Introduction
Squamous cell carcinoma of the head and neck (SCCHN)
is one of the most common cancers that result in mortality,
accounting for 6% of all cancers worldwide (1,2). The
overall 5-year survival rate for patients with this type of cancer
is among the lowest of the major cancer types (3). Despite the continuous improvement in
surgical procedures in the past few decades, the 5-year survival
rate of patients has not increased (4). One of the main reasons for this is the
lack of molecular understanding with regard to head and neck cancer
and the clinical benefits to patients. Therefore, a deeper
understanding of carcinogenesis associated with early diagnosis and
metastasis are required for the treatment of SCCHN.
Currently, the biological study focus is
transitioning from the cloning of novel genes to characterizing the
function of the protein product. The ECRG4 gene (GenBank accession
no. AF 325503) was initially identified and cloned in the State Key
Laboratory of Molecular Oncology and the Department of Etiology and
Carcinogenesis in Peking Union Medical College (Peking, China) from
normal human esophageal epithelium (5,6). The
ECRG4 gene was first described as a novel tumor suppressor gene
associated with prognosis in esophageal squamous cell carcinoma
(ESCC). ECRG4 RNA or protein was used as an independent prognostic
factor for ESCC (7,8). Subsequently, it was reported that
ECRG4 was also involved in certain tumors, including colorectal
carcinoma, prostate cancer, T-cell leukemia, gastric cancer and
glioma (9–13). Epigenetic alterations in cancer
include changes in the chromatin structure and in methylation of
cytosine residues in the DNA (14).
The head and neck epithelium is anatomically adjacent to the
esophageal epithelium and although the majority of head and neck
cancers are squamous carcinomas, no studies are available regarding
the effects of ECRG4 on SCCHN.
Thus, the present study demonstrated the role of
ECRG4 in the growth and invasiveness of SCCHN in vitro and
in vivo, in order to explore new approaches for the
diagnosis and treatment of SCCHN.
Materials and methods
Cell lines and cell culture
The SCCHN cell line, M2, is a metastatic cell line
capable of generating lymph node metastasis in vivo. M2
cells are derivatives of Tu686 created through repeated in
vivo selection in nude mice from a lymph node metastasis of the
same patient (15). Tu686 was
established from a primary tumor in the base of a human tongue. In
the present study, the M2 cells were a gift from the Emory
University Winship Cancer Institute, Atlanta, Georgia. The cell
lines were maintained as monolayer cultures in Dulbecco’s modified
Eagle’s medium (DMEM)/F12 medium (1:1) supplemented with 10% fetal
bovine serum (FBS), 100 IU/ml penicillin and 100 IU/ml streptomycin
at 37°C in a humidified atmosphere, with 5% CO2.
Construction of eukaryotic ECRG4
expression vector and stable transfection
The ECRG4 open reading frame was amplified from cDNA
clone IMAGE: 5260075 using the primers designed as follows:
forward, 5′gcaagcttatggctg cctcccccgcgcg3′ and reverse,
5′gcggatccttagtagtcatcgtagttga3′. Subsequent to being digested with
HindIII and BamHI, the coding region of the ECRG4
cDNA was subcloned into the eukaryotic expression vector,
pFLAG-CMV-2 (Sigma, St Louis, MO, USA). The reconstructive plasmid
was named pFLAG-CMV-2-ECRG4 and was fully sequenced to ensure that
no mutation was introduced during the PCR amplification. The M2
cells were transfected with pFLAG-CMV-2-ECRG4 using lipofectamine™
2000 (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. For constructing the stable clones,
cells were selected with 2000 to 3000 μg/ml G418
(Calbiochem, La Jolla, CA, USA) 48 h post-transfection. The
transfection efficiency was detected by RT-PCR and western blot
analysis as mentioned below.
Real-time PCR
The total RNA of the M2 cells which had undergone
varying treatments were isolated by Trizol (Invitrogen, Carlsbad,
CA, USA) The total RNA (1 μg) was reverse transcribed by a
RT-PCR kit (Toyobo Corporation, Osaka, Japan), according to the
manufacturer’s instructions. The primers were designed based on
previous studies (8,16) and were synthesized by Invitrogen.
The primers were designed as follows: ECRG4 forward,
5′-ttccttggcagcctgaagcg-3′ and reverse, 5′-ggctccatg
cctaaagccgt-3′; GAPDH forward, 5′-gtcagtggtggacctgacct-3′ and
reverse, 5′-tgaggaggggagattca-3′. RT products (1 μl) were
amplified by PCR at 95°C for 1 min, followed by 30 cycles at 95°C
for 10 sec, 60°C for 2 sec and 72°C for 30 sec and a final
extension at 4°C for 5 min. The PCR product (8 μl) was then
electrophoresed on a 1.2% agarose gel and the intensity of the
bands was quantified by FluorChem FC2 (Alpha Innotech, San Leandro,
CA, USA). The PCR experiments were repeated at least three
times.
