Introduction
Gliomas are the most frequent primary intracranial
tumors in adults, accounting for 80% of all primary malignant
tumors in the central nervous system (CNS) (1). The management of gliomas is sometimes
challenging due to the unconstrained growth and infiltrative nature
of glioma cells. Traditionally, debulking surgery and
post-operative radiation are the mainstay in the treatment of
gliomas but the prognosis is far from satisfactory. CNS cancers,
with a majority of gliomas, remain the second most common lethal
cancer in males younger than 40 years (2). Historically, the efficacy of
chemotherapy for gliomas is controversial due to the blockade of
chemotherapeutic agents by the blood-brain barrier (BBB) and the
insensitivity of gliomas to chemotherapy (3). However, the role of chemotherapy for
gliomas has been established with efforts in basic research and
clinical studies. In 1994, Cairncross et al demonstrated
that oligodendrial gliomas are sensitive to a chemotherapeutical
regimen containing procarbazine, lomustine and vincristine
(4). In a phase II trial, Yung
et al showed that chemotherapy with temozolomide (TMZ)
improved the prognosis of patients with anaplastic gliomas
(5). In 2005, the cornerstone
prospective randomized clinical trial performed by Stupp et
al revealed that TMZ combined with radiation significantly
improves the prognosis of newly diagnosed glioblastoma multiforme
(GBM), with a 5-year overall survival (OS) of 9.8%, compared with
that of 1.9% for radiotherapy alone (6). Chemotherapy has now become the
standard of care for malignant gliomas. In mainland China, numerous
patients with gliomas are treated every year and increasing
attention has been paid to chemotherapy. However, the history and
development of chemotherapy for gliomas in mainland China are not
well documented. In this study, a thorough literature search was
performed and a review of the field of glioma chemotherapy in
mainland China was conducted.
