Introduction
Accurate assessment of the presence of lymph node
metastasis is critical in predicting the clinical outcome of
patients who have undergone radical surgery for colorectal cancer.
The status of the lymph nodes also largely determines whether
adjuvant chemotherapy should be administered. Such adjuvant
chemotherapy has been shown unequivocally to provide disease-free,
as well as overall survival benefits, in patients with
node-positive disease (1). The
current literature indicates that the accuracy of staging and
overall survival in colon cancer increases proportionally with the
number of lymph nodes examined (2–4).
However, recent publications have suggested substantial variation
in nodal staging, attributable to surgical, pathological and
patient factors (3–5). These clinicopathological factors may
result in inaccurate staging and subsequent inappropriate therapy.
Previous studies focusing on stage II disease showed that for
patients with negative nodes, the survival rate is lower when
relatively few lymph nodes are recovered and examined, indicating
that these patients may be understaged (4,6,7).
Therefore, the current American Joint Committee on Cancer (AJCC)
guidelines suggest that a minimum of 12 nodes be present in the
surgical specimen (8).
By contrast, there are conflicting data regarding
lymph node retrieval in stage III disease. Prandi et
al(9) reported that overall and
relapse-free survival in stage III patients was not correlated to
the number of lymph nodes recovered. A similar conclusion was also
reported by Wong et al(10)
who stated that the number of lymph nodes following colectomy is
not associated with patient survival at a hospital level and that a
higher number of retrieved nodes might not be of public health
value.
The lymph node ratio (LNR), which is the ratio of
metastatic lymph nodes to examined lymph nodes, has been proposed
as a potentially more accurate predictor of overall survival (OS)
and disease-free survival (DFS) in colorectal as well as gastric
cancer. Noura et al reviewed the literature demonstrating
the clinicopathological significance of LNR in colorectal cancer
patients, and revealed that several reports have indicated the
advantage of considering the LNR compared with the number of lymph
nodes retrieved and/or lymph node status. The cut-off points for
LNRs were proposed in numerous studies; however, a consensus has
not yet been reached with regard to optimal thresholds for LNRs
(11).
C-reactive protein (CRP) is an acute phase reactant
that acts as a surveillance molecule for the activation of the
adaptive immune system. It is synthesized in hepatocytes and is
upregulated by cytokines such as interleukin (IL)-6 and tumor
necrosis factor-α (12). Several
studies have demonstrated that elevated CRP levels are associated
with an increased risk of early recurrence and poor outcome
following colorectal cancer resection (13–15).
Previously, we also reported that CRP levels reflect IL-6
production in colorectal cancer tissues and predict poor prognosis
in colorectal cancer patients, particularly in stage I or II
patients who are not usually candidates for postoperative adjuvant
chemotherapy (16,17).
Therefore, in this study, we examined whether
elevated CRP levels could be used to identify a subset of patients
with very poor prognosis in stage II or III colorectal cancer. We
also assessed whether elevated CRP levels could provide additional
information concerning stage migration, which is necessary when
inadequate retrieval of lymph nodes in stage II or III colorectal
cancer occurs.
Materials and methods
Patients
Overall, 193 patients with stage I–III colorectal
cancer who received potentially curative surgery at our institution
between January 1995 and January 2005 were enrolled in this
retrospective study. Curative resection was defined as the absence
of any gross residual tumor from the surgical bed and a surgical
resection margin that was pathologically negative for tumor
invasion. Data were retrieved from operative and pathological
reports. Follow-up data were obtained from the outpatient clinical
database. Blood collection and subsequent analyses were approved by
the Institutional Review Boards of Mie University Graduate School
of Medicine (protocol number: 2216).
Tumor characteristics
The study group comprised of 116 males and 77
females aged 29–91 years (median, 66 years; interquartile range,
58–73 years). Staging was principally based on the UICC/TNM
classification of colorectal cancer. Overall, 42 patients had stage
I disease, 74 patients had stage II and 77 had stage III disease.
