Introduction
Gastric cancer remains the fourth most commonly
diagnosed cancer and is the second leading cause of cancer related
mortality worldwide (1). Gastric
cancer is the most common cancer in Eastern Asia (2). Eradication of H. pylori in the
stomach by administration of oral antimicrobial agents results in
the resolution of H. pylori-infected chronic active
gastritis and significantly reduces the risk of gastric cancer
development (3). However, bacterial
eradication treatment has been lacking. The occurrence of
antibiotic-resistant H. pylori has been reported (4) and is occasionally associated with
adverse effects. Regular therapies such as chemotherapy, biotherapy
and radiotherapy have been previously applied, however, they have
unavoidable side effects (5).
Therefore, more effective alternative approaches for gastric cancer
prevention and therapies without undesirable side-effects are
needed.
It is widely accepted that phytochemical, especially
phenolic, compounds are associated with anticancer effects by
affecting molecular events in the initiation, promotion and
progression stages. Recent studies have demonstrated protective
effects of plant phenolic compounds against gastric cancer
(6–8). The expansion ability of tumor cells
depends on the rate of both cell proliferation and cell apoptosis.
The particular features of tumor cells allow them to evade
apoptosis, a cell suicide program that reduce the damaged or
mutated cells to maintain homeostasis (9).
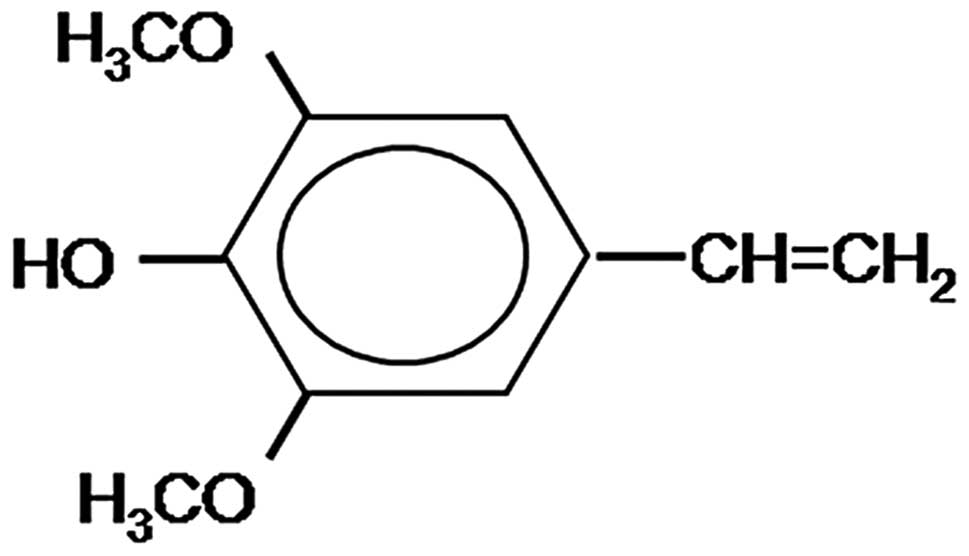
Canolol, 4-vinyl-2,6-dimethoxyphenol (Fig. 1), is purified from crude canola oil
and is a novel and potent antioxidant. Canolol has been proven to
prevent H. pylori-induced gastritis and carcinogenesis in an
animal model (10). However, its
potential anti-proliferative and proapoptotic effects on gastric
cancer cells and the possible mechanisms remain unknown.
The role of cyclooxygenase-2 (COX-2) inhibitors in
the chemoprophylaxis of gastric cancer has been investigated.
COX-2, the inducible isoform of COX, is undetectable in normal
tissues and highly expressed in gastric tumors (11). Experimental studies have identified
the correlation between COX-2 overexpression and the increased cell
proliferation and decreased cell apoptosis in malignant tumor cells
(12,13). COX inhibitors (Coxibs) are a series
of drugs with analgesic, antipyretic and anti-inflammatory
properties. Evidence suggests that COX-2 inhibitors correlate with
tumor inhibition in breast (14)
and endometrial cancer cell lines (15). Induction of apoptosis has
increasingly become important with regard to the mechanism of
cancer defense and prevention (16). However, the involvement of COX-2
inhibitors in gastric cancer prophylaxis remains to be determined,
as the long-term use of COX-2 inhibitors exerts side-effects on the
cardiovascular system and the digestive tract. A possible
correlation between COX-2 inhibition and cell apoptosis in gastric
cancer cell lines has yet to be examined.
