Introduction
Colorectal cancer is statistically recorded as the
second leading cause of mortality in Western countries and ∼50% of
mortalities which occur as a result of this type of cancer are
associated with progression (1). In
colorectal cancer treatment, the progression-free survival rate was
found to be shorter among patients with progressive metastatic
colorectal cancer who were treated with chemotherapeutic drugs
bevacizumab and cetuximab (CBC regimen) compared with patients who
received chemotherapy and bevacizumab alone (CB regimen) (2). However, metastasis continued to result
in a poor prognosis, with an average overall survival time of 20
months (3–7). The toxic side effects and symptoms
experienced by patients as a result of chemotherapy have led to the
development of less toxic therapies. Adoptive immunotherapy is one
of the therapies and patients have experienced its benefits for the
last 20 years (8).
Immunotherapy is based on the principle that the
host immune system is capable of generating immune responses
against cancer cells. There are plethoras of natural killer (NK)
cells and antigen-induced cytotoxic T-lymphocytes (CTLs) in the
human immune system. Initial efforts to generate anticancer immune
responses have focused on adaptive immunity, particularly on the
induction of tumor-specific CTLs. While it is appreciated that
tumor-specific CTLs are critical for successful immunotherapy, it
is becoming increasingly apparent that cellular components of the
innate and adaptive arms of the immune system are able to control
tumor growth (9).
NK cells are a component of the innate immune
system, which perform immunosurveillance via the recognition of
altered or missing ‘self’ surface markers in damaged, infected or
transformed malignant cells (10,11).
NK cells constitutively express lytic machinery that is able to
kill target cells independently of any previous exposure to cancer
antigens. These functional features are suggestive of their
identification and control of tumor growth and metastasic diffusion
in vivo.
The main functions of NK cells are to suppress tumor
cell initiation, growth and metastasis through mechanisms mediated
by perforin and the granzyme-containing granule-mediated death
receptor and interferon-γ-mediated pathways (12). These varied functions of NK cells
hold considerable potential for cell-based therapies which target
human malignancies (11,13–16).
Progress in immunobiotechnology, following extensive
investigation, has permitted clinical trials with in vitro
derived NK cells and CTLs, which may be adoptively transferred to
patients via a painless procedure for cancer treatment (17).
Autologous immune enhancement therapy (AIET) has had
a successful clinical history in Japan, Europe and the USA over the
past two decades. Immune cell therapy using autologous activated
lymphocytes (18,19) was first introduced in the laboratory
by Rosenberg et al of the National Institute of Health, USA.
In the late 1980s, Rosenberg et al published a key study
which reported a low tumor regression rate (2.6–3.3%) in 1,205
patients with metastatic cancer who had undergone different types
of active specific immunotherapy (ASI).
In the present study, we report the results from a
colorectal cancer patient who underwent 6 infusions of immune cell
therapy.
Case report
Patient history
A 50-year-old female diagnosed with stage IV colonic
cancer in May 2008 presented with a KRAS mutation. CT scans
revealed a malignancy in the liver and the carcinoembryonic antigen
(CEA) level was 500 U/ml. The patient was administered FOLFOX-6
between June 2008 and September 2008. The disease progressed and
selective internal radiation therapy (SIRT) was administered. After
the therapy, the patient remained healthy for 2 months and the CEA
level began to increase. Thus, Bevacizumab and FOLFIRI was
restarted between November 2008 and April 2009. The patient was
unable to tolerate the chemotherapeutic side effects and opted to
receive AIET.
