
activation receptor of NK cells (NKG2D), antigen, and possess
MHC-unrestricted cytotoxicity toward pancreatic cancer but not
toward normal targets (15,16). CIK cells are considered to be a type
of antitumor effector cells, and proliferate rapidly in
vitro, with stronger antitumor activity in a broad spectrum of
tumors (9,17). In addition, CIK cells regulate and
enhance cellular immune functions in patients with pancreatic
cancer by secretion of cytokines, such as interferon-γ, and a
number of chemokines, including RANTES, MIP-1α and MIP-1β (18,19).
The application of CIK cells as adoptive immunotherapy is important
in cancer treatment. The application of CIK cells as adoptive
immunotherapy in solid tumor treatment has been frequently reported
in the literature (10,20). In particular, Liu et al
applied CIK cells to treat 74 cases of metastatic renal cell
carcinoma patients in a randomized stage III clinical trial. The
mean PFS was prolonged by 4 months and the mean overall survival
time (mOS) was prolonged by 27 months compared with the control arm
(10). Shi et al applied CIK
cells to treat locally advanced gastric cancer patients and
indicated that adjuvant immunotherapy with CIK cells prolonged
disease-free survival (DFS) and significantly improved the mOS
(21). However, there are few data
concerned with applying CIK cells to treat pancreatic cancer in
clinical practice. The present case provides a novel treatment
option and further clinical practices are required to verify its
efficacy.
In conclusion, we describe the case of a 77-year old
female with pancreatic cancer who underwent surgery, although the
bilateral margins were positive. After one month, imaging
examination revealed that a low-density nodule had emerged at the
resection margin. Considering the patient’s poor condition, low KPS
scores and inability to tolerate the side-effects of chemotherapy
and/or radiotherapy, the patient was treated with CIK cell
immunotherapy. The patient achieved a PFS of >19 months, which
was longer than that demonstrated by using chemotherapy and/or
radiotherapy. In conclusion, CIK immunotherapy may be an effective
treatment method for advanced pancreatic cancer, particularly for
elderly patients.
References
|
1
|
Laheru D and Jaffee EM: Immunotherapy for
pancreatic cancer - science driving clinical progress. Nat Rev
Cancer. 5:459–467. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vizio B, Novarino A, Giacobino A, et al:
Potential plasticity of T regulatory cells in pancreatic carcinoma
in relation to disease progression and outcome. Exp Ther Med.
4:70–78. 2012.PubMed/NCBI
|
|
3
|
Franssen B and Chan C: Pancreatic Cancer:
The surgeons point of view. Rev Gastroenterol Mex. 76:353–361.
2011.PubMed/NCBI
|
|
4
|
Janes RJ, Niederhuber JE, Chmiel JS, et
al: National patterns of care for pancreatic cancer. Results of a
survey by the Commission on Cancer. Ann Surg. 223:261–272. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moss RA and Lee C: Current and emerging
therapies for the treatment of pancreatic cancer. Onco Targets
Ther. 3:111–127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koido S, Homma S, Takahara A, et al:
Current immunotherapeutic approaches in pancreatic cancer. Clin Dev
Immunol. 2011:2675392011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dougan M and Dranoff G: Immune therapy for
cancer. Annu Rev Immunol. 27:83–117. 2009. View Article : Google Scholar
|
|
8
|
Hontscha C, Borck Y, Zhou H, et al:
Clinical trials on CIK cells: first report of the international
registry on CIK cells (IRCC). J Cancer Res Clin Oncol. 137:305–310.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schwaab T, Schwarzer A, Wolf B, et al:
Clinical and immunologic effects of intranodal autologous tumor
lysate-dendritic cell vaccine with Aldesleukin (Interleukin 2) and
IFN-{alpha}2a therapy in metastatic renal cell carcinoma patients.
Clin Cancer Res. 15:4986–4992. 2009.PubMed/NCBI
|
|
10
|
Liu L, Zhang W, Qi X, et al: Randomized
study of autologous cytokine-induced killer cell immunotherapy in
metastatic renal carcinoma. Clin Cancer Res. 18:1751–1759. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmidt-Wolf IG, Lefterova P, Mehta BA, et
al: Phenotypic characterization and identification of effector
cells involved in tumor cell recognition of cytokine-induced killer
cells. Exp Hematol. 21:1673–1679. 1993.PubMed/NCBI
|
|
12
|
Werner J and Buchler MW: management of
pancreatic cancer: recent advances. Dtsch Med Wochenschr.
136:1807–1810. 2011.PubMed/NCBI
|
|
13
|
Ng J, Zhang C, Gidea-Addeo D, et al:
Locally advanced pancreatic adenocarcinoma: update and progress.
JOP. 13:155–158. 2012.PubMed/NCBI
|
|
14
|
Cardenes HR, Chiorean EG, Dewitt J, et al:
Locally advanced pancreatic cancer: current therapeutic approach.
Oncologist. 11:612–623. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karimi M, Cao TM, Baker JA, et al:
Silencing human NKG2D, DAP10, and DAP12 reduces cytotoxicity of
activated CD8+ T cells and NK cells. J Immunol. 175:7819–7828.
2005.PubMed/NCBI
|
|
16
|
Verneris MR, Karami M, Baker J, et al:
Role of NKG2D signaling in the cytotoxicity of activated and
expanded CD8+ T cells. Blood. 103:3065–3072. 2004.PubMed/NCBI
|
|
17
|
Li R, Wang C, Liu L, et al: Autologous
cytokine-induced killer cell immunotherapy in lung cancer: a phase
II clinical study. Cancer Immunol Immunother. 61:2125–2133. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schmidt-Wolf IG, Lefterova P, Mehta BA, et
al: Phenotypic characterization and identification of effector
cells involved in tumor cell recognition of cytokine-induced killer
cells. Exp Hematol. 21:1673–1679. 1993.PubMed/NCBI
|
|
19
|
Joshi PS, Liu JQ, Wang Y, et al:
Cytokine-induced killer T cells kill immature dendritic cells by
tcr-independent and perforin-dependent mechanisms. J Leukoc Biol.
80:1345–1353. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mesiano G, Todorovic M, Gammaitoni L, et
al: Cytokine-induced killer (cik) cells as feasible and effective
adoptive immunotherapy for the treatment of solid tumors. Expert
Opin Biol Ther. 12:673–684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi L, Zhou Q, Wu J, et al: Efficacy of
adjuvant immunotherapy with cytokine-induced killer cells in
patients with locally advanced gastric cancer. Cancer Immunol
Immunother. 61:2251–2259. 2012. View Article : Google Scholar : PubMed/NCBI
|