In the present study, the effects of canolol on
growth and apoptosis of human gastric adenocarcinoma SGC-7901 cells
were investigated. Human gastric mucosal epithelial (GES-1) cells
were used as the control cell model to examine the non-specific
cytotoxicity of canolol. The mRNA expression levels of COX-2, Bcl-2
and Bax were detected to further elucidate the possible mechanisms
involved.
Materials and methods
Materials and reagents
4-Vinyl-2,6-dimethoxyphenol (canolol with a
molecular mass of 180) was purchased from Junsei Chemical, Tokyo,
Japan. It was synthesized to at least 95% purity (confirmed by
nuclear magnetic resonance). The preparation was sealed under
helium or nitrogen and maintained at −80°C. Canolol was dissolved
in ethanol and diluted in a serum-free medium immediately before
the experiments. Gastric cancer SGC-7901 cells were obtained from
the Department of Pathogeny Biology, Norman Bethune Medical College
of Jilin University, China. Human gastric mucosal epithelial cell
line GES-1 was obtained from the Cancer Hospital of Beijing
University. The study protocol was approved by the ethics committee
of the First Hospital of Jilin University.
Cell culture and treatment
Human SGC-7901 gastric cancer cell line and human
GES-1 gastric mucosal epithelial cell line were cultured in
RPMI-1640 medium containing 10% heat-inactivated fetal bovine serum
(FBS) and 100 ng/ml each of penicillin and streptomycin in an
incubator (50 ml/l CO2) at 37°C. The medium was changed
every 2–3 days. Cells in the logarithmic growth phase were
collected for subsequent experiments. The cells were treated with
various concentrations of canolol for 24 h.
Cell viability assay
The method of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was employed to determine cell viability. Cultured SGC-7901
and GES-1 cells were detached using trypsinization, centrifuged at
1,000 × g for 5 min and resuspended in fresh RPMI-1640 medium. The
cells were plated at a density of 5×103 cells/well in
96-well microplates and treated with canolol ranging from 25 to
1,200 μmol/l for 24 h at 37°C. At the end of treatment, 20
μl of MTT stock solution was added to each well [(0.5 mg/ml
in phosphate-buffered saline (PBS)] for 4 h. The medium was
replaced with 150 μl DMSO to dissolve the converted purple
dye in the culture plates. Absorbance was measured at 570 nm on a
spectrophotometer microplate reader. Cell viability was assayed as
the relative formazan formation in treated compared with control
wells after correction for background absorbance. Four wells per
dose were counted in each experiment. Analyses were performed using
SPSS version 10.0 (SPSS Inc, Chicago, IL, USA). Data were evaluated
using one-way ANOVA. P<0.05 was considered statistically
significant.
Cell morphology
SGC-7901 and GES-1 cells were seeded at a density of
5×105 cells/well onto a cover slip loaded in 6-well
plates. Fresh RPMI-1640 medium containing different concentrations
of canolol was added. Cells were photographed with an inverted
microscope under ×200 magnifications to observe morphological
changes.
Annexin V-FITC/PI staining for flow
cytometry
SGC-7901 cells were collected and centrifuged at
1,000 × g for 5 min and resuspended in fresh RPMI-1640 medium at a
density of 2×105 cells/ml. Apoptotic and necrotic cells
were evaluated by Annexin V (AV) binding and propidium iodide (PI)
uptake using an AV-FITC-PI apoptosis assay kit (Pharmingen, San
Diego, CA, USA). Samples were analyzed by flow cytometry.