Ex vivo expansion of NK cells and
T-lymphocytes (TLs) from the peripheral blood
After informed consent was obtained from the
patient, 60 ml peripheral blood was withdrawn. The activated NK
cells and TLs were isolated from the peripheral blood mononuclear
cells (PBMCs) of the patient, and later were activated and expanded
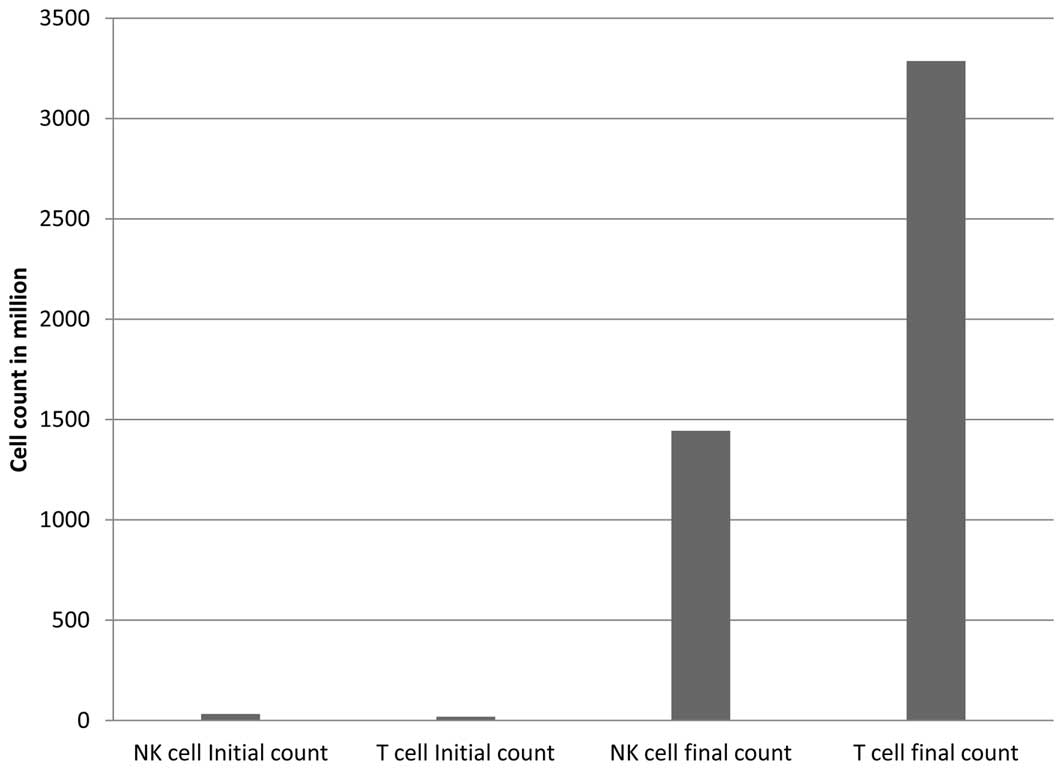
according to previously described methods (17), for 14 days. The average number of
PBMCs before the six times of expansion was 32.7×106 and
19.5×106 for NK cells and TLs, respectively. After 2
weeks of expansion, the average number of expanded lymphocytes for
six injections was 14.4×108 for NK cells and
32.9×108 for TLs. The average frequency of NK
(CD3−/CD56+) cells was 6.3% initially and
40.5% at final culture. For TLs (CD3+), the average
frequency was 88.9% initially and 62.1% at final culture, as