Western blot analysis
The whole cell lysates were prepared. A total of 50
μg protein from each sample was mixed with gel loading
buffer (2X: 125 mM Tris-HCl, pH 6.8, 4% SDS, 20% glycerol, 0.1%
bromophenol blue and 2.5% β-mercaptoethanol), boiled for 5 min,
separated by 8% SDS-polyacrylamide gel and then transferred onto
polyvinylidene difluoride membranes. The blotted membranes were
incubated with anti-ECRG4 (sc-135139, dilution 1:300), anti-cyclin
A (sc-271682, dilution 1:500), anti-cyclin E (sc-25303, dilution
1:500), anti-Bax (sc-20067, dilution 1:300), anti-Bcl-2 (sc-509,
dilution 1:300), anti-AKT (sc-5298, dilution 1:300), anti-p-AKT
(sc-101629, dilution 1:300) and anti-E-cadherin (sc-21791, dilution
1:500) at 4°C overnight (all antibodies were from Santa Cruz
Biotechnology Inc, Santa Cruz, CA, USA). Subsequent to being
washed, the membranes were incubated with HRP-labeled anti-rabbit
or anti-mouse for 1 h at room temperature. Bands were visualized by
employing the BeyoECL Plus Detection System (Beyotime Beyotime
Institute of Biotechnology, Jiangsu, China). Protein expression
levels were quantified by FluorChem FC2 and represented as the
densitometric ratio of the targeted protein to β-actin. Cell
protein lysates were assayed in triplicate.
Cell proliferation assays
An MTT assay was employed to detect the cell
proliferations. The transfected cells were seeded onto 96-well
plates at a density of 2.5×103 cells/well. The medium
was replaced with fresh medium containing 5 mg/ml MTT reagent
(Sigma, St. Louis, MO, USA) following various durations of
culturing (a total of 7 days). The cells were cultured for another
4 h at 37°C, then 100 μl DMSO was added to each well and
mixed vigorously to solubilize the colored crystals produced within
the living cells. The absorbance at 490 nm was measured using a
BIO-TEK microplate reader (Bio-Rad, Hercules, CA, USA). Each
experiment was repeated in triplicate.
Flow cytometric analysis of cell cycle
arrest and apoptosis
The transfected cells were seeded onto a 6-well
plate at a density of 105 cells per well in
RPMI-(DMEM)/F12 medium for 48 h. For the cell cycle analysis,
following incubation, the cells were harvested and fixed in
ice-cold 70% (v/v) ethanol for 15 min. The cells were then treated
with RNase A and stained with 50 μg/ml propidium iodide
(PI), followed by incubation at 37°C for 30 min in the dark.
Samples were analyzed by a FACScan flow cytometer (Becton
Dickinson, Franklin Lakes, NJ, USA), according to the
manufacturer’s instructions. The DNA content in the G1,
S and G/M phases was analyzed using BD FACSDiva™ software (Becton
Dickinson). The analysis of apoptosis was performed using an
ApopNexin™ FITC Apoptosis Detection kit (Millipore, Lake Placid,
NY, USA). Briefly, the cells were collected and washed twice with
cold PBS and re-suspended in 200 ml 1X binding buffer, prior to 10
μl annexin V-FITC and 20 μg/ml PI being added.
Following gentle vortex mixing, the cells were incubated in the
dark for another 20 min at room temperature. The samples were
analyzed by a FACScanto™ II flow cytometer (Becton Dickinson). All
experiments were performed in triplicate.
Matrigel™ invasion and scratch
assays
The cells were seeded at 2.5×104 cells
per well on Matrigel coated inserts (8 μm pores; BD
Bioscience) in serum free medium. Following a 48 h incubation, the
cells which penetrated the filters were stained with gentian
violet. The number of invasive cells was determined by counting all
cells attached to the bottom of the inserts under an inverted
microscope at ×10 magnification. All experiments were conducted
independently in triplicate.
For the scratch assay, each well of the 6-well
plates was marked with five straight black lines. The cells were
seeded onto the plates for 12 h in complete medium
(5×105 cells per well). Scratch wounds were applied in
each well with a 200-μl pipette tip and the non-adherent
cells were washed off with medium. Fresh serum free medium was
added to the wells and the cells were incubated for ≤48 h. Inverted
microscope images were captured at 0 and 48 h subsequent to
scratching. All experiments were conducted independently in
triplicate.
Nude mouse experiments
Male, 5-week-old BALB/c nude mice were purchased
from the Institute of Laboratory Animal Sciences (Beijing, China).
The mice were then observed daily for their diet consumption,
stools and mental state, and the tumor size and body weight were
measured every three days. A total of 50 ml cell solution from the
varying groups (control, vector and ECRG4, each containing
2.5×106 tumor cells) were injected into the mylohyoid
muscle of three groups of mice (each containing 8 mice) two weeks
after arrival. The lengths and widths of the tumors were measured
with Vernier calipers and calculated using the following formula:
Tumor volume = length × width2 × 0.5. The mice were
sacrificed 25 days later in accordance with institutional
regulations for animal experiments. The use of animals in the
present study complies with the Guide for the Care and Use of
laboratory Animals. The study was approved by the Institutional
Animal Care and Use Committee, Wuxi, Jiangsu, China.