Real-time quantitive PCR analysis
Total RNA of SGC-7901 cells was extracted using an
RNA extraction kit and primers used are shown in Table I. Following DNase treatment, the
first strand cDNA was synthesized. Quantitive PCR of Bcl-2, Bax and
COX-2 were performed with the Bio-Rad (Hercules, CA, USA) CFX
system. To exclude variations caused by RNA quantity and quality,
the GAPDH gene was used as an internal control. Analyses were
performed using SPSS version 10.0 (SPSS Inc). Data were evaluated
using one-way ANOVA. P<0.05 was considered a statistically
significant result.
 | Table IPrimer sequences used in real-time
quantitive PCR. |
Table I
Primer sequences used in real-time
quantitive PCR.
| Gene | Primer sequence | Annealing temperature
(°C) | Product size
(bp) |
|---|
| COX-2 | F:
CTCCCTTGGGTGTCAAAGGTA | 76 | 171 |
| R:
GCCCTCGCTTATGATCTGTC | | |
| Bcl-2 | F:
GAGTTCGGTGGGGTCATG | 83 | 186 |
| R:
GGAGAAATCAAACAGAGGC | | |
| Bax | F:
GGATGCGTCCACCAAGAA | 83.5 | 388 |
| R:
GAGCACTCCCGCCACAAA | | |
| GAPDH | F:
AACGGATTTGGTCGTATTG | 78.5 | 258 |
| R:
GGAAGATGGTGATGGGATT | | |
Results
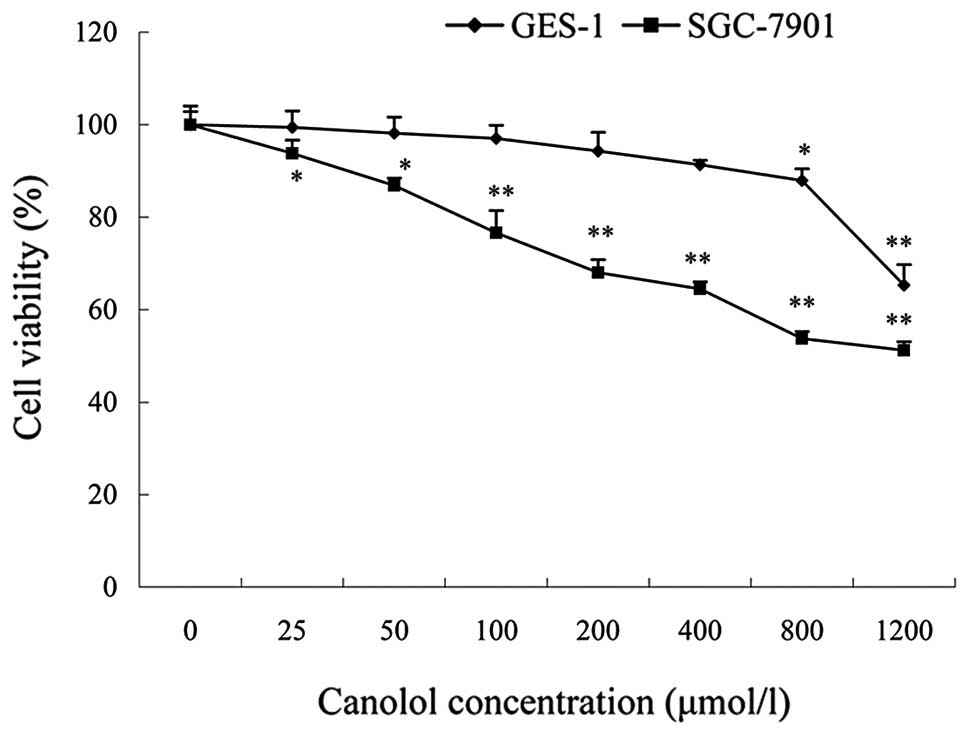
Canolol does not exhibit evident toxicity
to GES-1 cells
The proliferation effect of canolol was determined
using an MTT assay and GES-1 cells were used as a control to detect
the cell toxicity of canolol. Cells were treated with different
concentrations of canolol (0–1200 μmol/l). The data
indicated that canolol has no obvious cytotoxicity against normal
GES-1 cells. The percentage of cell viability was 99.38±3.57% at 25
μmol/l, 87.82±2.55% at 800 μmol/l and decreased to
65.31±4.44% at 1200 μmol/l (Fig.