evaluated by flow cytometry. The total infused cell numbers are
shown in Fig. 1. Before cells were
infused, a sterility test, using bacterial and fungal agar plates,
and an endotoxin test using Limulus amoebocyte lysate (Wako, Tokyo,
Japan) were carried out to confirm the asepsis of the products.
Effects of AIET
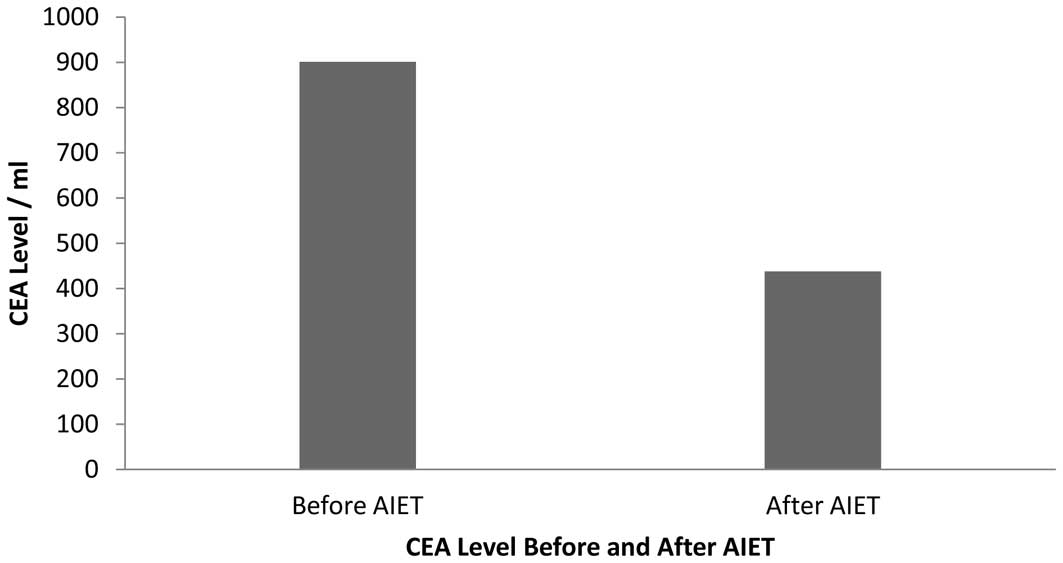
After 6 infusions of NK cells and TLs, the CEA level
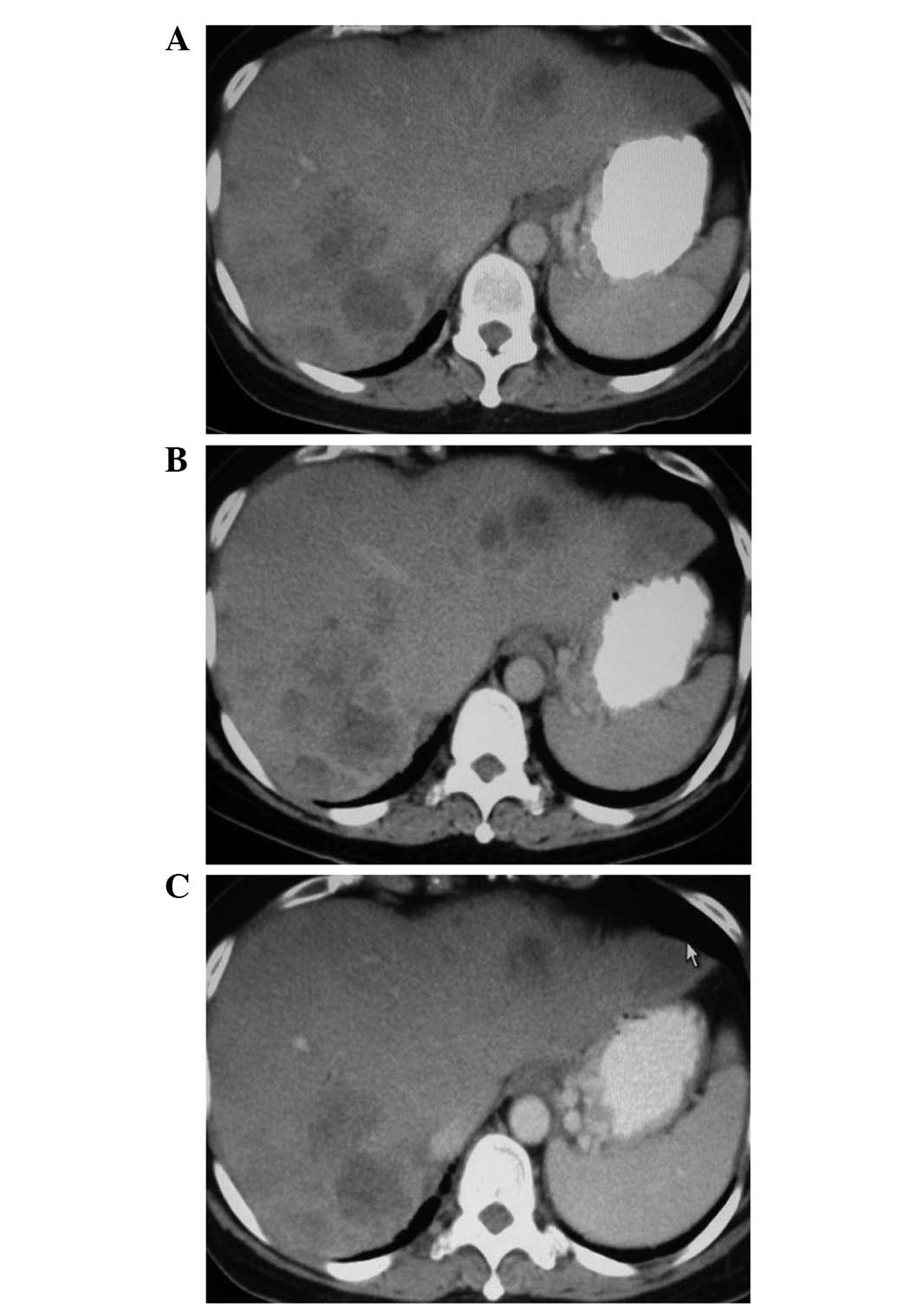
decreased considerably from 901 to 437 U/ml (Fig. 2). The CT scan revealed that the
patient with distant metastases (stage IV) responded to the
treatment with tumor reduction in one liver lobe. Tumor assessment
was carried out after the third and sixth infusion. The lesion in
the colon and lung (not shown) was stable; however, the size of the
liver metastasis was markedly reduced (Fig. 3).
