Introduction
Bladder cancer is a major clinical problem worldwide
and the incidence has increased over the last two decades, with
∼80% of the diagnosed tumors classified as non-muscle invasive
bladder cancer (NMIBC) (1).
Treatment for NMIBC includes transurethral resection (TUR) with or
without intravesical instillation therapy. The greatest problem in
management is the potential for local recurrence, with the
recurrence rate ranging between 50 and 70%. In addition, 10 to 20%
of NMIBC may further progress to muscle-invasive disease, thus
eventually leading to radical cystectomy and urinary diversion
(2,3). In the treatment of advanced-stage
bladder cancer, combination chemotherapy has exhibited promising
results. However, chemotherapy destroys the immune system of the
patient, resulting in serious problems (4). In this context, there is a clear
requirement to identify novel therapeutic agents which possess
immunoregulatory and anticancer effects in patients with bladder
cancer.
Engineered bacteria are able to aid in controlling
cancer (5). Bacille Calmette-Guérin
(BCG), a live-attenuated strain of Mycobacterium bovis, is
the most common intravesical therapy for NMIBC (6). A vaccine using Pseudomonas
aeruginosa-mannose-sensitive hemagglutinin (PA-MSHA) has been
shown to increase the antigen presenting function by activating the
proliferation and differentiation of dendritic cells by the body
(7). The PA-MSHA strain is a
peritrichous P. aeruginosa strain with MSHA fimbriae
established by Professor Xi-ya Mu. Furthermore, PA-MSHA possesses
cytotoxic qualities due to the addition of MSHA, which has been
shown to have anticarcinogenic activity against human
hepatocarcinoma and gastric, nasopharyngeal and breast cancer cells
(8–11). In addition, an intravesical
instillation of 10 ml PA-MSHA in 62 bladder cancer patients was
demonstrated to be effective and well tolerated (12). These findings suggest that the use
of PA-MSHA may be beneficial in bladder cancer treatment and that
it therefore represents a possible tool in adjuvant therapy
modalities.
However, the in vitro effects of PA-MSHA on
bladder cancer cells remain unclear. The present study was designed
to investigate the biological therapeutic potential of PA-MSHA
against bladder cancer and to further define the functional
mechanisms of PA-MSHA.
Materials and methods
Reagents
The PA-MSHA used in the present study were kindly
provided by Wanter Biopharma Company (Beijing, China), then
scale-diluted and stored at 4°C. Phosphate-buffered saline (PBS;
0.1 M; Gibco, Grand Island, NY, USA) was used as a blank control.
The following primary antibodies were all from Cell Signaling
Technology (Danvers, MA, USA): anti-caspase-8, anti-cleaved
caspase-8, anti-caspase-9, anti-cleaved caspase-9, anti-Fas,
anti-ERK (tERK), anti-phospho-ERK (pERK), anti-AKT (tAKT),
anti-phospho-AKT (pAKT), anti-MTOR (tMTOR), anti-phospho-MTOR
(pMTOR) and anti-β-actin.
Cells lines and culture conditions
The human bladder cancer cell lines, T24 and 5637,
were obtained from the Shanghai Institute of Cell Biology, Chinese
Academy of Science (Shanghai, China). The cells were cultured in
RPMI-1640 medium supplemented with 10% heat-inactivated fetal
bovine serum, penicillin (100 U/ml) and streptomycin (100 mg/l) at
37°C in a humidified atmosphere containing 5% CO2.
Cell growth/viability assay
Cell proliferation was analyzed using Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Gaithersburg,
MD, USA) assays. The cells were plated in 96-well plates
(1×104 cells/well) in a concentration- or time-dependent
manner. The following day, the medium was changed and fresh medium
and the indicated concentrations of PA-MSHA (10, 5, 2.5, 1 and
0.5×109 bacterial cells per ml) were added; the cells
were then incubated at 37°C for a further 0, 1, 2 or 3 days. The
inhibition of cell growth was determined 24 h later by a reduction
assay as follows: 10 μl of CCK-8 was added per well, then
the cells were incubated for an additional 4 h and the absorbance
at 450 nm was recorded using a 96-well plate reader (Sunrise
Microplate Reader, Tecan US, Inc., Charlotte, NC, USA). The
following formula was used: Cell viability (%) = [(As − Ab) / (Ac −
Ab)] × 100 (n=6, mean ± SD). As was the mean absorbance of the
wells with the various concentrations of the drugs added. Ac was
the mean absorbance of the diluent wells and Ab was the mean
absorbance of the blank wells (no cells, only RPMI-1640). Six
replicate wells were used for each analysis and at least three
independent experiments were performed.