Experienced pathologists from our institution participated in this
study and reassessed the quality of the original diagnosis. Of the
193 registered patients, 104 had tumors located in the colon and 89
had tumors in the rectum. The pathological tumor diameter indicated
the maximum micro-scopic length of the tumor, irrespective of the
depth. Differentiated tumors were histologically observed in 178
patients and undifferentiated tumors in 15 patients. Lymphatic
invasion was observed in 174 patients and vascular invasion in 97
patients. After 1997, 68 patients who gave informed consent
received adjuvant chemotherapies. Starting 4 weeks after curative
surgery, pyrimidine fluoride-based regimens were used for 6 months
to 1 year in patients classified as mainly stage III [stage II,
26/74 (35.1%); stage III, 42/77 (54.5%)].
Methods
Patients were followed up, using our standard
protocol, every 12–16 weeks for at least 5 years. This protocol
included tumor-marker studies, computed tomography, colorectal
fiber examinations, ultrasonography and chest radiography. Bone
scans were performed when bone metastasis was indicated. The mean
follow-up time was 59.5 months (95% CI for the mean was 54.5–64.5
months). The clinicopathological parameters studied for prognostic
value were tumor size, T classification, vessel involvement,
lymphatic invasion, lymph node metastases and serum
carcinoembryogenic antigen (CEA) concentration.
Blood samples were collected for routine laboratory
measurements of CRP prior to surgery. This is standard practice for
all cancer patients in our institution. The coefficient of
variation for these methods, over the range of measurement, was
<5%, as established by routine quality control procedures.
Patients who underwent non-elective surgery or preoperative
radiotherapy, who died within 30 days of surgery, or who showed
clinical evidence of infection or other inflammatory conditions,
were excluded from the study.
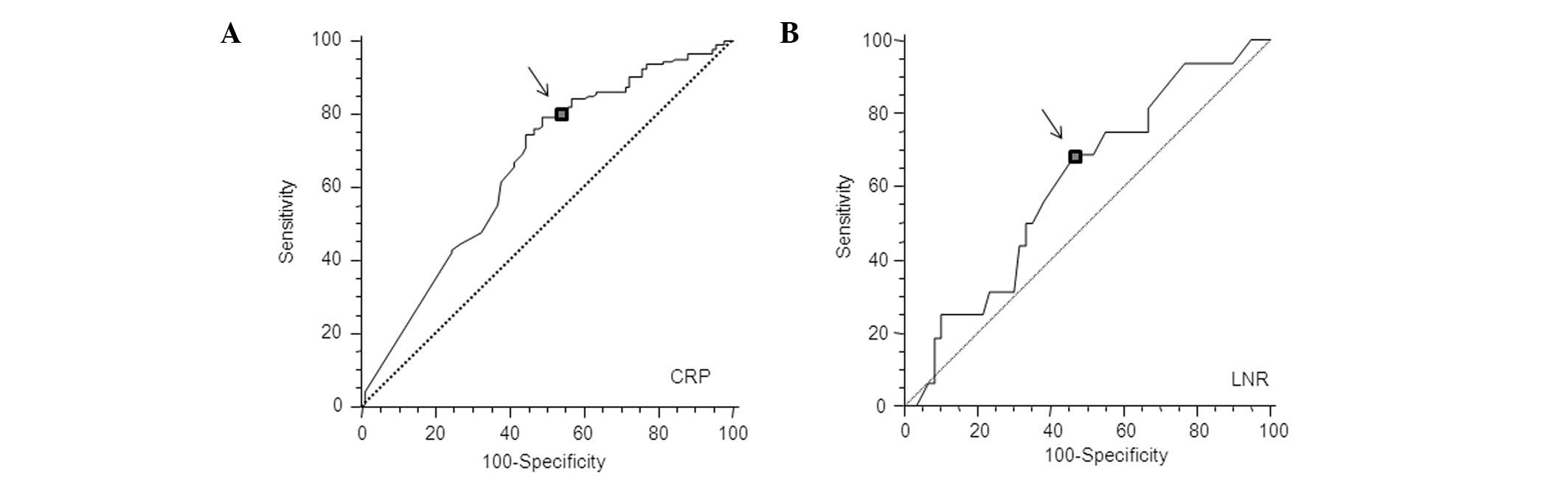
In the present study, elevated serum CRP levels were
defined according to the best predictive values calculated by
receiver operating characteristic (ROC) analyses, which found the
best pair of values for highest sensitivity and specificity based
on the peak and cut-off points (Fig.