Materials and methods
Literature search
In August 2011, an extensive literature search was
performed to identify clinical studies reporting outcomes of glioma
patients treated with chemotherapy in mainland China. The
electronic databases of Pubmed, China Knowledge Resource Integrated
Database, Chinese Medical Association Digital Periodicals and VIP
Database for Chinese Technical Periodicals were searched. Keywords
searched included ‘glioma’, ‘glial tumor’, ‘glioblastoma’,
‘astrocytoma’, ‘oligodendroglioma’, ‘oligodendroastrocytoma’,
‘chemotherapy’, ‘drug therapy’ and ‘drug treatment’.
Selection criteria
There were no language restrictions for the searched
articles. Titles and abstracts were first examined to exclude
irrelevant diseases and treatment, and duplicates were excluded.
Studies selected were in accordance with the following criteria: i)
A clinical study had been conducted on chemotherapy for
intracranial gliomas in mainland China; ii) The number of patients
was ≥5; iii) >70% of patients were adults (≥18 years); iv)
Patients with glioma comprised ≥70% of all cases.
Data extraction and analysis
Information of publications, patient and
chemotherapy information was extracted. Collected data were
analyzed and reviewed.
Results
Publication selection
A total of 333 potentially eligible publications
were found using the search strategy and by screening titles and
abstracts. A total of 210 articles were identified to be in line
with the selection criteria, of which 160 (76.2%) were
retrospective and 50 (23.8%) were prospective. An increasing number
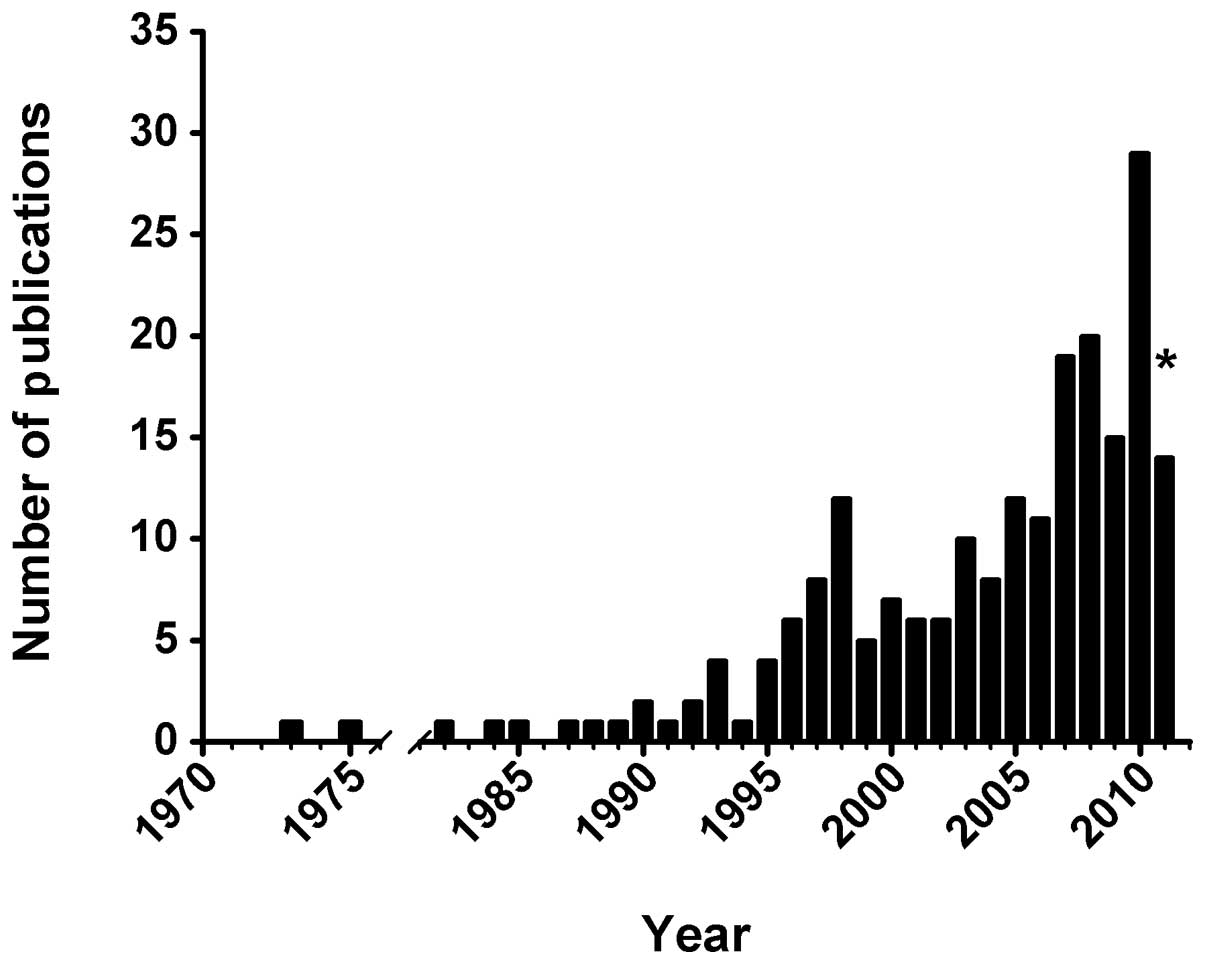
of publications have been published over time, with only 2 studies
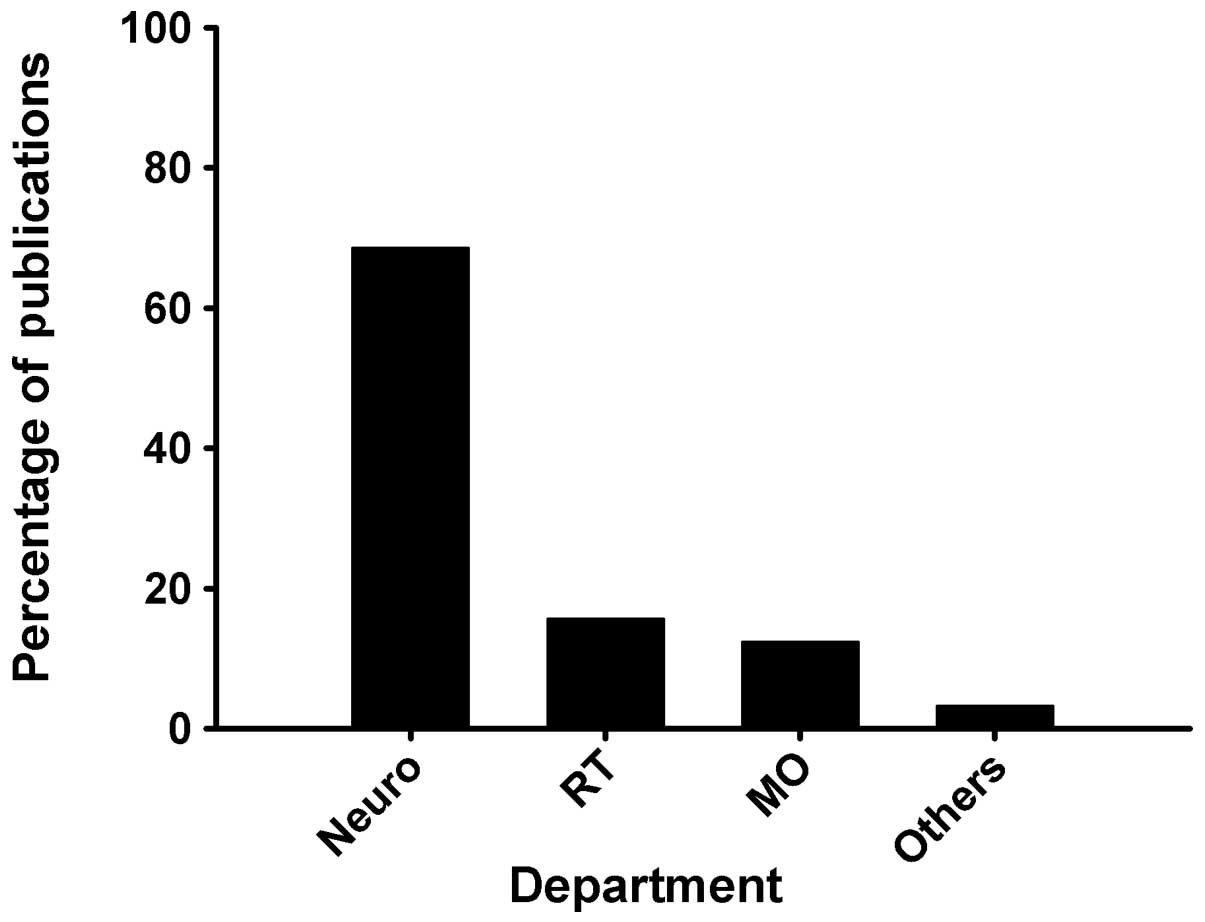
published before 1980 but 29 in 2010 (Fig. 1). Of the 210 studies, 144 (68.6%)
were performed in the Department of Neurosurgery, 33 (15.7%) in the
Department of Radiotherapy and 26 in the Department of Medical
Oncology (Fig. 2).