Statistical analysis
The SPSS 17.0 for Windows statistical analysis
software package (SPSS, Inc., Chicago, IL, USA) was employed for
the analysis of the data. The Student’s t-test and Mann-Whitney U
test were used for the statistical analysis of the data. P<0.05
was considered to indicate a statistically significant
difference.
Results
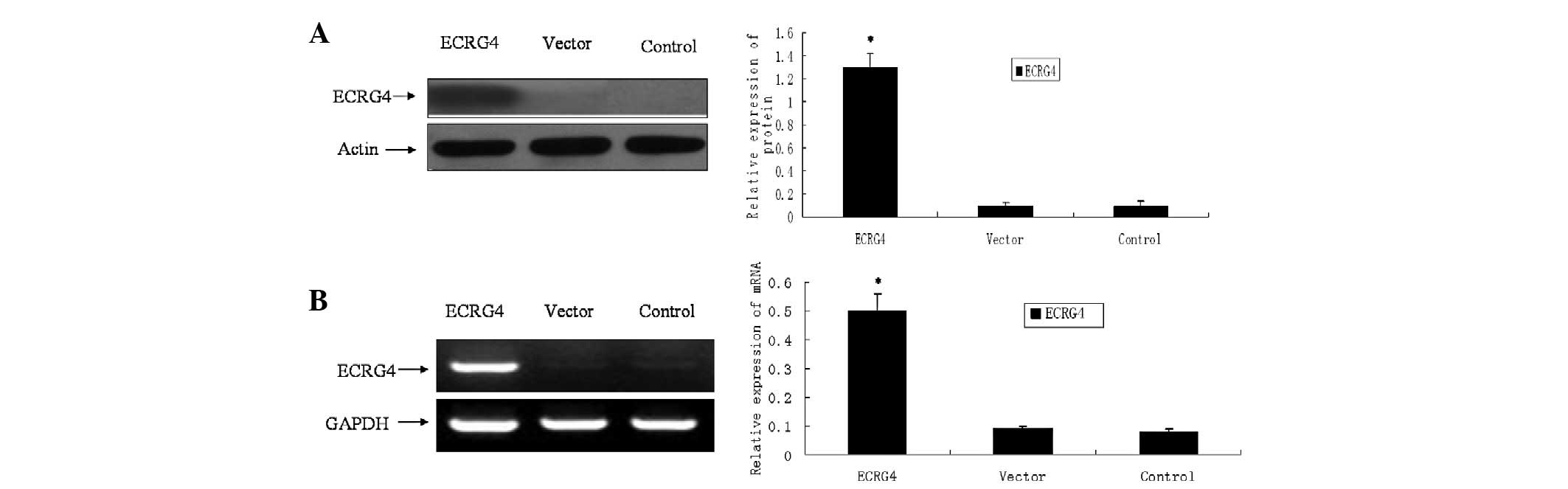
Overexpression of ECRG4 in SCCHN cell
lines following stable transfection
pFLAG-CMV-2-ECRG4 was stably transfected into M2
cells to upregulate the expression of the ECRG4 gene. The control
or vector consisted of cells without interference or those
transfected with pFLAG-CMV-2, respectively. As shown in Fig 1, the selected clones were able to
overexpress ECRG4. In the control and vector cells, the ECRG4 mRNA
and protein were minimally expressed in the cells. However, the two
were significantly upregulated following transfection with
pFLAG-CMV-2-ECRG4 (Fig. 1).
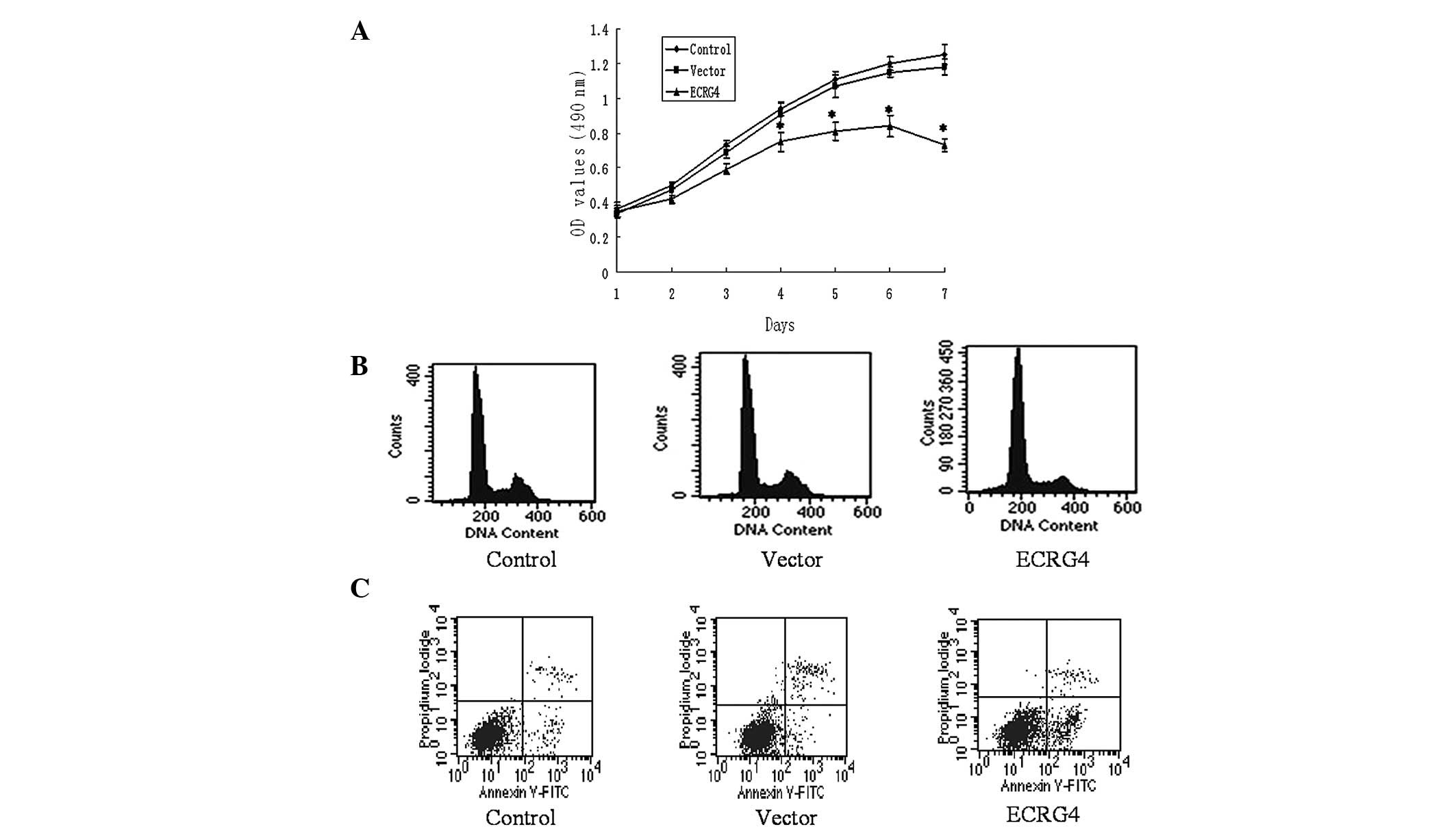
Overexpression of ECRG4 inhibits cell
proliferation of M2 cells in vitro
In order to reveal the effect of the ECRG4 gene on
SCCHN cell proliferation in vitro, an MTT assay was applied