Discussion
In this study, we evaluated the safety and efficacy
of the ex vivo adoptively transferred, activated and
expanded NK cell s and TLs derived from the peripheral blood of a
colorectal cancer patient. The patient was followed up for 8 months
from the first infusion of AIET. CEA levels were recorded before
and after injection of the AIET (Fig.
2). The average initial and final cell counts were recorded at
the time of each sample collection and expansion (Fig. 1).
In this course of treatment, interleukin-2 (IL-2)
was not administered. Following standard procedures, the effector
cells were cultured with IL-2, which ranged from 350 to 700 IU/ml
over the duration of 0–14 days. For all six infusions, the fold
expansion of lymphocytes from PBMCs was ∼44 and 168 for NK cells
and TLs, respectively. With regard to this fold expansion, it was
concluded that the total number of activated and expanded cells are
highly dependent on the initial lymphocyte population of PBMCs of
the whole blood. Notably, inverted phase contrast microscopy (data
not shown) revealed that cell viability and the frequency of the
healthy population was clear in each sample processing during the
culture of PBMCs.
The expanded cells were administered intravenously.
We determined that the infusion of heterogeneous TL and NK cell
populations induced a decrease in tumor mass in this case, as shown
by the CT scan results (Fig. 3C).
We concluded that the acceleration of tumor growth was arrested by
the infused effector cells, since there were no other supportive
therapies administered with or in between AIET. This was further
supported by a testimonial from the patient which described
improved physical strength and an active lifestyle.
Several case reports demonstrate that CTLs and NK
cells improve the cytotoxic effects in cellular immune responses to
similar levels as those identified in in vitro studies
(20–23). NK cell infiltration is an indicator
in patients with colorectal carcinoma. Colorectal carcinoma
displays ligands for the activating receptors, loss of HLA class I
molecules and dismissed MHC class I, which shows the susceptibility
for NK cell-mediated lysis (11,24).
CTLs also play a critical role in the adaptive
immune system. CTLs and NK cells secrete granzyme and perforin,
which are packaged in cytoplasmic granules (25) to lyse cancerous cells. For a decade,
it has been widely accepted that CD8+ T cells are
correlated with an effective antitumor response (26), patient survival (27) and the control of tumor invasion and
metastasis (28).
A number of studies support the sensitivity of CTLs
against colorectal cancer in vitro and in vivo.
Todaro et al(29) showed
that colon cancer stem cells were sensitive to γδT cells, a small
subpopulation of TLs which have the ability to target cancerous
cells. It has been hypothesized that tumor reduction and an
improved quality of life may have been derived from the presence of
ex vivo derived CTLs and γδT cells. CTLs and γδT cells kill
their targets via the secretion of perforin and granzyme B, which
supports the clinical observations in the present case.
However, the cancer stem cell population and its
dysfunction in the presence of CTLs and γδT cells requires further
investigation in clinical studies. Due to the minor population of
γδT cells in vivo, whether γδT cells are able to effectively
recognize cancer cell populations is questionable. This has been
clarified by a large cohort study. Ogino et al(30) showed that lymphocytic reactions to
tumors were associated with longer survival in colorectal cancer
patients. Although the study did not thorougly analyze the
subpopulations of inflating lymphocytes, it confirms the importance
of immune targeting therapies.
Numerous studies which explored NK cells and TLs
have demonstrated the impact of immune contexture in human tumors
and its clinical outcome. In a study involving colon cancer
patients with a history of surgery, 20–25% of patients had
recurrence of their disease, suggesting that occult metastases are
already present at the time of surgery (31). Adjuvant therapy contributed to the
survival of 86.2% of patients after 5 years, whereas 72% of
patients with a low immune score had disease recurrence and only
27.5% of these survived after 5 years (32). Thus, immune enhancement may be a
solution with regard to improving survival rates.