Patient data
In all, 10,105 patients with glioma were enrolled in
the 210 studies. The mean age of patients was 21–56 years and the
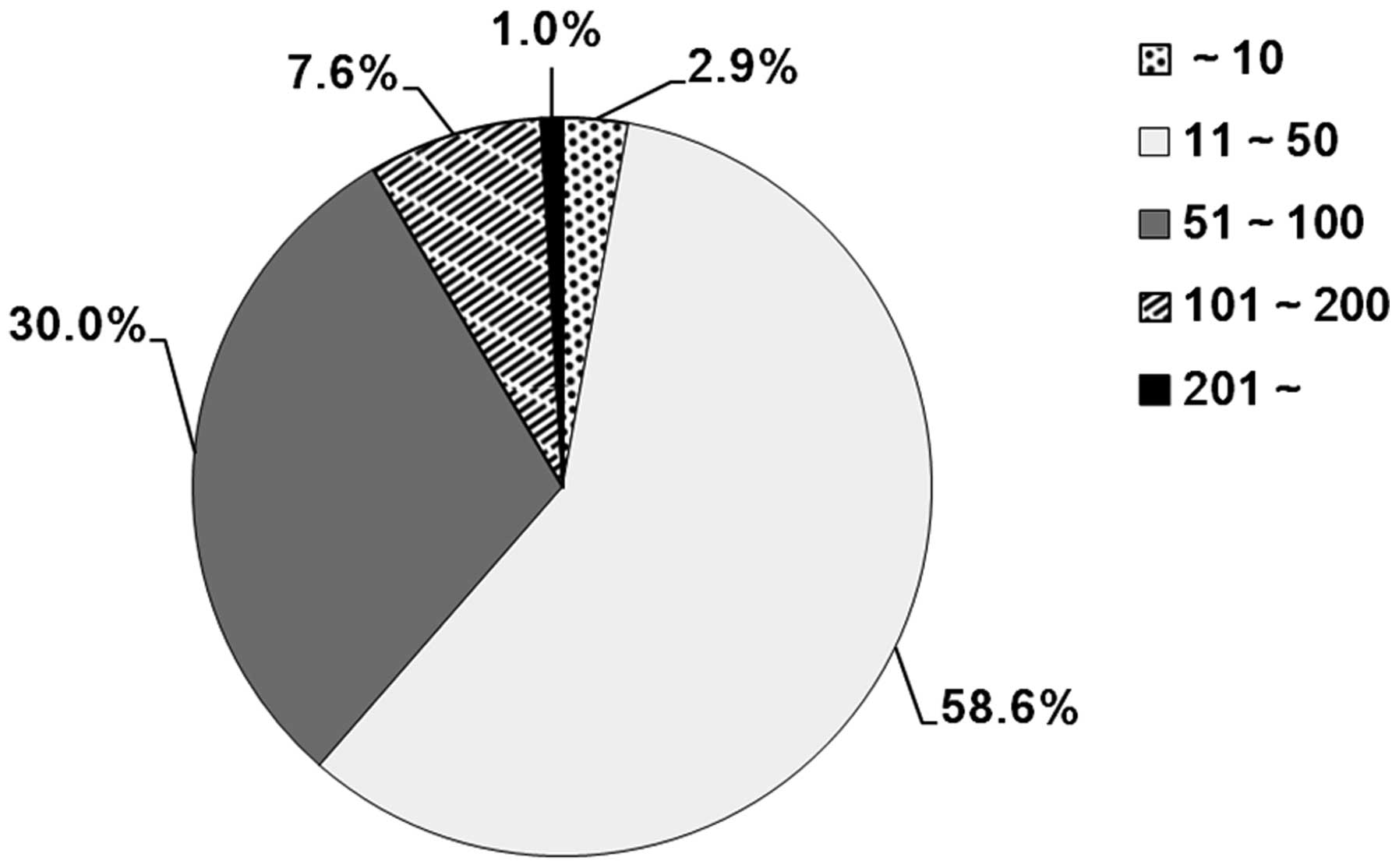
male/female ratio was 1.5:1. Of the 210 studies, 192 (91.4%)
enrolled fewer than 100 patients and only 18 (8.6%) had >100
cases in each study (Fig. 3).