1A). Based on this analysis, the cut-off value for CRP was
calculated to be 0.5 mg/dl.
Statistical methods
The data were presented as the means ± standard
deviation (SD). Comparisons were made using the non-parametric
Mann-Whitney U test for continuous variables and the χ2
test for categorical data. Correlations were analyzed by Spearman’s
coefficient analysis. ROC analyses were performed to calculate the
cut-off values according to the most accurate value obtained using
Medcalc 7.2 for Windows (Mariakerke, Belgium). The survival
probabilities were calculated using the product limit method of the
Kaplan-Meier method of analysis, considering treatment-related
mortality and mortality caused by colorectal cancer. The
differences between the two groups were determined using the
log-rank test. The effect of each significant predictor identified
by the log-rank tests was assessed by multivariate analysis using
Cox’s proportional hazard model. Statistical analyses were carried
out using StatView 5.0 (SAS Institute Inc., Cary, NC, USA) for
Windows. Two-sided p-values of <0.05 were considered to indicate
statistical significance.
Results
Correlation between CRP and
clinicopathological characteristics in patients undergoing
potentially curative resection for colorectal cancer
During the observation period, 31 patients succumbed
to colorectal cancer. Overall, 45 patients had elevated CRP levels
(>0.5 mg/dl). The mean number of lymph nodes examined was
12.6±0.75 in stage I–III colorectal cancer. The mean number of
positive lymph nodes and LNR in stage III colorectal cancer were
2.6±0.2 and 0.23±0.02, respectively. Table I shows the correlation between
clinicopathological characteristics and CRP status in patients with
stage I–III colorectal cancer. Gender, vascular or lymphatic
invasion, lymph node metastasis, pathological differentiation and
TNM classification were not significantly associated with CRP
concentration. However, age, serosal invasion and CEA levels were
significantly associated with CRP concentration.
 | Table ICorrelation between CRP status and