These results encourage most attempts at cancer
immunization, which directly involve ex vivo clonal
expansion of CD8+ T cells and CD4+ T cells to
activate and endure CD8+ killer cells (33). A number of studies note that the
infusion of ex vivo derived therapies are effective with a
reasonable dose of IL-2 or other combined therapies (34,35).
Although the administration of IL-2 provided successful outcomes,
its toxicity could not be tolerated by patients and in certain
cases, patients succumbed to this toxicity (36).
In conclusion, considering the immune contexture,
CTLs and NK cells may be useful tools for the benefit of patients
by enhancing their internal tumor infiltrating lymphocytes.
Adoptive therapy demonstrated long-term disease-free survival, a
notable improvement in quality of life and also an improved overall
survival and therefore, may be recommended to patients with no
other treatment options. Although AIET has been shown to benefit
patients, longer follow-up is required before a final conclusion
may be determined.
References
|
1
|
Gill S, Thomas RR and Goldberg RM: Review
article: colorectal cancer chemotherapy. Aliment Pharmacol Ther.
18:683–692. 2003. View Article : Google Scholar
|
|
2
|
Kalady MF, Dejulius KL, Sanchez JA, et al:
BRAF mutations in colorectal cancer are associated with distinct
clinical characteristics and worse prognosis. Dis Colon Rectum.
55:128–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldberg RM, Sargent DJ, Morton RF, et al:
A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan, and oxaliplatin combinations in patients with
previously untreated metastatic colorectal cancer. J Clin Oncol.
22:23–30. 2004. View Article : Google Scholar
|
|
4
|
Medinger M, Steinbild S and Mross K:
Adjuvant and palliative anticancer treatment of colon carcinoma in
2004. Praxis (Bern 1994). 93:1633–1644. 2004.(In German).
|
|
5
|
Köhne CH: Palliative therapy of colorectal
cancer. Onkologie. 26(Suppl 7): 41–47. 2003.(In German).
|
|
6
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tournigand C, André T, Achille E, et al:
FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: a randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goto S, Shirotani N, Hatakeyama M, et al:
Clinical benefit of non-toxic therapy in patients with advanced
cancer (opinion). Anticancer Res. 22:2461–2464. 2002.PubMed/NCBI
|
|
9
|
Gattinoni L, Powell DJ, Rosenberg SA and
Restifo NP: Adoptive immunotherapy for cancer: building on success.
Nat Rev Immunol. 6:383–393. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
zum Büschenfelde CM, Hermann C, Schmidt B,
Peschel C and Bernhard H: Antihuman epidermal growth factor
receptor 2 (HER2) monoclonal antibody trastuzumab enhances
cytolytic activity of class I-restricted HER2-specific T
lymphocytes against HER2-overexpressing tumor cells. Cancer Res.
62:2244–2247. 2002.
|
|
11
|
Terunuma H, Deng X, Dewan Z, Fujimoto S
and Yamamoto N: Potential role of NK cells in the induction of
immune responses: implications for NK cell-based immunotherapy for
cancers and viral infections. Int Rev Immunol. 27:93–110. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smyth MJ, Hayakawa Y, Takeda K and Yagita
H: New aspects of natural-killer-cell surveillance and therapy of
cancer. Nat Rev Cancer. 2:850–861. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsu KC and Dupont B: Natural killer cell
receptors: regulating innate immune responses to hematologic
malignancy. Semin Hematol. 42:91–103. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kiessling R, Klein E and Wigzell H:
‘Natural’ killer cells in the mouse. I. Cytotoxic cells with
specificity for mouse Moloney leukemia cells Specificity and
distribution according to genotype. Eur J Immunol. 5:112–117.
1975.
|
|
15
|
Kärre K, Ljunggren HG, Piontek G and
Kiessling R: Selective rejection of H-2-deficient lymphoma variants
suggests alternative immune defence strategy. Nature. 319:675–678.
1986.
|
|
16
|
Ruggeri L, Capanni M, Urbani E, et al:
Effectiveness of donor natural killer cell alloreactivity in
mismatched hematopoietic transplants. Science. 295:2097–2100. 2002.
View Article : Google Scholar
|
|
17
|
Takada M, Terunuma H, Deng X, et al:
Refractory lung metastasis from breast cancer treated with
multidisciplinary therapy including an immunological approach.