Chemotherapy information
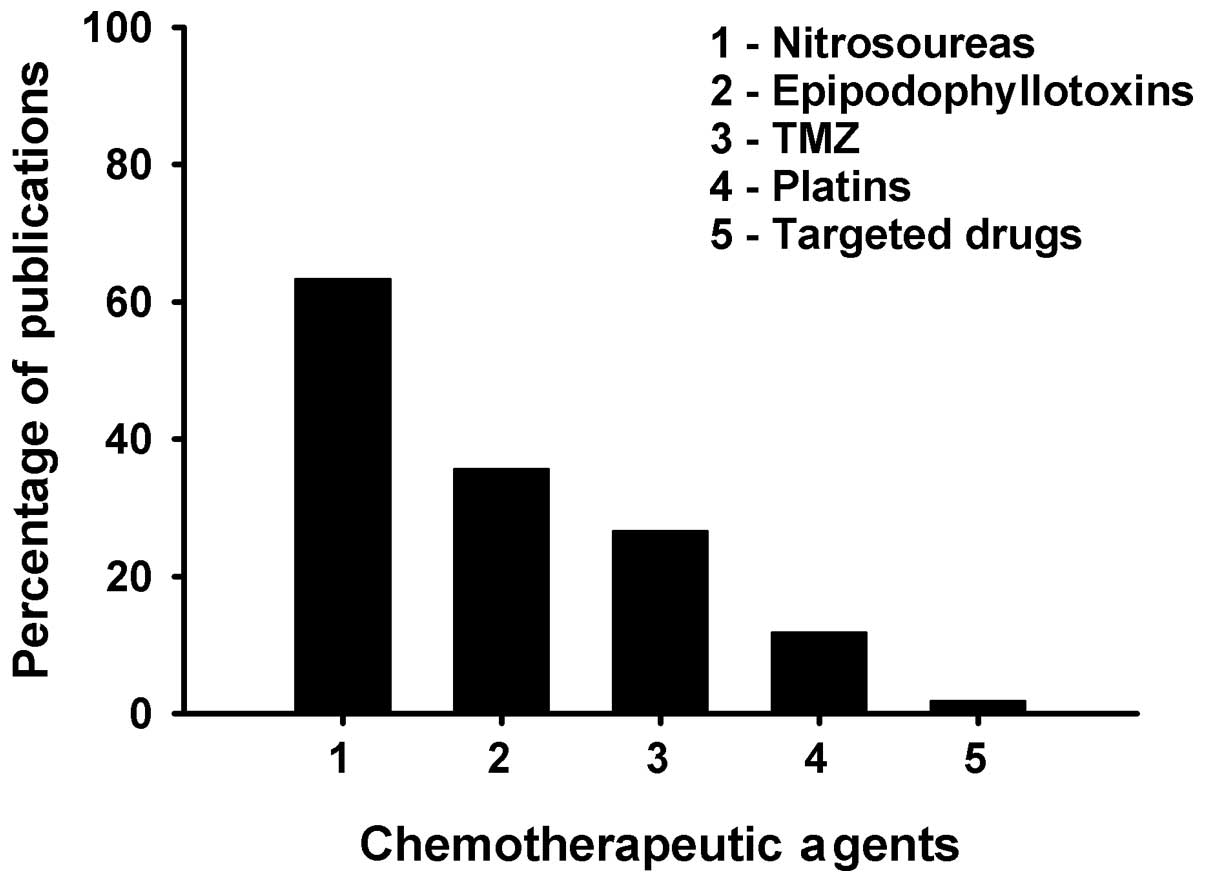
Nitrosourea drugs including nimustine (ACNU),
carmustine (BCNU), lomustine (CCNU) and semustine (MeCCNU) were the
most frequently used chemotherapeutic agents and were found in 133
(63.3%) studies. The epipodophyllotoxins were used in 75 (35.7%)
studies, TMZ in 56 (26.7%), platins in 25 (11.9%) and targeted
agents in 4 (1.9%) studies, respectively (Fig. 4). Nitrosourea-containing regimens
decreased from 100% of studies before 1990 to 59.4% of publications
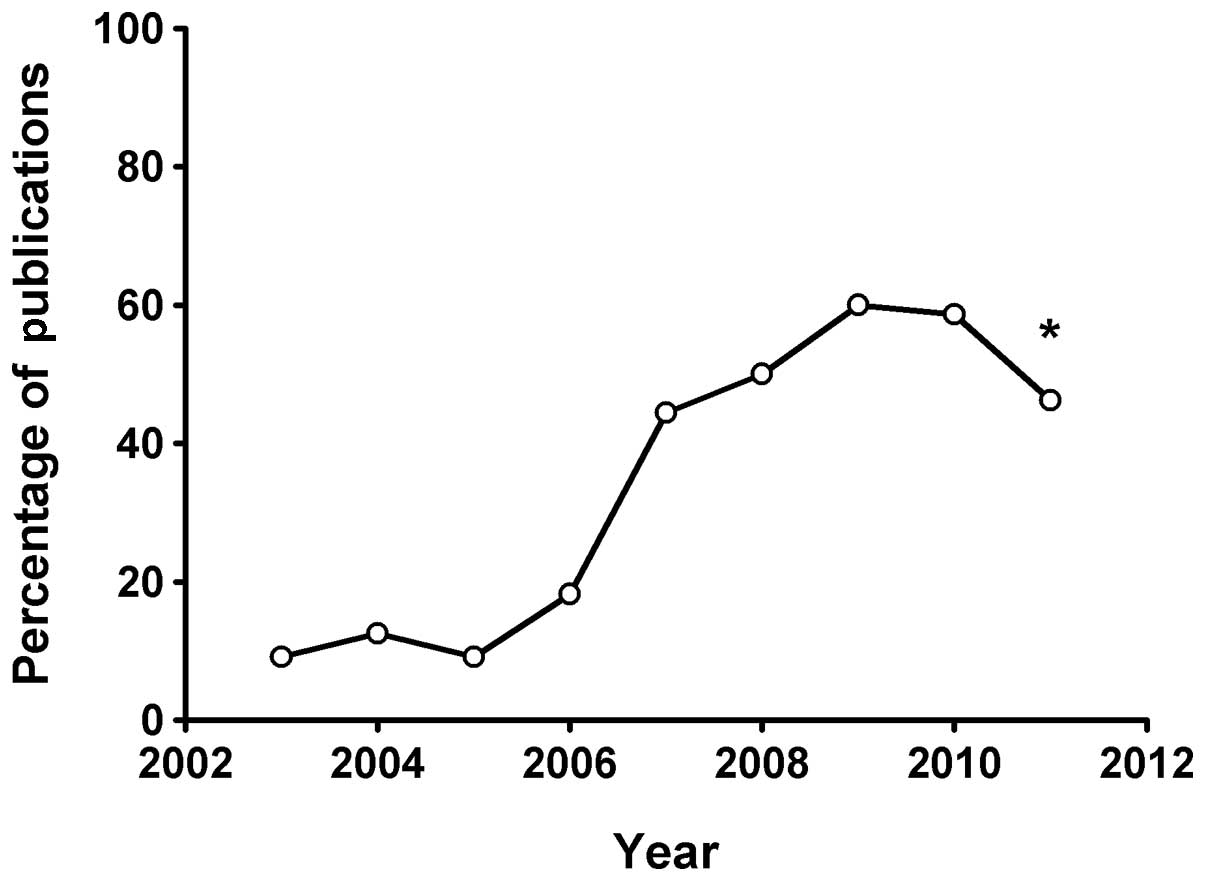
since 2000. Since 2003, TMZ has been used to treat gliomas in
mainland China and the number of publications regarding TMZ for
gliomas has gradually increased (Fig.