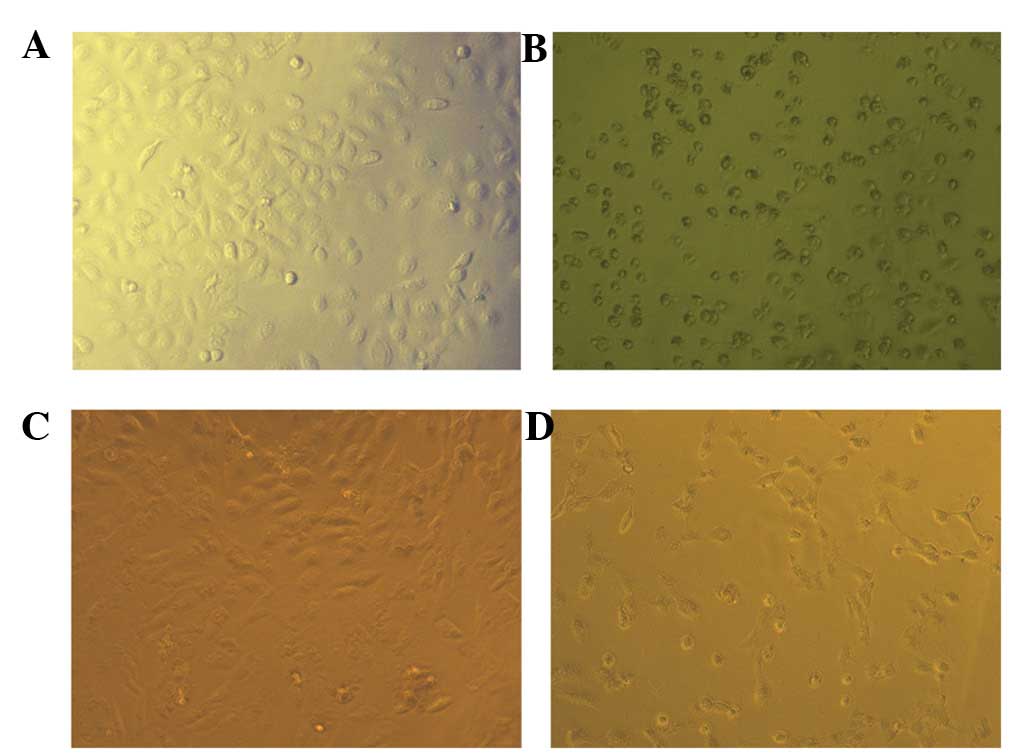
2). Cell morphology using an inverted microscope also showed
that cell structures were intact and were well established after
1,200 μmol/l canolol treatment (Fig. 3).
Canolol inhibits proliferation and
induces apoptosis of SGC-7901 cells
SGC-7901 cells were treated with different
concentrations of canolol (0–1200 μmol/l). The percentages
of cell viability at various canolol doses were determined as the
percentage of viable treated cells in comparison with viable
untreated cells. The results provided solid evidence that the
inhibitory effects on the proliferation of canolol to SGC-7901
cells were dose-dependent (Fig. 2);
the percentage of cell viability was 89.80±2.83% at 25
μmol/l, 73.73±1.51% at 800 μmol/l (P<0.05)
and 51.22±1.82% at 1,200 μmol/l (P<0.01).
Consistent with the MTT assay results, the adherent SGC-7901 cells
were markedly decreased and showed apoptosis under the treatment of
1,200 μmol/l canolol (Fig.
3).