Flow cytometry with annexin-V-fluorescein
isothiocyanate (FITC) and propidium iodide (PI) staining
The cells were pretreated with the indicated
concentrations of PA-MSHA (1, 2.5 or 5×109/ml) for 24 h
and single-cell suspensions containing at least 1×106
cells were created. The cell cycle and apoptotic analyses were
performed using flow cytometry as described previously, using a
FACScalibur system (Becton Dickinson Biosciences, San Diego, CA,
USA) (13). The apoptotic cells
were analyzed using quadrant statistics on the PI-negative and
annexin V-positive cells. Data for the cell cycle analysis were
analyzed using ModFit LTTM software (Verity Software House, Inc.,
Topsham, ME, USA) to determine the proportion of cells in the
G0/G1, S and G2/M fractions of the
cell cycle. The mean ± SD was calculated for the cell populations
from triplicate data.
Western blot analysis
Cells were lysed according to the standardized
protocol (26). Equal amounts of
protein lysate at various concentrations were electrophoresed in
SDS-PAGE, followed by electroblotting onto polyvinylidene fluoride
(PVDF; Immobion™; Millipore, Bedford, MA, USA), for 1 h at 100 V.
The membranes were blocked for 1 h at room temperature or overnight
at 4°C in 5% skimmed milk in TBS with 0.1% Tween 20. The blot was
incubated with the primary antibody (1:1,000), then incubated with