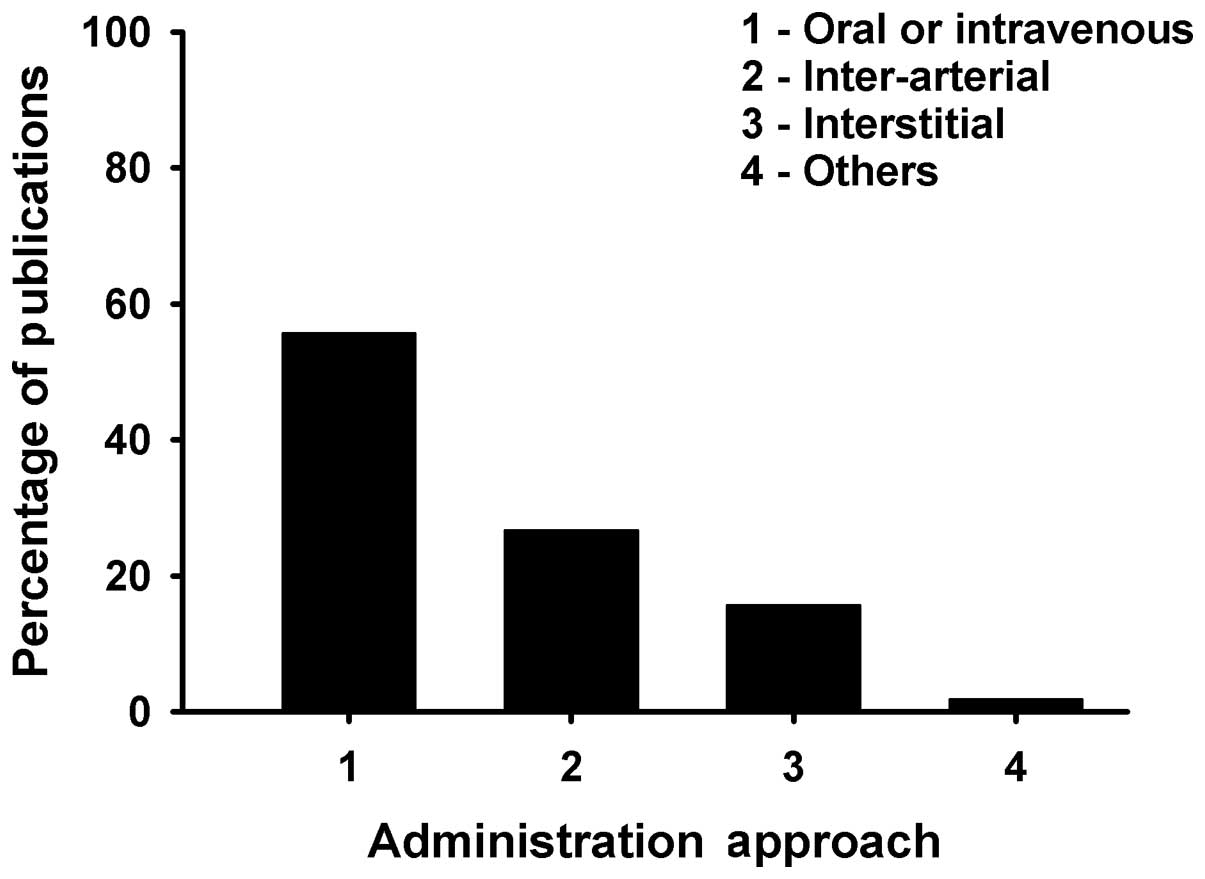
5). The majority of chemotherapy was administered orally or
intravenously, which was found in 117 (55.7%) studies.