clinicopatho logical characteristics of stage I–III patients
undergoing potentially curative resection for colorectal
cancer. |
Table I
Correlation between CRP status and
clinicopatho logical characteristics of stage I–III patients
undergoing potentially curative resection for colorectal
cancer.
| Factors | CRP >0.5 | CRP <0.5 | p-value |
|---|
| Age | | | |
| >66 | 32 | 64 | |
| <66 | 13 | 84 | 0.0019 |
| Gender | | | |
| Male | 19 | 90 | |
| Female | 26 | 58 | 0.8493 |
| T-stage | | | |
| I–II | 8 | 50 | |
| III–IV | 37 | 98 | 0.0622 |
| T4 tumor | | | |
| Negative | 38 | 141 | |
| Positive | 7 | 7 | 0.0337 |
| Venous
invasion | | | |
| Negative | 20 | 76 | |
| Positive | 25 | 72 | 0.5214 |
| Lymphatic
invasion | | | |
| Negative | 3 | 16 | |
| Positive | 42 | 132 | 0.5951 |
| Lymph node
metastasis | | | |
| Negative | 28 | 89 | |
| Positive | 17 | 59 | 0.9388 |
| Pathology | | | |
|
Differentiated | 43 | 135 | |
|
Undifferentiated | 2 | 13 | 0.526 |
| TNM
classification | | | |
| I | 6 | 36 | |
| II | 22 | 52 | |
| III | 17 | 60 | 0.1585 |
| CEA | | | |
| <0.5 | 50 | 98 | |
| >0.5 | 29 | 16 | 0.0005 |
Univariate and multivariate analyses in
relation to mortality in patients with stage I–III colorectal
cancer
The results of the univariate and multivariate
analyses of postoperative mortality are shown in Table II. Based on Cox’s univariate
proportional hazard model, serosal invasion (p= 0.03),
undifferentiated tumors (poorly differentiated and mucinous
adenocarcinoma; p= 0.013), elevated serum CEA levels (p= 0.0008)
and CRP positivity (p<0.0001) were significant prognostic
factors for poor survival in patients with stage I–III colorectal
cancer. Multivariate analysis revealed that undifferentiated tumors
(p=0.002) and CRP detection (p<0.0001) were the only independent
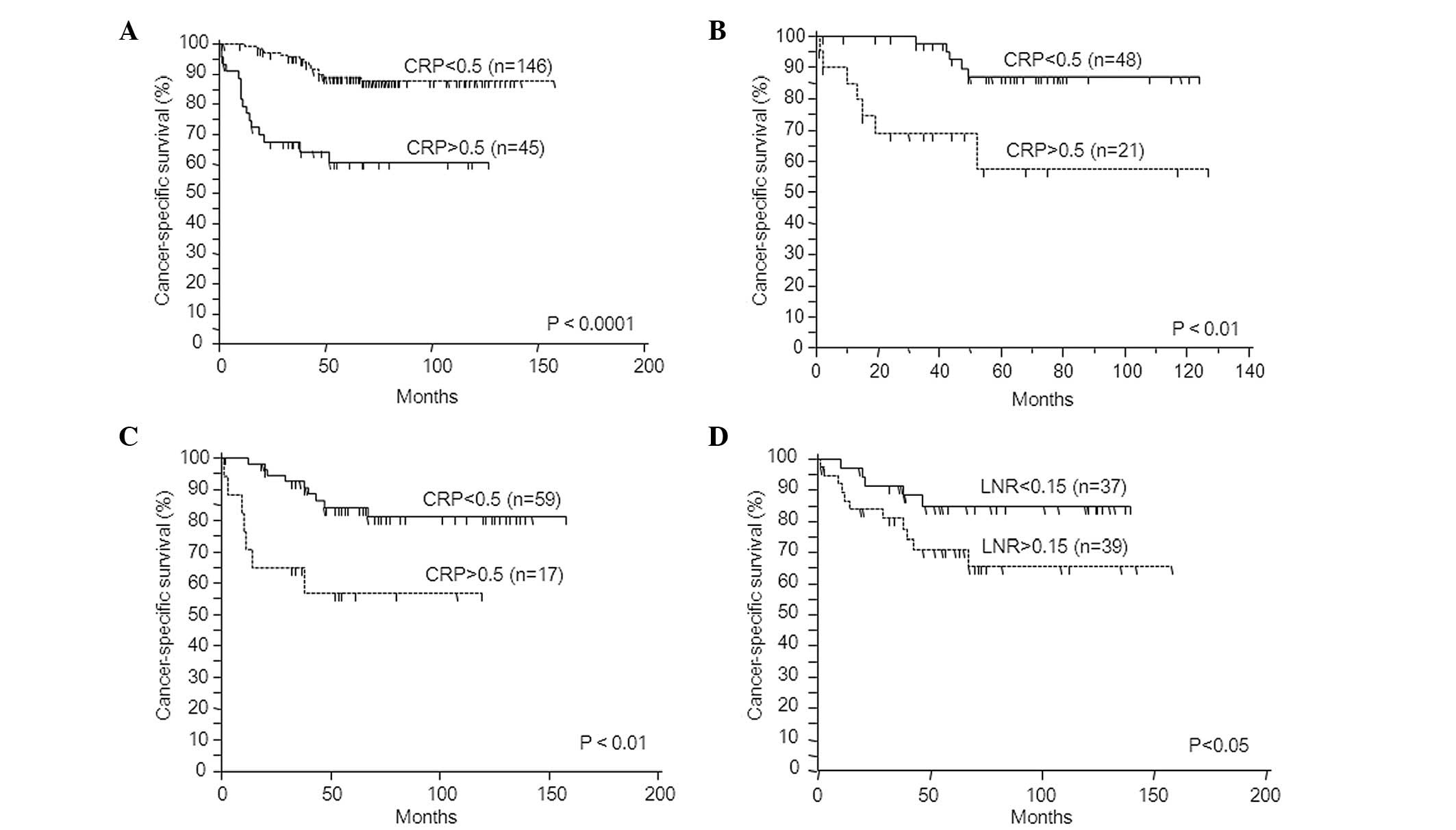
risk factors for predicting poor prognosis. Fig. 2A shows cancer-specific survival
according to the CRP status. CRP-positive patients had a
significantly worse prognosis than patients whose levels were below
the cut-off value (log-rank test, p<0.0001).