a horseradish-peroxidase conjugated secondary antibody (1:3,000;
DAKO, Carpinteria, CA, USA). The chemiluminescent detection of
antibody binding was performed using an Enhanced Chemiluminescence
(ECL) Detection kit (Amersham Pharmacia Biotech, Uppsala, Sweden)
and images were captured using the the FUJIFILM LAS-1000 system
(Fujifilm, Tokyo, Japan). To ensure that equal amounts of proteins
were loaded, the blot was reprobed with rabbit anti-β-actin
monoclonal antibodies (1:1,000). A densitometry analysis was
performed using the Quantity One software (Bio Rad, Hercules, CA,
USA). The relative protein expression levels were normalized by
dividing the level of target proteins by the level of β-actin for
each sample.
Statistical analysis
Statistical analysis was performed using the
Statistical Package for the Social Sciences (SPSS) software version
15 for Windows (SPSS Inc, Chicago, IL, USA). The differences
between pairs of groups were analyzed by two-tailed Student’s
t-tests. Differences between multiple groups were evaluated by
one-way analysis of variance (ANOVA). P<0.05 was considered to
indicate a statistically significant difference.
Results
PA-MSHA inhibits the proliferation of
bladder cancer cell lines
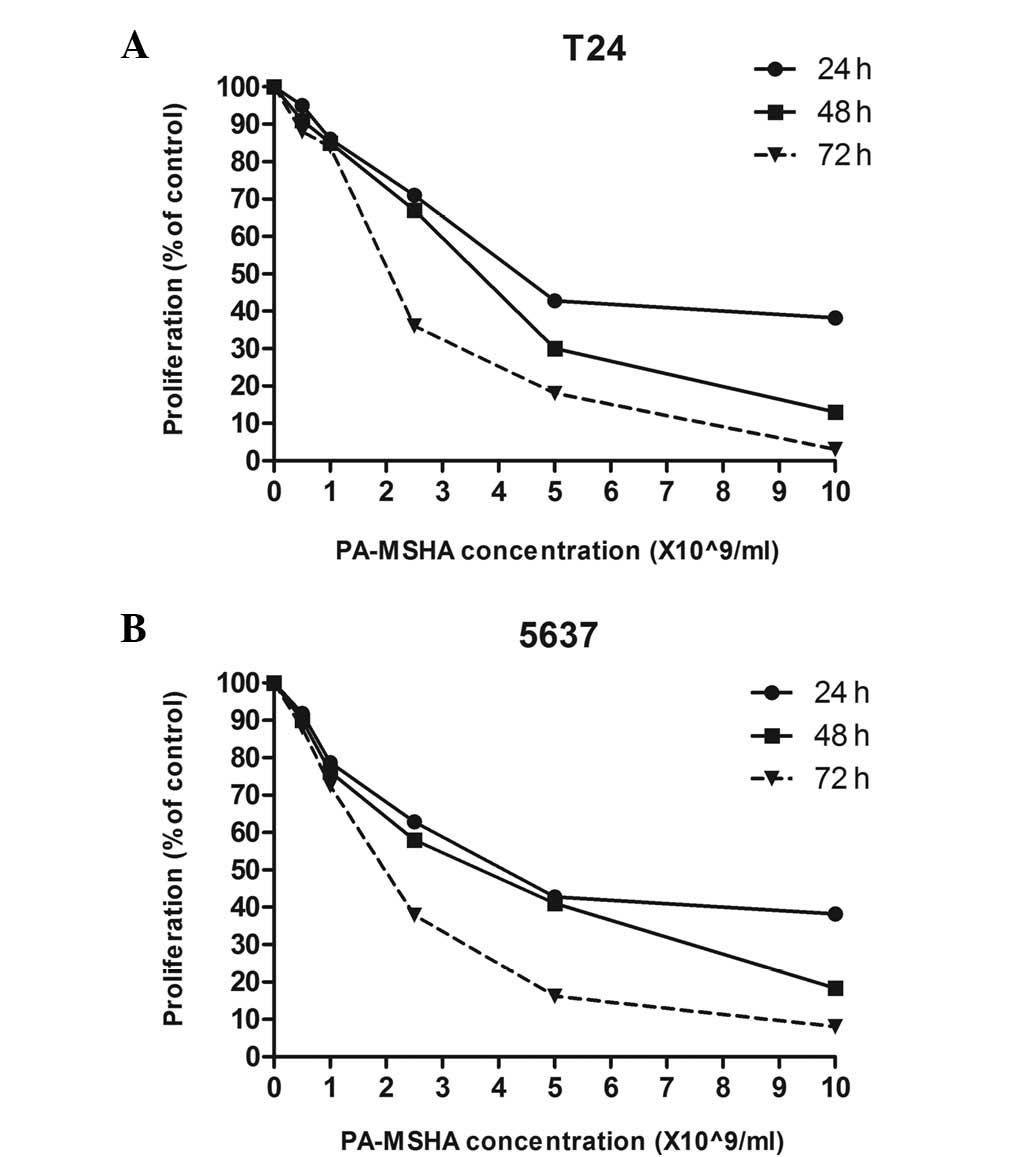
To determine whether PA-MSHA is a potential bladder