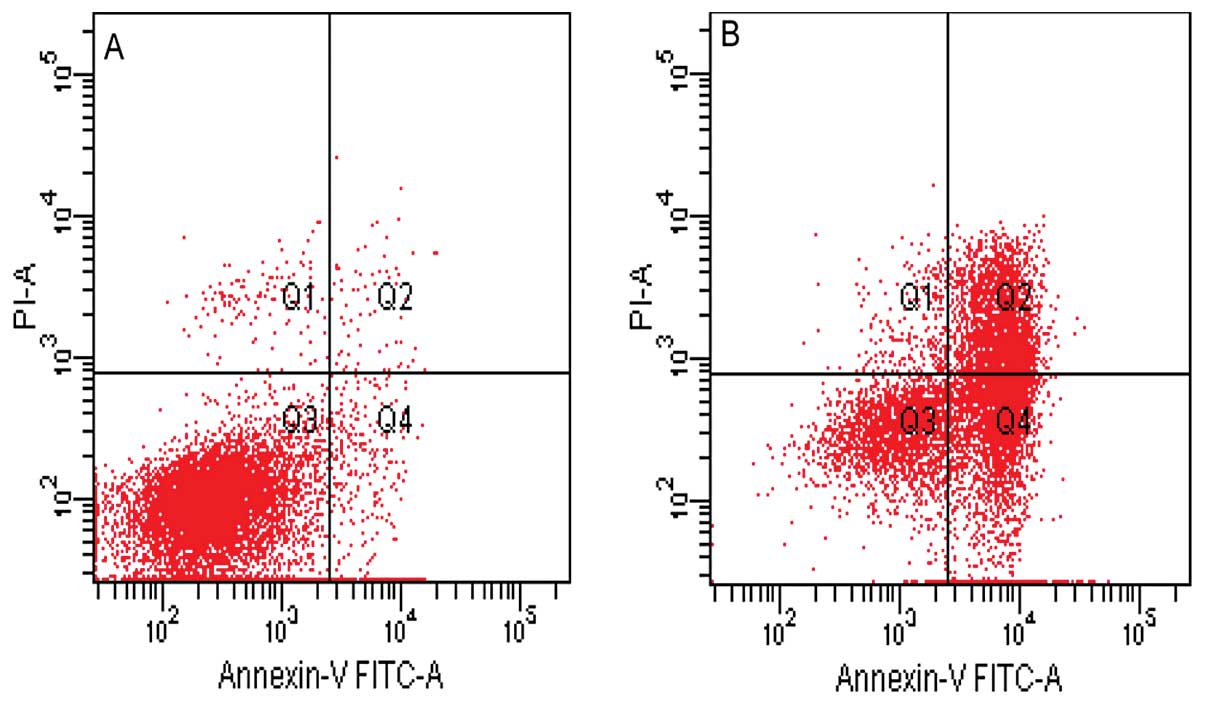
Furthermore, a flow cytometric analysis was used to
quantify the rate of cell apoptosis using double staining of
Annexin V-FITC and PI. As shown in Fig.
4, the lower right field (high Annexin V, low PI staining)
represents the early apoptotic cells due to the strong affinity of
Annexin V-FITC with phosphatidylserine, which transports from the
inner to the outer surface of the plasma membrane during early
apoptosis. By contrast, the higher left field (high PI, low Annexin
V staining) represents the necrotic cells, since PI, which binds to
nucleic acids, only cross through the compromised membrane of dead
cells or late apoptotic cells (17). Viable cells are shown in the lower
left field (low Annexin V and PI staining) and the higher right
field (high Annexin V and PI staining), indicating late apoptotic
cells. The results showed that canolol was able to induce the
apoptosis of SGC-7901 cells and the rate of early apoptosis, late
apoptosis and necrosis of SGC-7901 cells were increased under 400
μmol/l canolol (Fig. 4).