Intra-arterial and interstitial administration were found in 56
(26.7%) and 33 (15.7%) studies, respectively (Fig. 6). Important characteristics of
patients were reported in most but not all studies. Of the 210
studies, 11 studies contained gliomas of only one pathological
grade and 190 studies had gliomas of at least two pathological
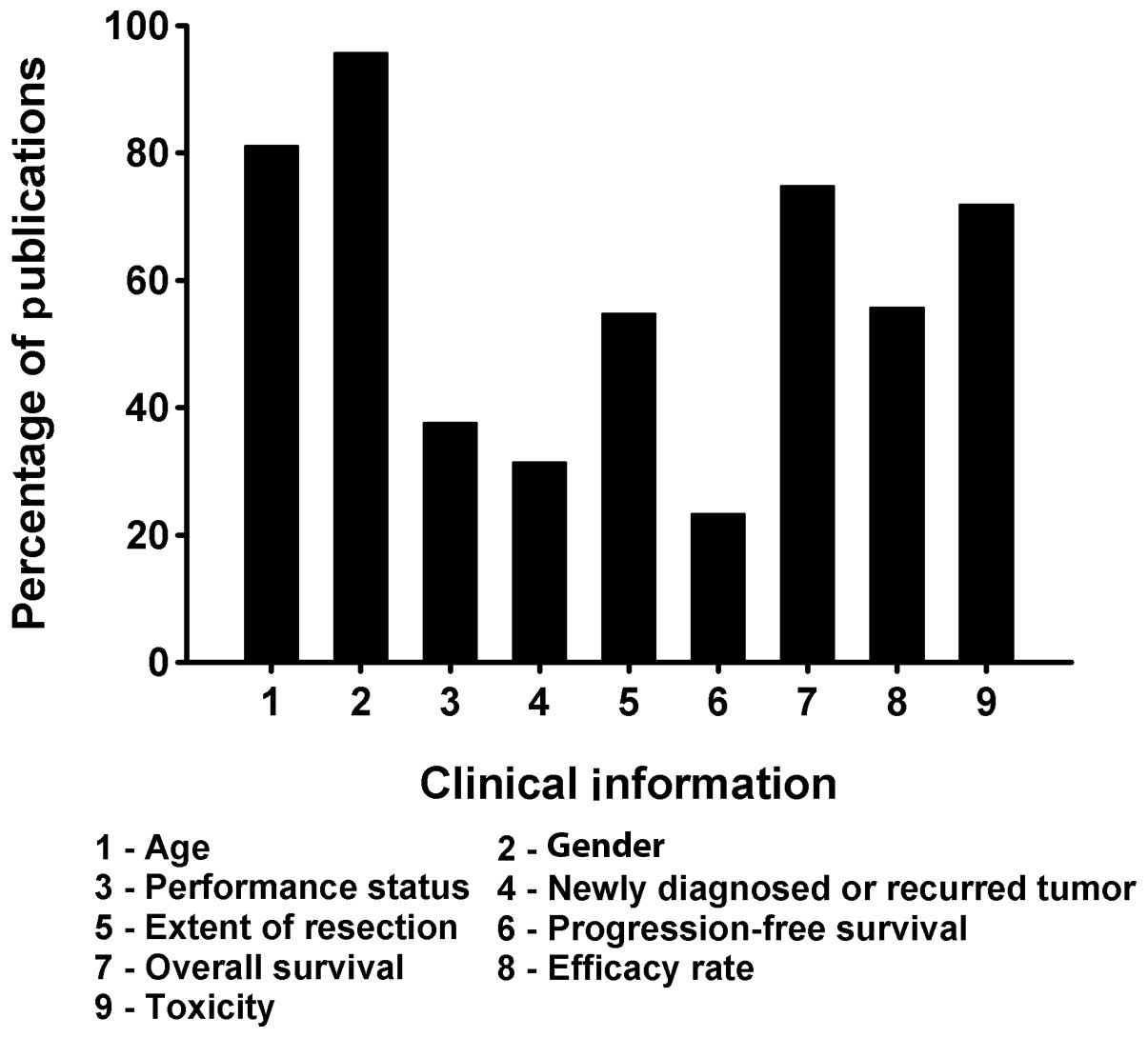
grades. Among the 210 studies, patient age was reported in 170
(81.1%) studies, gender in 201 (95.7%), Karnofsky performance score
(KPS) in 79 (37.6%), newly diagnosed or recurring tumors in 66
(31.4%), extent of tumor resection in 115 (54.8%), progression-free
survival (PFS) in 49 (23.3%), overall survival (OS) in 157 (74.8%),
efficacy rate in 117 (55.7%) and toxicity in 151 (71.9%) studies,
respectively (Fig. 7).
Discussion
Although chemotherapy has been employed as an
adjuvant treatment for glioma for more than three decades, its
efficacy as chemotherapy for glioma has been controversial. In
2002, Stewart performed a systematic review of 12 randomized trials
and demonstrated that chemotherapy significantly prolonged the
survival of adult patients with high-grade gliomas (7). In 2005, a randomized clinical trial
demonstrated the efficacy of TMZ, making TMZ the standard of care
for GBM (8). In addition, the
success of biodegradable BCNU wafers (Gliadel) and bevacizumab
(Avastin) strengthened the role of chemotherapy in the management
of gliomas (9,10). Increasing attention has been paid to
studies on glioma chemotherapy worldwide. There have been no
studies to date describing the history and development of
chemotherapy for gliomas in mainland China. In this study, the
major electronic medical databases were searched for studies on
glioma chemotherapy in mainland China and an analysis was conducted
to provide an overview.
Studies on chemotherapy for gliomas began at the
beginning of the 1970s in mainland China. In 1973, a group from
Tianjin Medical School introduced their experience of using BCNU to
treat brain tumors (11). In 1975,
a study published by neurosurgeons from Suzhou Medical School
presented preliminary results of the management of gliomas with
CCNU (12). These two retrospective
studies demonstrated the potential antitumor activity of
nitrosoureas in Chinese glioma patients and described the most
common side-effects, including gastrointestinal adverse reactions
and myelosuppression. In the following years, publications of
glioma chemotherapy in mainland China increased significantly. Only
eight studies were published during the 20 years from 1970–1990
while 29 studies were identified in 2010 alone.
Similar to the situation in most Asian countries,
chemotherapy in mainland China was mainly administered by
neurosurgeons. Among the 210 studies identified, 144 (68.6%) were
carried out in the Department of Neurosurgery, followed by 33
(15.7%) in the Department of Radiation Oncology and 26 (12.4%) in
the Department of Medical Oncology. One of the reasons for this may
be that neurosurgeons are capable of administering chemotherapeutic
agents through routes other than oral and intravenous, such as
arterial and interstitial delivery. Another reason may be that
neurological complications during chemotherapy and disease
progression may be managed by neurosurgeons with more confidence
and efficiency. With the development of neuro-oncology in mainland
China in the past 10 years, physicians with expertise of oncology
have been trained and recruited by the Department of Neurosurgery
to administer chemotherapy to glioma patients. The increasing
number of neuro-oncologists in mainland China will provide glioma
patients with better and more specialized care and focus on both
clinical and basic research in the field of neuro-oncology.