cancer treatment agent, its effects on the growth of T24 and 5637
cells were studied. Changes in cell number caused by PA-MSHA were
assessed every 24 h using a non-radioactive CCK-8 cell
proliferation assay. The 50% percent inhibitory concentrations
(IC50) are shown in Table
I. The exposure of tumor cells to PA-MSHA for up to 72 h had a
cumulative effect on T24 and 5637 cell proliferation (Fig. 1A and B, respectively) in a
concentration- and time-dependent manner within the same time and
concentration range for each cell line.
 | Table IIC50 values of PA-MSHA in
various bladder cancer cell lines. |
Table I
IC50 values of PA-MSHA in
various bladder cancer cell lines.
| IC50
(×109/ml)
|
|---|
| Cell lines | 24 h | 48 h | 72 h |
|---|
| T24 | 4.2±0.21 | 3.6±0.23 | 2.1±0.19 |
| 5637 | 4.3±0.27 | 3.8±0.25 | 2.1±0.22 |
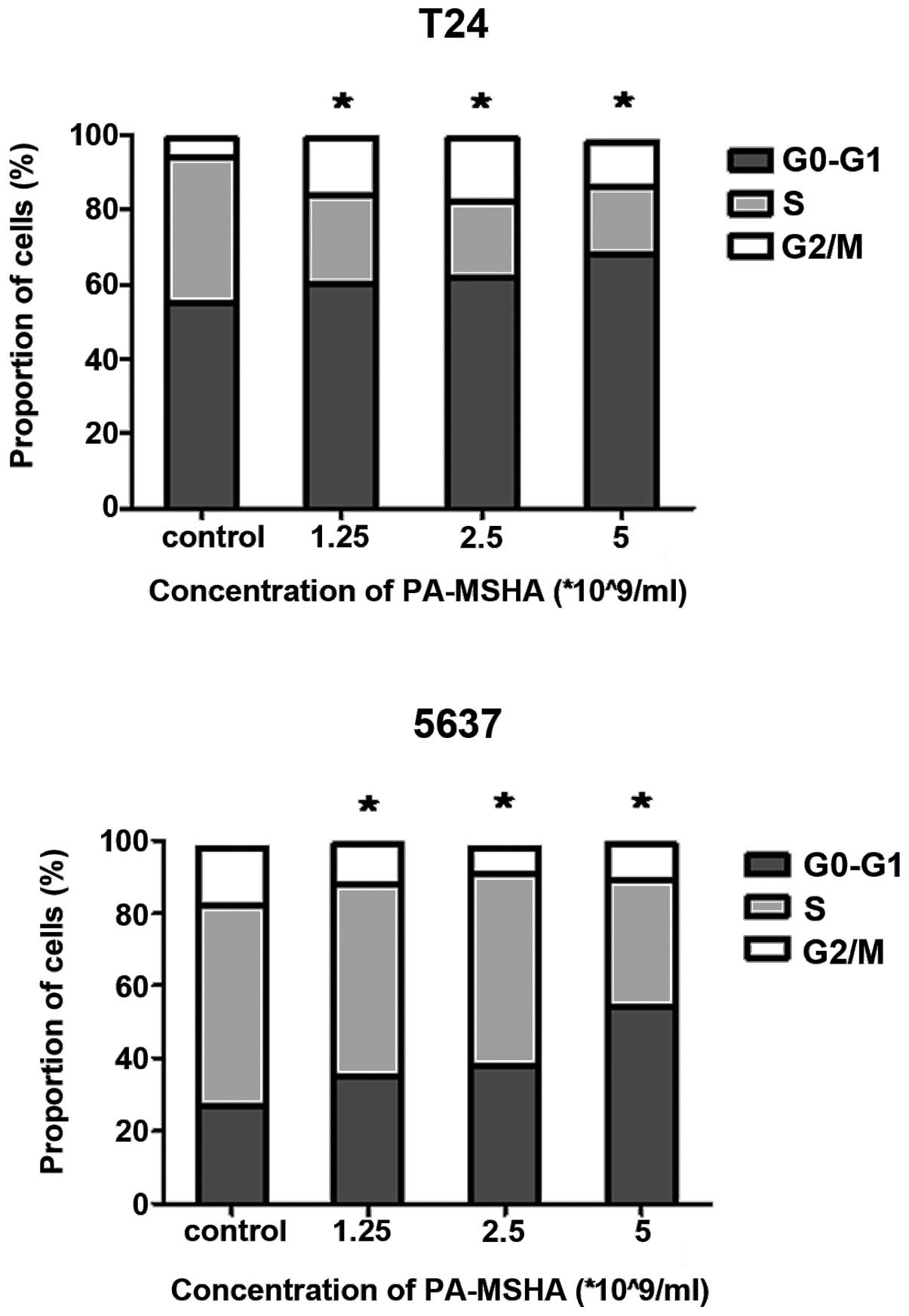
PA-MSHA arrests bladder cancer cell lines
in the G0/G1 phase of the cell cycle
Since PA-MSHA slowed the proliferation of cells, the
mechanism by which it exerted these growth-regulatory effects was
investigated. The cells were treated with PA-MSHA for 24 h, stained
with PI and analyzed using flow cytometry. Treating the T24 and
5637 with increasing concentrations of PA-MSHA (1, 2.5 or
5×109/ml) dose-dependently arrested the cells in the