to the M2 cells and the growth curve was obtained. As presented in
Fig. 2A, the cells of the
experimental group grew significantly slower than the cells of the
control and vector groups. This indicates that ECRG4 may inhibit
the proliferation of SCCHN cells.
Overexpression of ECRG4 induces cell
cycle arrest in G0/G1 phase and promotes apoptosis in M2 cells
To further demonstrate the mechanism of
ECRG4-induced cell growth inhibition in the M2 cells, flow
cytometry was performed to examine the cell cycle and apoptosis.
The cytometric analysis showed that the percentage of
G0/G1 phase cells in the experimental group
was 76.74±3.22, which was significantly more than those identified
in the control and vector groups (59.31±2.04 and 61.49±1.82%,
respectively; P<0.05). The apoptotic rate of the cells
significantly increased post-transfection (16.30±4.36 vs. 4.07±4.70
and 3.91±3.59%; P<0.05; Fig. 2B and
C).
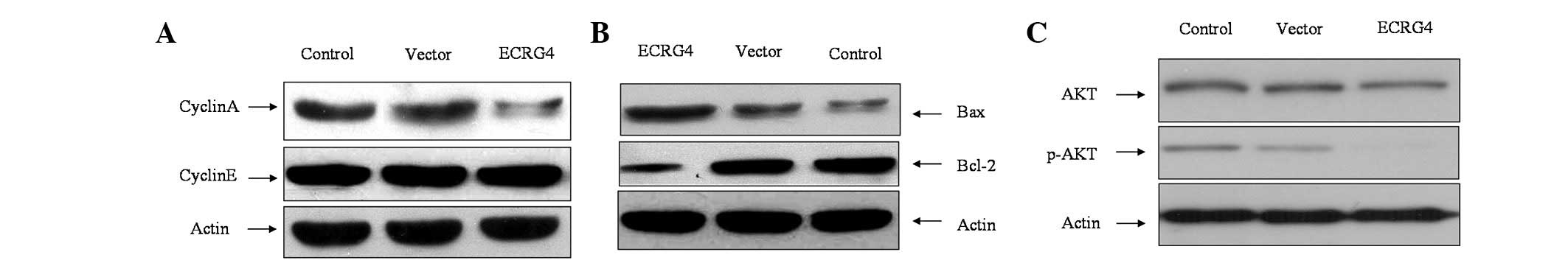
Effect of ECRG4 gene on cell cycle and
apoptosis-related proteins
To investigate the mechanism involved in ECRG4
gene-induced G0/G1 cell cycle arrest, the
cyclins were examined. As shown in Fig.