Nitrosoureas were the most common agents used as
chemotherapy for Chinese glioma patients. The earliest two studies
identified explored the efficacy of nitrosoureas (11,12).
Nitrosoureas were predominantly employed in the majority of
studies, accounting for 63.3% of the 210 studies. However, due to
the toxicity and the chemoresistance of glioma cells, the number of
reports of nitrosourea-containing regimens has declined from 100%
of studies published before 1990 to 59.4% of publications after
2000. Other chemotherapeutic agents have been adopted to treat
Chinese glioma patients. Of the 210 studies, epipodophyllotoxins
were used in 75 (35.7%) and platins in 25 (11.9%). The earliest
report focusing on TMZ, a novel oral alkylating agent with low
toxicity for the management of Chinese patients with gliomas was
published in 2003. In the study, the authors retrospectively
investigated the anti-tumor activity of TMZ in 17 patients with
malignant gliomas. An objective response rate of 47.1% and a
6-month survival rate of 58.8% were observed (13). Once the efficacy of TMZ was
confirmed by a large well-designed clinical trial by Stupp et
al, the publications of chemotherapy with TMZ in Chinese glioma
patients significantly increased (6). In 2003, 9.1% of studies explored
chemotherapy with TMZ while 58.6% of publications in 2010 used
TMZ-containing regimens.
Multiple approaches have been investigated to
deliver chemotherapeutic agents for gliomas in mainland China.
Among the 210 studies examined, oral or intravenous administration
was found in 55.7% of studies, intra-arterial administration in
26.7% and interstitial administration in 15.7% of studies. There
are pharmacological rationales for intra-arterial administration.
When a drug is delivered intraarterially, it results in an
augmentation of the local peak plasma concentration and the area
under the curve (AUC) of the drug, compared with the conventional
intravenous dose. Mathematical modeling predicted up to a five-fold
increase in drug delivery by intra-arterial administration as
defined by the concentration-time integral (14). A study using positron emission
tomography and 11C-labeled BCNU demonstrated that
superselective intra-arterial infusion in patients with recurrent
gliomas resulted in an ∼50-fold increase in drug administration
compared with intravenous delivery (15). Although retrospective intra-arterial
studies reported some positive results in mainland China, no
survival benefit of intra-arterial infusion for glioma patients has
been proven in phase III trials overseas (16). Thus, intra-arterial chemotherapy for
patients with glioma is diminishing in mainland China.
Interstitial chemotherapy is also an appealing
method to administer chemotherapeutic drugs because local delivery
of drugs in the tumor bed potentially overcomes systemic toxicities
and limitations in traversing the blood-brain barrier by systemic
agents. Until now, Gliadel wafers have been the most successful
commercially available interstitial chemotherapy drug for gliomas.
A large phase III clinical trial demonstrated that this BCNU-loaded
polymer prolonged the median survival of patients with primary
malignant glioma from 11.6 to 13.9 months compared with the control
group without significant toxicity (17). Based on its safety and efficacy,
Gliadel wafers are recommended for newly diagnosed and recurrent
GBM by National Comprehensive Cancer Network (NCCN) guidelines. In
mainland China, the following interstitial drug delivery methods in
glioma patients were investigated: i) Administration of
chemotherapeutic agents through stereotaxic surgery (18); ii) Implantation of materials
containing drugs in the tumor bed during neurosurgery (19); iii) Infusion of chemotherapeutic
agents to the post-operative cavity through a subcutaneous
reservoir (20). Potential efficacy
was observed in the interstitial chemotherapy studies. The small
sample size and the retrospective nature of these studies, however,
makes prospective trials necessary to confirm the results.
Recently, unpublished phase I trial data revealed that
biodegradable BCNU-loaded polymers, developed by Chinese
investigators, with a higher dosage and more stable release of drug
compared with Gliadel wafers, are well-tolerated in patients with
recurrent malignant gliomas. A large randomized trial has been
launched to evaluate the efficacy of this interstitial
chemotherapy.
There are challenges in chemotherapy studies for
glioma patients in mainland China. Firstly, the majority of the
identified studies were retrospective with a small sample size. Of
the 210 studies, only 50 (23.8%) were prospective. In addition,
despite a total of 10,105 cases enrolled, 91.4% of all 210 studies
enrolled fewer than 100 patients and only two studies had a sample
size of >200 patients. The retrospective nature and small sample
size will result in bias and weaken the scientific strength of the
studies. Secondly, improvement in the reporting of studies is
required. A proper report of the methodology and detailed results
of a study are necessary in order to understand the significance of
the study and to compare it with others. In the studies identified,
certain important information was missing. For example, performance
status and extent of tumor resection were only reported in 37.6 and
54.8% of all 210 studies, respectively. These are well-known
prognostic factors for glioma patients (21). If this information is missing, the
quality and reliability of a study may potentially be impaired.
Thirdly, more effort is required for studies on targeted therapy in
glioma patients in mainland China. With advances in molecular
biology, disruption of signaling pathways has been revealed to be
responsible for the development, progression and treatment
resistance of gliomas. Strategies specifically targeting
abnormalities in molecular pathways theoretically have better
efficacy and safety profiles than systemic cytotoxic chemotherapy
(22). The success of bevacizumab,
a monoclonal antibody targeting angiogenic pathways, has created an
evolving therapeutic landscape for gliomas. Many international
clinical trials on the efficacy of small molecule drugs are
ongoing. Studies on targeted therapy for glioma patients in
mainland China has just begun. Only 1.9% of the studies identified
according to our selection criteria involved inhibitors targeting
epidermal growth factor (EGF) or other potential angiogenic
pathways.