G0/G1 phase of the cell cycle, thereby
decreasing the proportion of cells in the S phase (Fig. 2).
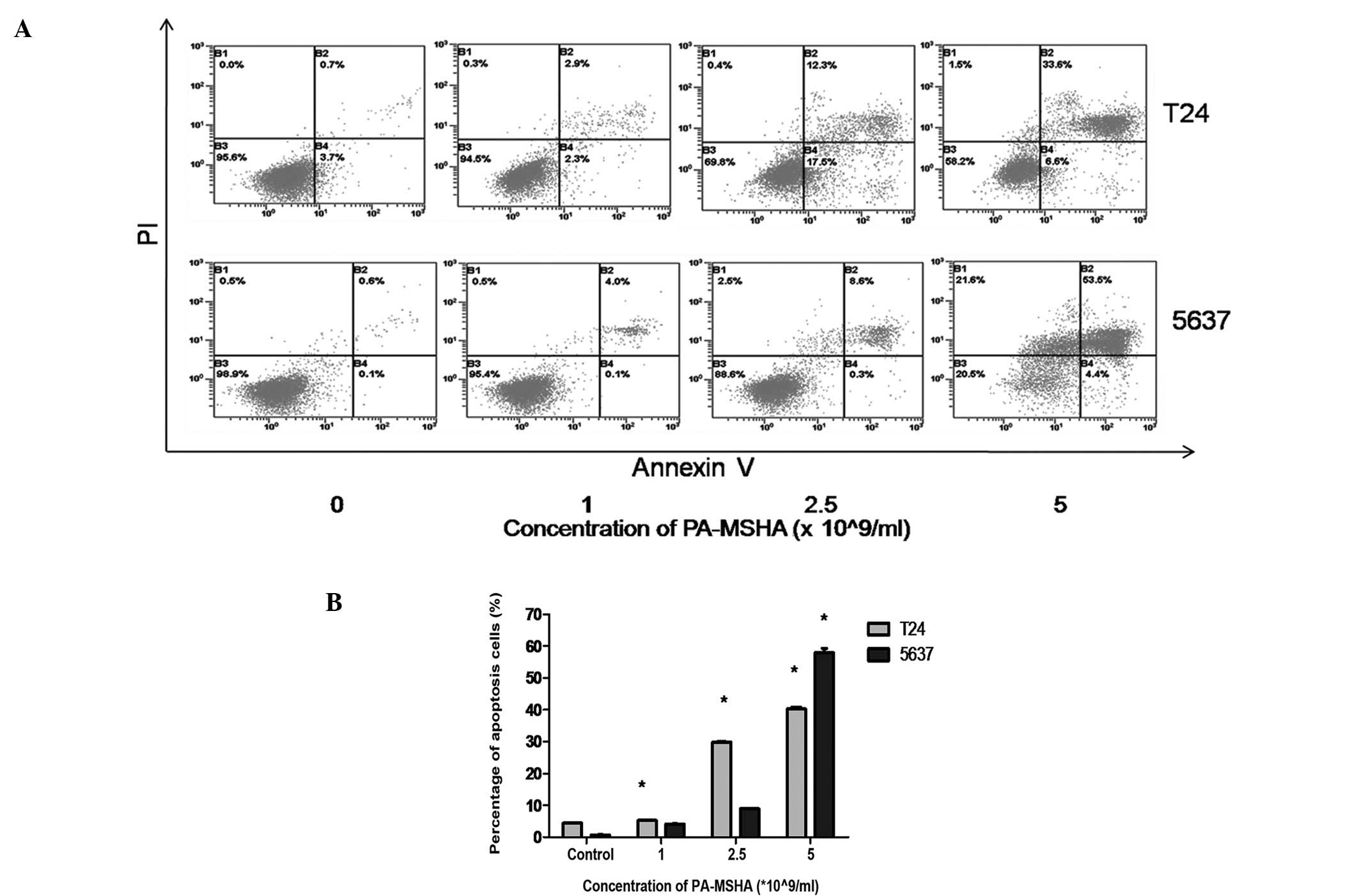
PA-MSHA induces apoptosis in bladder
cancer cell lines
In the presence of a low dose of PA-MSHA
(1×109/ml), slightly elevated numbers of apoptotic cells
were detected among the T24 and 5637 cells compared with the
controls (Fig. 3A). The apoptotic
cell number increased markedly following treatment with high
concentrations (2.5 or 5×109/ml) of PA-MSHA (Fig. 3B). It was demonstrated that the T24
and 5637 cells treated with PA-MSHA for 24 h underwent apoptosis in
a dose-dependent manner.
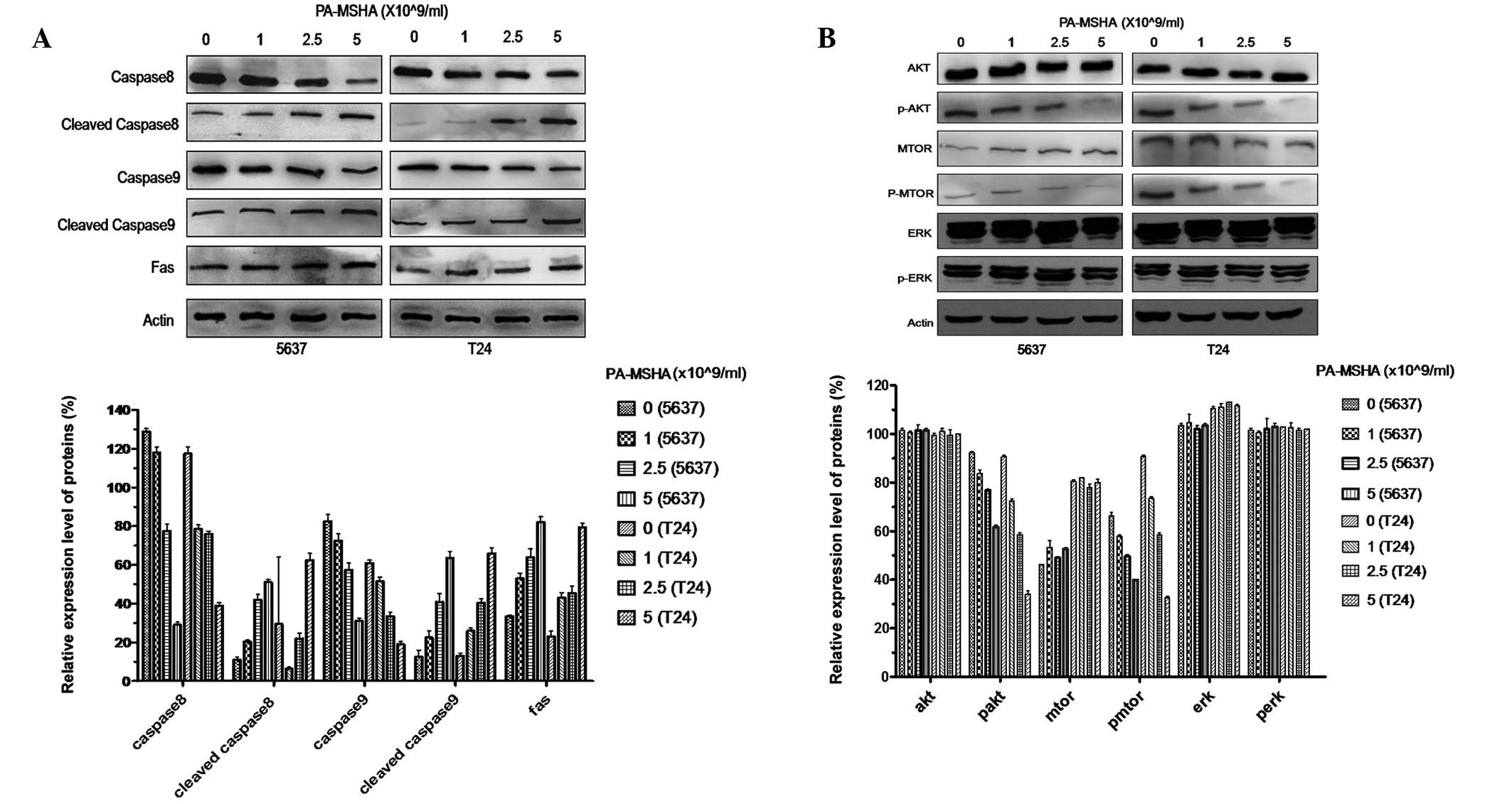
PA-MSHA induces apoptosis using caspase
cascade proteins
In order to further investigate the mechanisms
behind PA-MSHA-induced apoptosis, the T24 and 5637 cells were
treated with PA-MSHA to study the activation of caspase-associated
proteins. The lysates were analyzed using antibodies against
caspase-8 and -9, the cleaved forms of the caspases and Fas
protein. As expected, when the T24 and 5637 cells were exposed to
PA-MSHA for >24 h, there were dose-dependent losses of
procaspase-8 and -9, as well as a concentration-dependent increase
in the Fas and cleaved caspase proteins (Fig. 4A), indicating the proteolytic
processing of the proenzymes to their active enzyme subunits.