3A, Cyclin A expression decreased significantly, while there
was no difference in the expression of Cyclin E. To investigate the
potential mechanism involved in ECRG4-induced apoptosis, the
expression of Bax and Bcl-2 was detected. The results showed that
following transfection, Bax expression was significantly increased
and Bcl-2 expression was decreased (Fig. 3B).
ECRG4 inhibits phosphorylation of
AKT
To further investigate the potential molecular
mechanism involved in ECRG4-induced cell growth inhibition, the
expression of p-AKT was examined. It was shown that AKT
phosphorylation was significantly inhibited, which suggests that
ECRG4 may be involved in the PI3K/AKT pathway in SCCHN (Fig. 3C).
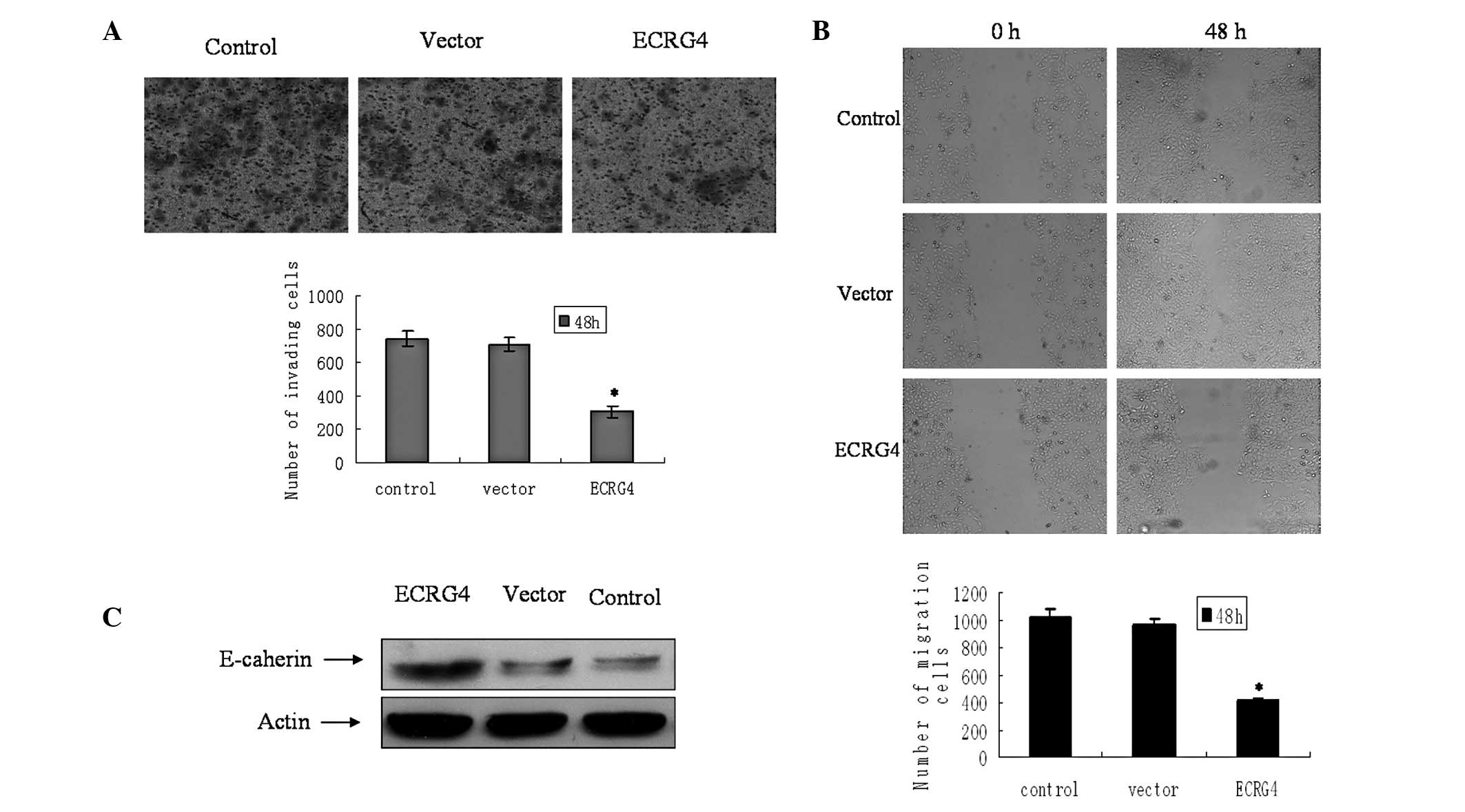
ECRG4 inhibits cell invasiveness and
migration in M2 cells
The effect of ECRG4 on cell invasion was measured in
the M2 cells. The cells which penetrated through the filters to the
other side of the inserts in the experimental group were fewer in
number compared with those in the control and vector groups
(Fig. 4A). The capability for
migration following transfection was then evaluated. The cells
transfected with ECRG4 showed lower motility and achieved less
wound closure at 48 h (Fig. 4B).