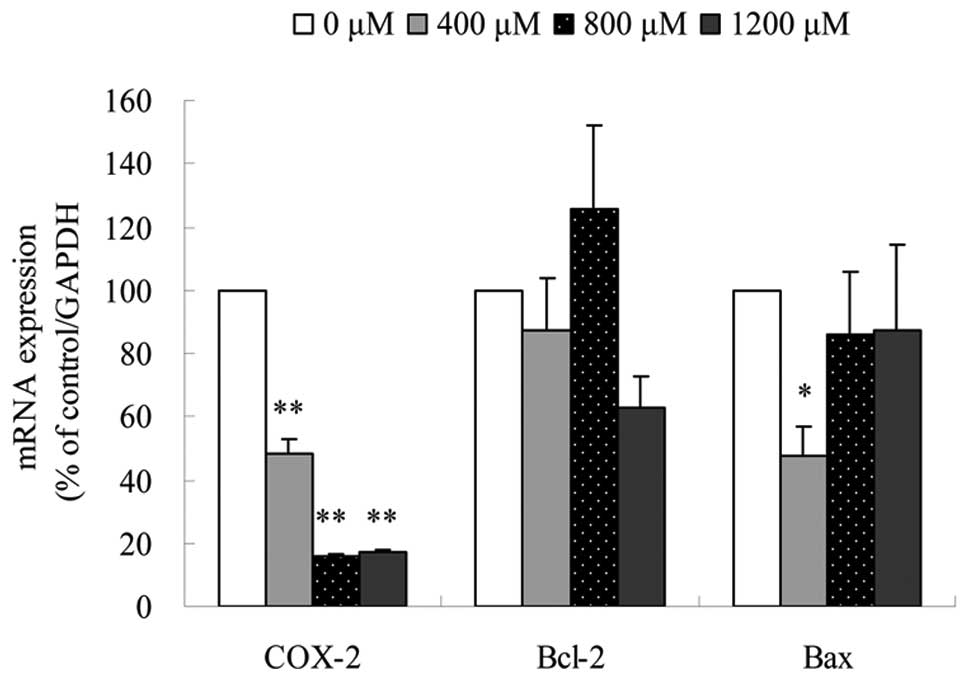
Canolol downregulates the mRNA expression
level of COX-2
To clarify the mechanisms of SGC-7901 cell apoptosis
under canolol treatment, the mRNA expression level of COX-2, Bcl-2
and Bax was evaluated using real-time quantitive PCR. The sequences
of these primers were shown in Table
I. The results showed that in SGC-7901 cells, the relative mRNA
expression level of COX-2 was decreased to 48.50±4.67% in 400
μmol/l, 16.08±0.75% in 800 μmol/l and 17.22±0.88% in
1,200 μmol/l canolol. The effect of canolol on COX-2
expression was downregulated (P<0.01); However, the
expression levels of Bcl-2 and Bax fluctuated slightly (Fig. 5). These data suggested that the
inhibition of COX-2 might play an important role in the apoptosis
of SGC-7901 cells.
Discussion
Gastric cancer is one of the most prevalent
malignant tumors and its morbidity is the highest in China.
Currently, many natural and synthesized compounds are used in the
chemoprovention and treatment of gastric cancer (18,19).
Canolol, 4-vinyl-2,6-dimethoxyphenol, which is extracted from crude
canola oil, has the ability to prevent H. pylori-infected
gastric carcinogenesis in gerbils (10). In the present study, it was
demonstrated that canolol prevented proliferation and induced
apoptosis of SGC-7901 cells dose-dependently in vitro.
Additionally, it had low toxicity to immortalized GES-1 cells
(Figs. 2 and 3). The results indicated that canolol has
the potential to be developed as a new natural anti-gastric
carcinoma agent.
COX-2 is important in the conversion of arachidonic
acid to prostaglandin H2. Accumulating evidence suggests
that the constitutive overexpresion of the inducible COX-2 gene is
involved in a diverse array of cancers and Harris et
al(20) demonstrated that COX-2
overexpresion initiated and promoted carcinogenesis through: i)
mutagenesis, i.e., the production of certain reactive oxygen
species that are carcinogenic; ii) mitogenesis, i.e., cell
proliferation promoted by PGE-2 and other factors; iii)
anti-apoptosis, i.e., cell differentiation and apoptosis reduced by
PGE-2 and other factors; iv) angiogenesis, metastasis and
immunosuppression (20). The
real-time quantitive PCR in this study showed that COX-2 expression
was downregulated under canolol treatment (P<0.01)
(Fig. 5). It was postulated that
inhibition of COX-2 expression may result in blockade of the
prostaglandin cascade and a decrease in reactive oxygen species
(ROS), thus stimulating apoptosis of malignant cells and preventing
neoplastic growth. The scavenging potency of canolol against
ROO• is much higher than that of well-known
antioxidants, such as α-tocopherol, vitamin C and β-carotene
(21). A previous study in this
laboratory showed canolol decreased serum 8-OHdG, a key biomarker
of oxidative DNA damage relevant to carcinogenesis (10). Other natural phenolic extracts, such
as BCE (black currant extract) and dioscin, reduce the risk of
gastric cancer owing to their antioxidative functions (22–24).