Breast Cancer. 18:64–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui Z, Willingham MC, Hicks AM, et al:
Spontaneous regression of advanced cancer: identification of a
unique genetically determined, age-dependent trait in mice. Proc
Natl Acad Sci USA. 100:6682–6687. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hicks AM, Riedlinger G, Willingham MC, et
al: Transferable anticancer innate immunity in spontaneous
regression/complete resistance mice. Proc Natl Acad Sci USA.
103:7753–7758. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gumperz JE and Parham P: The enigma of the
natural killer cell. Nature. 378:245–248. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hercend T, Farace F, Baume D, Charpentier
F, Droz JP, Triebel F and Escudier B: Immunotherapy with
lymphokine-activated natural killer cells and recombinant
interleukin-2: a feasibility trial in metastatic renal cell
carcinoma. J Biol Response Mod. 9:546–555. 1990.PubMed/NCBI
|
|
22
|
Rabinowich H, Sedlmayr P, Herberman RB and
Whiteside TL: Increased proliferation, lytic activity, and purity
of human natural killer cells cocultured with mitogen-activated
feeder cells. Cell Immunol. 135:454–470. 1991. View Article : Google Scholar
|
|
23
|
Harada H, Saijo K, Watanabe S, Tsuboi K,
Nose T, Ishiwata I and Ohno T: Selective expansion of human natural
killer cells from peripheral blood mononuclear cells by the cell
line, HFWT. Jpn J Cancer Res. 93:313–319. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Coca S, Perez-Piqueras J, Martinez D, et
al: The prognostic significance of intratumoral natural killer
cells in patients with colorectal carcinoma. Cancer. 79:2320–2328.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stinchcombe JC and Griffiths GM: Secretory
mechanisms in cell-mediated cytotoxicity. Annu Rev Cell Dev Biol.
23:495–517. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
DiLillo DJ, Yanaba K and Tedder TF: B
cells are required for optimal CD4+ and CD8+ T cell tumor immunity:
therapeutic B cell depletion enhances B16 melanoma growth in mice.
J Immunol. 184:4006–4016. 2010.
|
|
27
|
Naito Y, Saito K, Shiiba K, Ohuchi A,
Saigenji K, Nagura H and Ohtani H: CD8+ T cells infiltrated within
cancer cell nests as a prognostic factor in human colorectal
cancer. Cancer Res. 58:3491–3494. 1998.
|
|
28
|
Yu P and Fu YX: Tumor-infiltrating T
lymphocytes: friends or foes? Lab Invest. 86:231–245. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Todaro M, D’Asaro M, Caccamo N, et al:
Efficient killing of human colon cancer stem cells by gammadelta T
lymphocytes. J Immunol. 182:7287–7296. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ogino S, Nosho K, Irahara N, et al:
Lymphocytic reaction to colorectal cancer is associated with longer
survival, independent of lymph node count, microsatellite
instability, and CpG island methylator phenotype. Clin Cancer Res.
15:6412–6420. 2009. View Article : Google Scholar
|
|
31
|
Fridman WH, Pagès F, Sautès-Fridman C and
Galon J: The immune contexture in human tumours: impact on clinical
outcome. Nat Rev Cancer. 12:298–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pagès F, Kirilovsky A, Mlecnik B, et al:
In situ cytotoxic and memory T cells predict outcome in patients
with early-stage colorectal cancer. J Clin Oncol. 27:5944–5951.
2009.PubMed/NCBI
|
|
33
|
Sun JC and Bevan MJ: Defective CD8 T cell
memory following acute infection without CD4 T cell help. Science.
300:339–342. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fehniger TA, Bluman EM, Porter MM, et al:
Potential mechanisms of human natural killer cell expansion in vivo
during low-dose IL-2 therapy. J Clin Invest. 106:117–124. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rosenberg SA, Lotze MT, Yang JC, et al:
Combination therapy with interleukin-2 and alpha-interferon for the
treatment of patients with advanced cancer. J Clin Oncol.
7:1863–1874. 1989.PubMed/NCBI
|
|
36
|
Atzpodien J, Kirchner H, Hänninen EL,
Deckert M, Fenner M and Poliwoda H: Interleukin-2 in combination
with interferon-alpha and 5-fluorouracil for metastatic renal cell
cancer. Eur J Cancer. 29A(Suppl 5): S6–S8. 1993. View Article : Google Scholar : PubMed/NCBI
|