PA-MSHA inhibits the PI3K-AKT-mTOR
signaling pathway
The PI3K-AKT-mTOR signaling pathways are important
survival pathways for the regulation of cell survival and
proliferation. The present study tested whether treatment with
PA-MSHA was able to reduce basal Akt and mTOR phosphorylation. The
T24 and 5637 cells were incubated for 48 h in the presence of 1,
2.5 or 5×109/ml PA-MSHA. The treatment with PA-MSHA
caused a dose-dependent decrease in pAKT and pmTOR levels but not
in pERK, whereas the total levels of AKT, mTOR and ERK remained the
same (Fig. 4B). Densitometry
indicated that the treatment with 1, 2.5 or 5×109/ml
PA-MSHA caused decreases of 17, 32 and 64%, respectively, in
phosphorylated Akt and 16, 21 and 68% decreases in phosphorylated
mTOR in the T24 cells.
Discussion
The immunoregulatory effect of PA-MSHA has been well
validated. PA-MSHA has been shown to be effective in improving the
immune response of patients with several types of cancer and
certain other conditions including trauma and infection and chronic
diseases such as hepatic fibrosis (14–18).
PA-MSHA has also been shown to be effective as a vaccine in plasma
phospholipid metabolic profiling and in correcting the ratio of
Th2/Th1 cells within the immune organs of mice with IgA nephropathy
(19). However, studies on the
direct anti-cancer cytotoxic effect of PA-MSHA are limited. Only
four studies have demonstrated its anticancer cytotoxicity effect
when using hepatocarcinoma, gastric cancer, nasopharyngeal cancer
and breast cancer cells (8–11).
The present study is the first systematic attempt to
assess the cytotoxic effects of PA-MSHA in bladder cancer cells. It
was demonstrated that PA-MSHA is able to inhibit cell
proliferation, arrest cells in the G0/G1
phase and induce cell apoptosis in the T24 and 5637 cells in a
dose- and time-dependent manner. In addition, PA-MSHA induced
caspase-mediated apoptosis in vitro and inhibited the
PI3K-AKT-mTOR signaling pathway.
The periods of time and sequence of events from one
cell division to the next are collectively referred to as the cell
cycle. In the cell cycle analysis, a significant increase in the
cell population at the G0/G1 phase was
observed at increased concentrations of PA-MSHA. This result is in
agreement with previous findings that showed that PA-MSHA was able
to arrest tumor cells in the G0/G1 phase in
breast and nasopharyngeal cancer cells (9,11).
Cell cycle checkpoint controls at the G1 to S transition
prevent the cell cycle from progressing when DNA is damaged. We
therefore hypothesize that the G1 phase arrest is at
least partially explained by the PA-MSHA-induced growth inhibition
of the bladder cancer cells. It was also observed that the 24 h
IC50 doses for the T24 and 5637 cell lines were
<4.5×109/ml (Table
I), indicating that the tumor cells were sensitive to
PA-MSHA.
Caspases have been shown to be activated during
apoptosis in numerous cells and are critical in the initiation and
execution of apoptosis. Currently, there are two recognized points
at which caspases are activated to initiate apoptosis. In the
extrinsic pathway, initiator caspase-8 is activated by
adapter-mediated recruitment to the death receptor’s cytosolic face
following Fas ligation (20).
Alternatively, in the intrinsic pathway, initiator caspase-9 is
activated following the release of mitochondrial components to form
the Apaf complex (21). It has
previously been shown that live P. aeruginosa is able to
induce apoptosis via the extrinsic and intrinsic apoptosis pathways
(22,23). The present data suggested that
PA-MSHA also acted by triggering the intrinsic and the extrinsic
apoptosis pathways, which was consistent with studies performed in
breast cancer cell lines (11).