Selective and non-selective COX-2 inhibitors may be
involved in the intervention and chemoprevention of carcinogenesis
(25–27). A series of epidemiologic studies
found that the COX-2 inhibition levels of coxibs were consistent
with their chemopreventive effects in cancers of the breast, colon,
prostate and lung (20). Ma et
al(28) have demonstrated that
PGE2 acts with a family of G-protein-coupled receptors
participating in multiple signal tranduction pathways.
The Bcl-2 family, such as Bax, Bad, Bid, Bcl-2 and
Bcl-x, is one of the most extensively studied groups of proteins
involved in cell apoptosis. Bax, Bad and Bid were shown to activate
apoptosis, while Bcl-2 and Bcl-x were shown to inhibit the process
(29). Transfection of COX-2
constitutive expression vector into the BCC cell line significantly
upregulated Bcl-2 expression and this indicated that Bcl-2 might
participate in COX-2 mediated anti-apoptic processes (30). In addition, the expression level of
Bax, a member of a pro-apopotic protein family was downregulated in
a transgenic mouse model (31).
However, in the present study, no correlation between Bcl-2/Bax and
COX-2 expression was found (Fig.
5).
The relationship between apoptosis and COX-2
downregulation in this gastric adenocarcinoma cells should be
studied. COX-2 is a potential pharmacologic target that may be used
in the prevention and treatment of various types of
malignancies.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (Nos. 30972476 and
81273065). The authors would like to thank Dr Yu Chen for technical
support.
References
|
1
|
Ferlay J, Shin H R, Bray F, et al:
Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int
J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu X, Zhang B, Guo Y, et al:
Down-regulation of AP-4 inhibits proliferation, induces cell cycle
arrest and promotes apoptosis in human gastric cancer cells. PLoS
ONE. 7:e370962012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chuah SK, Tsay FW, Hsu PI and Wu DC: A new
look at anti-Helicobacter pylori therapy. World J
Gastroenterol. 17:3971–3975. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Graham D Y: Antibiotic resistance in
Helicobacter pylori: implications for therapy.
Gastroenterology. 115:1272–1277. 1998.
|
|
5
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics. CA Cancer J Clin. 62:10–29. 2012.
|
|
6
|
Kountouri AM, Kaliora AC, Koumbi L and
Andrikopoulos NK: In-vitro gastric cancer prevention by a
polyphenol-rich extract from olives through induction of apoptosis.
Eur J Cancer Prev. 18:33–39. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alvarez-Suarez JM, Dekanski D, Ristić S,
et al: Strawberry polyphenols attenuate ethanol-induced gastric
lesions in rats by activation of antioxidant enzymes and
attenuation of MDA increase. PLoS One. 6:e258782011. View Article : Google Scholar
|
|
8
|
Jaganathan SK and Supriyanto E:
Antiproliferative and molecular mechanism of eugenol-induced
apoptosis in cancer cells. Molecules. 17:6290–6304. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vaux DL and Korsmeyer SJ: Cell death in
development. Cell. 96:245–254. 1999. View Article : Google Scholar
|
|
10
|
Cao XY, Tsukamoto T, Seki T, et al:
4-Vinyl-2,6-dimethoxyphenol (canolol) suppresses oxidative stress
and gastric carcinogenesis in Helicobacter pylori-infected
carcinogen-treated Mongolian gerbils. Int J Cancer. 122:1445–1454.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Harris RE, Chlebowski RT, Jackson RD, et
al: Breast cancer and nonsteroidal anti-inflammatory drugs:
prospective results from the Women’s Health Initiative. Cancer Res.
63:6096–6101. 2003.
|
|
12
|
Kilic G, Gurates B, Garon J, et al:
Expression of cyclooxygenase-2 in endometrial adenocarcinoma. Eur J
Gynaecol Oncol. 26:271–274. 2005.PubMed/NCBI
|
|
13
|
Targosz A, Brzozowski T, Pierzchalski P,
Szczyrk U, Ptak-Belowska A, Konturek SJ and Pawlik W:
Helicobacter pylori promotes apoptosis, activates
cyclooxygenase (COX)-2 and inhibits heat shock protein HSP70 in
gastric cancer epithelial cells. Inflamm Res. 61:955–966. 2012.