The epithelial marker E-cadherin was also significantly upregulated
(Fig. 3C).
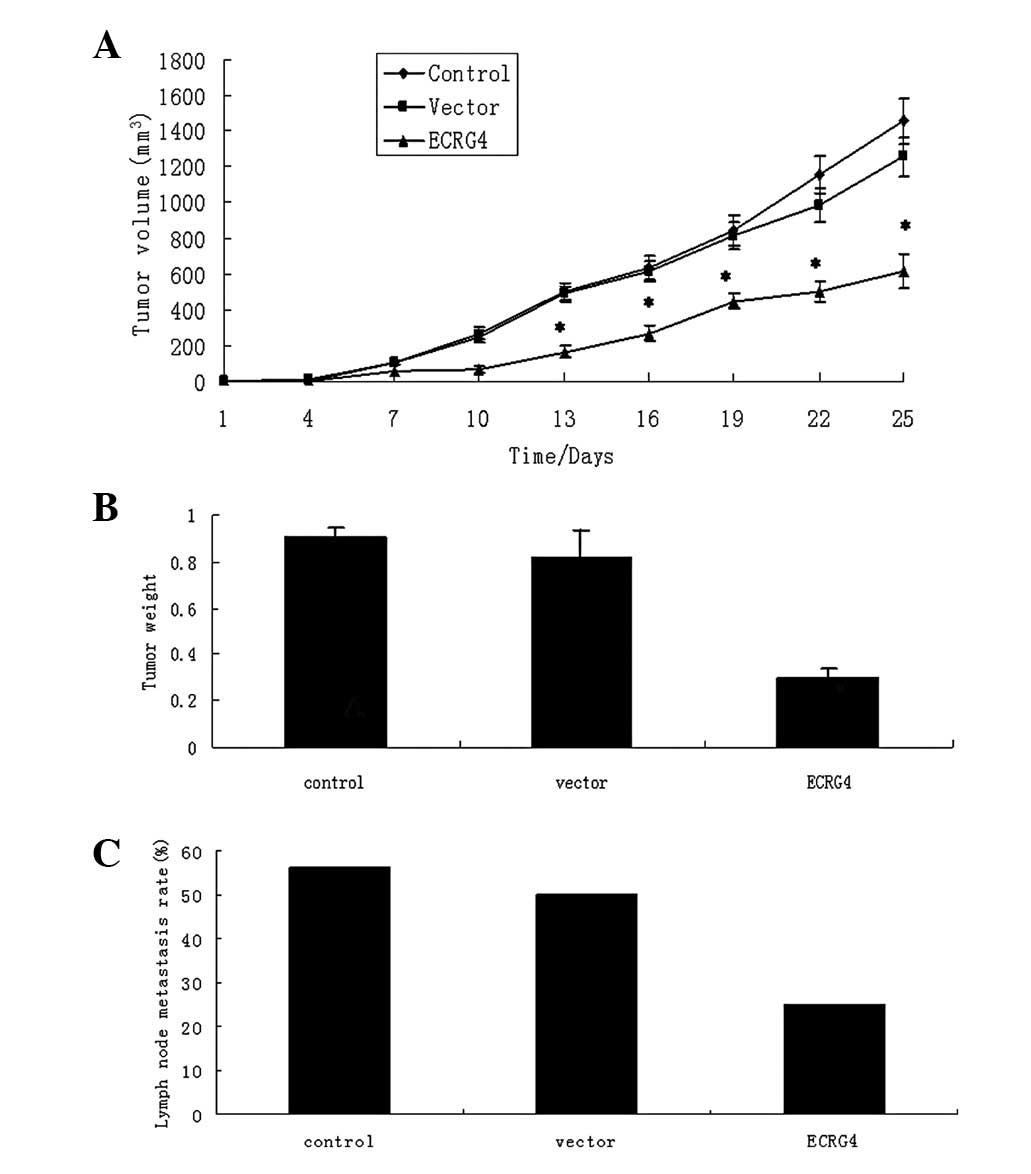
Overexpression of ECRG4 suppresses growth
and metastasis of xenografts in nude mice
The M2 cells were applied to the in vivo
experiment due to their high invasiveness. The three groups of
cells (control, vector and ECRG4) were injected into the mylohyoid
muscle of the nude mice and the tumor formation was carefully
observed. The tumor weights and volumes were measured at 25 days
following cell injection. The growth rate of the
ECRG4-overexpressed tumors was significantly decreased. The tumor
volumes and weights of the mice in the experimental group were
significantly less than those of the control and vector tumors
(P<0.05). However, no significant difference was detected in
either the tumor volume or weight between the control and vector
groups (both P>0.05; Fig. 5).