In this study, an overview of chemotherapy for
glioma in mainland China is presented. The role of chemotherapy in
the management of gliomas is well-recognized. The investigation
into the efficacy of chemotherapy in glioma patients began in the
1970s in mainland China and multiple disciplines participate.
Different chemotherapeutic agents and various methods of drug
delivery have been tested. The rarity of randomized controlled
trials (RCTs) with high quality results is a major limitation. Much
effort and collaboration should, therefore, be made to conduct
well-designed multicenter RCTs on chemotherapy, with the aim of
improving the prognosis of patients with glioma.
References
|
1
|
Schwartzbaum JA, Fisher JL, Aldape KD and
Wrensch M: Epidemiology and molecular pathology of glioma. Nat Clin
Pract Neurol. 2:494–503; quiz 1 p following 516. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
3
|
Minniti G, Muni R, Lanzetta G, Marchetti P
and Enrici RM: Chemotherapy for glioblastoma: current treatment and
future perspectives for cytotoxic and targeted agents. Anticancer
Res. 29:5171–5184. 2009.PubMed/NCBI
|
|
4
|
Cairncross G, Macdonald D, Ludwin S, et
al: Chemotherapy for anaplastic oligodendroglioma. National Cancer
Institute of Canada Clinical Trials. Group J Clin Oncol.
12:2013–2021. 1994.PubMed/NCBI
|
|
5
|
Yung WK, Prados MD, Yaya-Tur R, et al:
Multicenter phase II trial of temozolomide in patients with
anaplastic astrocytoma or anaplastic oligoastrocytoma at first
relapse. Temodal Brain Tumor. Group J Clin Oncol. 17:2762–2771.
1999.PubMed/NCBI
|
|
6
|
Stupp R, Hegi ME, Mason WP, et al: Effects
of radiotherapy with concomitant and adjuvant temozolomide versus
radiotherapy alone on survival in glioblastoma in a randomised
phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet
Oncol. 10:459–466. 2009.
|
|
7
|
Stewart LA: Chemotherapy in adult
high-grade glioma: a systematic review and meta-analysis of
individual patient data from 12 randomised trials. Lancet.
359:1011–1018. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stupp R, Mason WP, van den Bent MJ, et al:
Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hart MG, Grant R, Garside R, Rogers G,
Somerville M and Stein K: Chemotherapy wafers for high grade
glioma. Cochrane Database Syst Rev CD. 0072942011.PubMed/NCBI
|
|
10
|
Friedman HS, Prados MD, Wen PY, et al:
Bevacizumab alone and in combination with irinotecan in recurrent
glioblastoma. J Clin Oncol. 27:4733–4740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tianjin Medical School Affliated Hospital:
Preliminary study on BCNU in the treatment of intracranial tumors:
report of 45 cases. Tianjin Med J. 15:26–28. 1973.(In Chinese).
|
|
12
|
The First Affliated Hospital to Suzhou
Medical School: Preliminary observation of treating gliomas in
central nervous system with CCNU. Chin J Nerv Ment Dis. 1:51–53.
1975.(In Chinese).
|
|
13
|
Zeng X and Yang S: Clinical observation in
chemotherapy with temozolomide alone in postoperative malignant
primary cerebral glioma. Mod J Neurol Neurosurg. 3:270–273.
2003.
|
|
14
|
Newton HB: Intra-arterial chemotherapy of
primary brain tumors. Curr Treat Options Oncol. 6:519–530. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tyler JL, Yamamoto YL, Diksic M, et al:
Pharmacokinetics of superselective intra-arterial and intravenous
[11C]BCNU evaluated by PET. J Nucl Med. 27:775–780. 1986.PubMed/NCBI
|
|
16
|
Shapiro WR, Green SB, Burger PC, et al: A
randomized comparison of intra-arterial versus intravenous BCNU,
with or without intravenous 5-fluorouracil, for newly diagnosed
patients with malignant glioma. J Neurosurg. 76:772–781. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Westphal M, Hilt DC, Bortey E, et al: A
phase 3 trial of local chemotherapy with biodegradable carmustine
(BCNU) wafers (Gliadel wafers) in patients with primary malignant
glioma. Neuro Oncol. 5:79–88. 2003.PubMed/NCBI
|
|
18
|
Lin Y, Chen W, Zhuge Q, et al: Combined
CT-guided stereo-tactic interstitial radiotherapy of 32P and MTX
chemotherapy for treatment of deep brain gliomas. Chin J Neurosurg.
12:216–218. 1996.(In Chinese).
|
|
19
|
Qi S and Qiu B: Treatment of recurrent
malignant brain gliomas by surgical excision combined with
biodegradable polymers of interstitial chemotherapy. Zhonghua Zhong
Liu Za Zhi. 26:58–61. 2004.(In Chinese).
|
|
20
|
Li A, Zhang X, Yi Y, et al: Interstitial
chemotherapy for malignant chemotherapy. Chin J Clin Neurosurg.
4:24–26. 1999.(In Chinese).
|
|
21
|
Buckner JC: Factors influencing survival
in high-grade gliomas. Semin Oncol. 30:10–14. 2003. View Article : Google Scholar
|
|
22
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: the avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010.PubMed/NCBI
|