However, it is not yet known which pathway is dominant.
The PI3K, AKT and mTOR signal transduction pathways
regulate cell survival, proliferation and invasion, all key
functions in the progression of bladder cancer (24). In a previous study, the activation
of the PI3K-AKT-mTOR pathway correlated with tumor progression and
reduced survival in patients with urothelial carcinoma of the
urinary bladder (25). In addition,
downregulation of the AKT-mTOR signaling pathway predisposed the
bladder cancer cells to become apoptotic, indicating that the
AKT-mTOR pathway may be an important treatment target for bladder
cancer (26). The present study
demonstrated that PA-MSHA inhibits the PI3K-AKT-mTOR signaling
pathway in a dose-dependent manner. In a previous study in breast
cancer cells, Liu et al also noted that PA-MSHA inhibited
the EGFR and AKT signaling pathways (27). Since targeting the PI3K-AKT-mTOR
signaling pathway induces cascade-dependent apoptosis and
G0/G1 cell cycle arrest (28–30),
we hypothesize that PA-MSHA exerts it’s antitumor cytotoxic effect
by blocking the PI3K-AKT-mTOR signaling pathway. However, further
in-depth and detailed experiments are required to verify this
theory.
Taken together, the present data demonstrate that
PA-MSHA inhibits proliferation and induces apoptosis in the T24 and
5637 bladder cancer lines by modulating caspase family proteins and
affecting the cell cycle regulation machinery. The PI3K-AKT-mTOR
signaling pathway may have an important role in the direct
anticancer cytotoxic effect of PA-MSHA. We propose that PA-MSHA,
either alone or in combination with standard therapy, may be a
novel strategy for the management of bladder cancer. However,
further studies are required to validate the present findings in
appropriate animal models.
Acknowledgements
This study was supported by a grant
from the Fudan University Shanghai Cancer Center (YJ-201211).
References
|
1
|
Nargund VH, Tanabalan CK and Kabir MN:
Management of non-muscle-invasive (superficial) bladder cancer.
Semin Oncol. 39:559–572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mansoor M, Ali S, Fasihuddin Q and Baloch
MU: Superficial bladder tumours: recurrence and progression. J Coll
Physicians Surg Pak. 21:157–160. 2011.PubMed/NCBI
|
|
3
|
Herr HW: High-risk superficial bladder
cancer: transurethral resection alone in selected patients with T1
tumor. Semin Urol Oncol. 15:142–146. 1997.PubMed/NCBI
|
|
4
|
Mead GM, Russell M, Clark P, et al: A
randomized trial comparing methotrexate and vinblastine (MV) with
cisplatin, methotrexate and vinblastine (CMV) in advanced
transitional cell carcinoma: results and a report on prognostic
factors in a Medical Research Council study. MRC Advanced Bladder
Cancer Working Party. Br J Cancer. 78:1067–1075. 1998.
|
|
5
|
Jain RK and Forbes NS: Can engineered
bacteria help control cancer? Proc Natl Acad Sci USA.
98:14748–14750. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kresowik TP and Griffith TS: Bacillus
Calmette-Guerin immunotherapy for urothelial carcinoma of the
bladder. Immunotherapy. 1:281–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mu XY: Success in establishing the
MSHA-positive Pseudomonas aeruginosafimbrial strain. Wei
Sheng Wu Xue Bao. 26:176–179. 1986.(In Chinese).
|
|
8
|
Cao Z, Shi L, Li Y, et al: Pseudomonas
aeruginosa: mannose sensitive hemagglutinin inhibits the growth
of human hepatocarcinoma cells via mannose-mediated apoptosis. Dig
Dis Sci. 54:2118–2127. 2009. View Article : Google Scholar
|
|
9
|
Wang J, Wu D and Chen L: Pseudomonas
aeruginosavaccine inhibits the proliferation of human
nasopharyngeal cancer cells in vitro. Nan Fang Yi Ke Da Xue Xue
Bao. 32:544–547. 2012.(In Chinese).
|
|
10
|
Ling W, Liu H, Cao H, Yu FR and Xu J:
Effects of PA-MSHA vaccine on gastric cancer cells in vitro.
Zhonghua Zhong Liu Fang Zhi Za Zhi. 15:1381–1385. 2008.(In
Chinese).
|
|
11
|
Liu ZB, Hou YF, Min-Dong, et al: PA-MSHA
inhibits proliferation and induces apoptosis through the
up-regulation and activation of caspases in the human breast cancer
cell lines. J Cell Biochem. 108:195–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song XX, Jiang T, Wu DJ, et al:
Intravesical instillation of PA-MSHA for preventing postoperative
recurrence of bladder cancer. Zhonghua Bi Niao Wai Ke Za Zhi.