Bilateral and unilateral cervical lymph node metastasis was
exhibited by only one and three mice, respectively, in the ECRG4
group (lymph node metastasis rate 4/16; 25%); by two and four mice,
respectively, in the vector group (lymph node metastasis rate,
8/16; 50%); and by three and three mice, respectively, in the
control group (lymph node metastasis rate, 9/16; 56.25%). The
metastasis rate of the ECRG4 group was significantly lower than
that of the control and vector groups (both P<0.05), while there
was no significant difference between the control and vector groups
(P>0.05; Fig. 5). No lung
metastasis was identified from serial sections in any of the
groups. These data indicate that the overexpression of ECRG4
decreases cervical lymph node metastasis in vivo.
Discussion
Recent studies on a novel gene, ECRG4, in other
types of carcinomas (8–13) have attracted attention to its
potential role in SCCHN. The present study demonstrated the effect
of the ECRG4 gene on the growth and metastasis of SCCHN in
vitro and in vivo, and revealed a prognostic marker that
contributes to the malignant properties of SCCHN. To the best of
our knowledge, this was the first study associated with the
mechanism of function for ECRG4 in SCCHN.
pFLAG-CMV-2-ECRG4 successfully upregulated the
expression of ECRG4 in the M2 cells. Cell cycle arrest and the
induction of apoptosis are major mechanisms involved in anti-cancer
treatments (17). The present study
showed that ECRG4 inhibited cell growth and induced
G0/G1 cycle arrest and apoptosis in the M2
cell lines. This was consistent with the study by Li et al
with regard to ECRG4-induced cell cycle arrest in esophageal
carcinoma (18). Although other
studies have investigated the process of cell cycle arrest and
apoptosis in other cancers, the analysis of the exact mechanism was
not thorough (6–14). Cell cycle progression is tightly
regulated by cyclin/CDK complexes. The kinase activity of CDKs is
modulated by their regulatory subunits known as cyclins. The
expression level of the cyclins is also an important determinant in
cell cycle progression, particularly during G1/S and
G2/M transitions (19).
In the present study, ECRG4 decreased the expression of cyclin A
but not cyclin E, indicating that cyclin A may play a key role in
ECRG4-induced cancer cell arrest at the G0/G1
cell cycle phase. Bcl-2 family members appear to play a significant
role in the regulation of the intrinsic pathway of apoptosis
(14). Changes in the ratio of
Bcl-2 and Bax proteins in mitochondria may cause a loss of membrane
potential, the release of cytochrome-c and the activation of
caspase-9 (20). Li et al
demonstrated that ECRG4 inhibits glioma proliferation and induces
cell apoptosis through the NF-κb signaling pathway (18). Matsuzaki et al revealed that
ECRG4 is a novel antiapoptotic gene involved in the negative
regulation of caspase-8-mediated apoptosis in T-leukemia cells
(12). However, the present study
identified a novel signaling pathway for ECRG4-induced apoptosis.
The results showed that ECRG4 increased the expression of Bax and
decreased the expression of Bcl-2 in the M2 cell line, which
revealed that ECRG4 may trigger the mitochondrial pathway of
apoptosis through Bax/Bcl-2. Disruption of normal PI3K signaling
has been documented as a frequent occurrence in several human
cancers and appears to play a significant role in their progression
(21,22). The suppressed phosphorylation of AKT
may provide a mechanism to explain the role of ECRG4 in controlling
SCCHN cell proliferation.
Cell invasion and migration play significant roles
in cancer metastasis. SCCHN has a characteristically high rate of
relapse and a high facility for metastasis (23). The present study showed that ECRG4
was able to suppress SCCHN cell invasion and migration, implicating
its potential involvement in cancer metastasis. This observation
was consistent with a study on gliomas in which ECRG4 was observed
to reduce cell invasion (18).
Epithelial-mesenchymal transition (EMT) has previously been
implicated to be a key mechanism involved in cancer metastasis
(23). A hallmark of EMT is the
loss of E-cadherin expression. Our preliminary experiment (24) showed that as a high metastatic
derivative of Tu686, M2 cells demonstrate a lower expression of
E-cadherin. In the present study, this was reversed following the
transfection with ECRG4. This indicates that ECRG4 may suppress the
invasion of SCCHN cells by reversing the progress of EMT.
Although numerous studies (6–14) have
focused on the effects of ECRG4 in other cancers using the clinical
and in vitro levels, studies on animals are rare. The
present in vivo study further demonstrated that ECRG4 was
able to markedly diminish the tumorigenicity of SCCHN.
Submandibular injections of SCCHN M2 cells were successfully able
to induce lymph node metastasis, while injections of
pFLAG-CMV-2-ECRG4-interfered cells caused apparent inhibition of
cervical lymph node metastasis. These results may profoundly
demonstrate a critical role for ECRG4 in SCCHN cancer, thus
providing strong evidence for a clinical diagnosis and
treatment.
To the best of our knowledge, this is the first
study to demonstrate the effect of the ECRG4 gene on the growth and
metastasis of SCCHN in vitro and in vivo. The results
revealed that ECRG4 gene expression results in the suppression of
the growth rate and metastasis of SCCHN tumors. Increasing evidence
indicates that ECRG4 inhibits cell growth and invasion in other
human malignancies, however, the molecular mechanisms involved
require further research.