View Article : Google Scholar
|
|
14
|
Ashok V, Dash C, Rohan TE, Sprafka JM and
Terry PD: Selective cyclooxygenase-2 (COX-2) inhibitors and breast
cancer risk. Breast. 20:66–70. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ozalp SS, Eren CY, Bostancioğlum RB and
Koparal AT: Induction of apoptosis and inhibition of cell
proliferation by the cyclooxygenase enzyme blocker-nimesulide in
the Ishikawa endometrial cancer cell line. Eur J Obstet Gyn Reprod
Biol. 164:79–84. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun SY, Hail JRN and Lotan R: Apoptosis as
a novel target for cancer chemoprevention. J Natl Cancer Inst.
96:662–672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tripathi M, Singh BK, Mishra C, Raisuddin
S and Kakkar P: Involvement of mitochondria mediated pathways in
hepato-protection conferred by Fumaria parviflora Lam.
Extract against nimesulide induced apoptosis in vitro. Toxicol In
Vitro. 24:495–508. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu XY, Wang YL, Wang HW, et al: Quinacrine
inhibits cell growth and induces apoptosis in human gastric cancer
cell line SGC-7901. Cur Ther Res Clin Exp. 73:52–64. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ji YB, Qu ZY and Zou X: Juglone-induced
apoptosis in human gastric cancer SGC-7901 cells via the
mitochondrial pathway. Exp Toxicol Pathol. 63:69–78. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harris RE, Beebe J and Alshafie GA:
Reduction in cancer risk by selective and nonselective
cyclooxygenase-2 (COX-2) inhibitors. J Exp Pharmacol. 6:491–496.
2012.
|
|
21
|
Dong X, Li ZR, Wang W, Zhang WJ, Liu SZ
and Zhang XM: Protective effect of canolol from oxidative
stress-induced cell damage in ARPE-19 cells via an ERK mediated
antioxidative pathway. Mol Vis. 17:2040–2048. 2011.PubMed/NCBI
|
|
22
|
Jia N, Xiong YLL, Kong BH, Liu Q and Xia
XF: Radical scavenging activity of black currant (Ribes nigrum
L) extract and its inhibitory effect on gastric cancer cell
proliferation via induction of apoptosis. J Funct Foods. 4:382–390.
2011.
|
|
23
|
Gao LL, Li FR, Jiao P, et al: Paris
chinensis dioscin induces G2/M cell cycle arrest and apoptosis in
human gastric cancer SGC-7901 cells. World J Gastroenterol.
17:4389–4395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu MM, Xu LN, Yin LH, et al: Cytotoxicity
of dioscin in human gastric carcinoma cells through death receptor
and mitochondrial pathways. J Appl Toxicol. View Article : Google Scholar : 2012.PubMed/NCBI
|
|
25
|
Fu SL, Wu YL, Zhang YP, Qiao MM and Chen
Y: Anti-cancer effects of COX-2 inhibitors and their correlation
with angio-genesis and invasion in gastric cancer. World J
Gastroenterol. 10:1971–1974. 2004.PubMed/NCBI
|
|
26
|
Harris RE: Cyclooxygenase-2 (COX-2)
blockade in the chemoprevention of cancers of the colon, breast,
prostate, and lung. Inflammopharmacology. 17:55–67. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chan AT, Ogino S and Fuchs CS: Aspirin use
and survival after diagnosis of colorectal cancer. JAMA.
302:649–658. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma X, Kundu N, Rifat S, Walser T and
Fulton AM: Prostaglandin E receptor EP4 antagonism inhibits breast
cancer metastasis. Cancer Res. 66:2923–2927. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsujimoto Y and Shimizu S: Bcl-2 family:
life-or-death switch. FEBS Lett. 466:6–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tjiu JW, Liao YH, Lin SJ, et al:
Cyclooxygenase-2 overexpression in human basal cell carcinoma cell
line increases antiapoptosis, angiogenesis, and tumorigenesis. J
Invest Dermatol. 126:1143–1151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu CH, Chang SH, Narko K, et al:
Overexpression of cyclooxygenase-2 is sufficient to induce
tumorigenesis in transgenic mice. J Biol Chem. 276:18563–18569.
2001. View Article : Google Scholar : PubMed/NCBI
|