22:597–598. 2001.(In Chinese).
|
|
13
|
Wang JS, Wang FB, Zhang QG, Shen ZZ and
Shao ZM: Enhanced expression of Rab27A gene by breast cancer cells
promoting invasiveness and the metastasis potential by secretion of
insulin-like growth factor-II. Mol Cancer Res. 6:372–382. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z, Hao D, Zhang H, Ren L, Yang Y, Li L,
Chai J, Zhou X and Fu L: A clinical study on PA-MSHA vaccine used
for adjuvant therapy of lymphoma and lung cancer. Hua Xi Yi Ke Da
Xue Xue Bao. 31:334–337. 2000.(In Chinese).
|
|
15
|
Sun WP, Fu HW, Liu N, et al: Clinical
investigation of PA-MSHA strain vaccine on the improvement of
immune functions of cancer patients. Min Guo Wei Sheng Wu Ji Mian
Yi Xue Za Zhi. 20:373–376. 2000.
|
|
16
|
Shan HW, Lin ZF, Zhao L, Yang YX and Yuan
Z: Effect of PA-MSHA vaccine on the prevention of pulmonary
infection in severe traumatic patients. Zhongguo Ji Jiu Yi Xue.
27:375–377. 2007.(In Chinese).
|
|
17
|
Cheng GZ, Jia CX and Yang HR: Studies on
mechanism of immunization against recurrent urinary tract infection
with MSHA-piliated strain vaccine of Pseudomonas aeruginosa.
Zhongguo Wei Sheng Tai Xue Za Zhi. 12:331–333. 2000.(In
Chinese).
|
|
18
|
Jia LW, Li CM, Wang YD, Hou JP and Mu XY:
Protective effect of PA-MSHA vaccine on experimental hepatic
fibrosis. Basic Med Sci Clin. 1:73–78. 1999.(In Chinese).
|
|
19
|
Jia L, Wang C, Kong H, et al: Effect of
PA-MSHA vaccine on plasma phospholipids metabolic profiling and the
ratio of Th2/Th1 cells within immune organ of mouse IgA
nephropathy. J Pharm Biomed Anal. 43:646–654. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muzio M, Stockwell BR, Stennicke HR,
Salvesen GS and Dixit VM: An induced proximity model for caspase-8
activation. J Biol Chem. 273:2926–2930. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu X, Kim CN, Yang J, Jemmerson R and
Wang X: Induction of apoptotic program in cell-free extracts:
requirement for dATP and cytochrome c. Cell. 86:147–157. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cannon CL, Kowalski MP, Stopak KS and Pier
GB: Pseudomonas aeruginosa-induced apoptosis is defective in
respiratory epithelial cells expressing mutant cystic fibrosis
transmembrane conductance regulator. Am J Respir Cell Mol Biol.
29:188–197. 2003. View Article : Google Scholar
|
|
23
|
Jendrossek V, Grassmé H, Mueller I, Lang F
and Gulbins E: Pseudomonas aeruginosa-induced apoptosis
involves mitochondria and stress-activated protein kinases. Infect
Immun. 69:2675–2683. 2001. View Article : Google Scholar
|
|
24
|
Chen M, Cassidy A, Gu J, et al: Genetic
variations in PI3K-AKT-mTOR pathway and bladder cancer risk.
Carcinogenesis. 30:2047–2052. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun CH, Chang YH and Pan CC: Activation of
the PI3K/Akt/mTOR pathway correlates with tumour progression and
reduced survival in patients with urothelial carcinoma of the
urinary bladder. Histopathology. 58:1054–1063. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yohn NL, Bingaman CN, DuMont AL and Yoo
LI: Phosphatidylinositol 3′-kinase, mTOR, and glycogen synthase
kinase-3β mediated regulation of p21 in human urothelial carcinoma
cells. BMC Urol. 11:192011.
|
|
27
|
Liu ZB, Hou YF, Zhu J, et al: Inhibition
of EGFR pathway signaling and the metastatic potential of breast
cancer cells by PA-MSHA mediated by type 1 fimbriae via a
mannose-dependent manner. Oncogene. 29:2996–3009. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang S, Zhang Y, Zhuang Y, et al: Matrine
induces apoptosis in human acute myeloid leukemia cells via the
mitochondrial pathway and Akt inactivation. PLoS One. 7:e468532012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yuan L, Wang J, Xiao H, Xiao C, Wang Y and
Liu X: Isoorientin induces apoptosis through mitochondrial
dysfunction and inhibition of PI3K/Akt signaling pathway in HepG2
cancer cells. Toxicol Appl Pharmacol. 265:83–92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gong C, Liao H, Wang J, et al: LY294002
induces G0/G1 cell cycle arrest and apoptosis of cancer stem-like
cells from human osteosarcoma via down-regulation of PI3K activity.
Asian Pac J Cancer Prev. 13:3103–3107. 2012. View Article : Google Scholar : PubMed/NCBI
|