Acknowledgements
The authors thank Dr Zhuo Chen for
providing the cell lines. The present study was supported by grants
from the Foundation of Nanjing Medical University (no. 2011NJMU112)
and the key program of Medical Science and Technology Development
Fund of the Medical Control Center in Wuxi City (no. YGZ1111).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar
|
|
3
|
Hardisson D: Molecular pathogenesis of
head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol.
260:502–508. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gil Z and Fliss DM: Contemporary
management of head and neck cancers. Isr Med Assoc J. 11:296–300.
2009.PubMed/NCBI
|
|
5
|
Su T, Liu H and Lu S: Cloning and
identification of cDNA fragments related to human esophageal
cancer. Zhonghua Zhong Liu Za Zhi. 20:254–257. 1998.(In
Chinese).
|
|
6
|
Yue CM, Deng DJ, Bi MX, Guo LP and Lu SH:
Expression of ECRG4, a novel esophageal cancer-related gene,
downregulated by CpG island hypermethylation in human esophageal
squamous cell carcinoma. World J Gastroenterol. 9:1174–1178.
2003.PubMed/NCBI
|
|
7
|
Mori Y, Ishiguro H, Kuwabara Y, et al:
Expression of ECRG4 is an independent prognostic factor for poor
survival in patients with esophageal squamous cell carcinoma. Oncol
Rep. 18:981–985. 2007.PubMed/NCBI
|
|
8
|
Li LW, Yu XY, Yang Y, Zhang CP, Guo LP and
Lu SH: Expression of esophageal cancer related gene 4 (ECRG4), a
novel tumor suppressor gene, in esophageal cancer and its
inhibitory effect on the tumor growth in vitro and in vivo. Int J
Cancer. 125:1505–1513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Götze S, Feldhaus V, Traska T, et al:
ECRG4 is a candidate tumor suppressor gene frequently
hypermethylated in colorectal carcinoma and glioma. BMC Cancer.
9:4472009.PubMed/NCBI
|
|
10
|
Vanaja DK, Ehrich M, Van den Boom D, et
al: Hypermethylation of genes for diagnosis and risk stratification
of prostate cancer. Cancer Invest. 27:549–560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang YB and Ba CF: Promoter methylation of
esophageal cancer-related gene 4 in gastric cancer tissue and its
clinical significance. Hepatogastroenterology. 59:1696–1698.
2012.PubMed/NCBI
|
|
12
|
Matsuzaki J, Torigoe T, Hirohashi Y, et
al: ECRG4 is a negative regulator of caspase-8-mediated apoptosis
in human T-leukemia cells. Carcinogenesis. 33:996–1003. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sabatier R, Finetti P, Adelaide J, et al:
Down-regulation of ECRG4, a candidate tumor suppressor gene, in
human breast cancer. PLoS One. 6:e276562011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li LW, Li YY, Li XY, Zhang CP, Zhou Y and
Lu SH: A novel tumor suppressor gene ECRG4 interacts directly with
TMPRSS11A (ECRG1) to inhibit cancer cell growth in esophageal
carcinoma. BMC Cancer. 11:522011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Liu Y, Gilcrease MZ, et al: A
lymph node metastatic mouse model reveals alterations of
metastasis-related gene expression in metastatic human oral
carcinoma sublines selected from a poorly metastatic parental cell
line. Cancer. 95:1663–1672. 2002. View Article : Google Scholar
|
|
16
|
Lis R, Capdet J, Mirshahi P, et al:
Oncologic trogocytosis with Hospicells induces the expression of
N-cadherin by breast cancer cells. Int J Oncol. 37:1453–1461.
2010.PubMed/NCBI
|
|
17
|
Tang J, Feng Y, Tsao S, Wang N, Curtain R
and Wang Y: Berberine and Coptidis rhizoma as novel antineoplastic
agents: a review of traditional use and biomedical investigations.
J Ethnopharmacol. 126:5–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W, Liu X, Zhang B, et al:
Overexpression of candidate tumor suppressor ECRG4 inhibits glioma
proliferation and invasion. J Exp Clin Cancer Res. 29:892010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morgan DO: Principles of CDK regulation.
Nature. 374:131–134. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao JJ, Cheng H, Shidong J, et al: The
p110a isoform of PI3K is essential for proper growth factor
signaling and oncogenic transformation. Proc Natl Acad Sci USA.
103:16296–16300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inoki K, Zhu T and Guan KL: TSC2 mediates
cellular energy response to control cell growth and survival. Cell.
115:577–590. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yao M, Epstein JB, Modi BJ, Pytynia KB,
Mundt AJ and Feldman LE: Current surgical treatment of squamous
cell carcinoma of the head and neck. Oral Oncol. 43:213–223. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu T, Yu CY, Sun JJ, et al: Bone
morphogenetic protein-4-induced epithelial-mesenchymal transition
and invasiveness through Smad1-mediated signal pathway in squamous
cell carcinoma of the head and neck. Arch Med Res. 42:128–137.
2011. View Article : Google Scholar : PubMed/NCBI
|