 | Table IIUni- and multivariate Cox’s
proportional hazard model for cancer-specific survival in stage
I–III colorectal cancer. |
Table II
Uni- and multivariate Cox’s
proportional hazard model for cancer-specific survival in stage
I–III colorectal cancer.
| Factors | HR | 95% CI | p-value |
|---|
| Univariate
analysis | | | |
| CRP
(>0.5) | 5.03 | 2.49–10.18 | <0.0001 |
| CEA (>5) | 3.43 | 1.62–7.25 | 0.0008 |
| Total number of
dissected lymph nodes (<12) | 1.92 | 0.89–4.42 | 0.08 |
| Lymph node
metastasis (positive) | 1.72 | 0.85–3.46 | 0.13 |
| T-stage (III,
IV) | 3.17 | 1.11–9.01 | 0.03 |
| Lymphatic
invasion (positive) | 3.46 | 0.48–25.11 | 0.22 |
| Vessel invasion
(positive) | 1.31 | 0.65–2.65 | 0.45 |
| Pathology
(undifferentiated type) | 3.08 | 1.27–7.51 | 0.013 |
| Multivariate
analysis | | | |
| CRP
(>0.5) | 4.90 | 2.33–10.32 | <0.0001 |
| CEA (>5) | 2.15 | 0.98–4.72 | 0.054 |
| T-stage (III,
IV) | 2.02 | 0.62–5.96 | 0.21 |
| Pathology
(undifferentiated type) | 4.25 | 1.68–10.76 | 0.002 |
Correlation between survival and
clinicopathological findings, including lymph node number examined
and CRP status, in stage II colorectal cancer
The cancer-specific survival rate was not
significantly higher for patients with 12 or more lymph nodes
examined compared with those with <12 lymph nodes examined
(log-rank test, p= 0.09; ≥12, 92.0%; <12, 77.2%). The results of
univariate and multivariate analysis of postoperative mortality in
stage II are shown in Table III.
Based on Cox’s univariate proportional hazard model, serosal
invasion (p= 0.024) and CRP presence (p= 0.005) were significant
prognostic factors for poor overall survival. Multivariate analysis
revealed that CRP (p= 0.02) was the only independent risk factor
for predicting poor prognosis. Fig.
2B shows the cancer-specific survival according to the CRP
status. CRP-positive patients had a significantly poorer prognosis
than patients whose levels were below the cut-off value (log-rank
test, p<0.01; cancer-specific survival rate: CRP-positive,
89.5%; CRP-negative, 66.6%).
 | Table IIIUni- and multivariate Cox’s
proportional hazard model for cancer-specific survival in stage II
colorectal cancer. |
Table III
Uni- and multivariate Cox’s
proportional hazard model for cancer-specific survival in stage II
colorectal cancer.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|---|
| Factors | HR | 95% CI | p-value | HR | 95% CI | p-value |
|---|
| CRP (>0.5) | 5.20 | 1.64–16.48 | 0.005 | 4.20 | 1.26–14.00 | 0.02 |
| CEA (>5) | 1.61 | 0.52–5.06 | 0.41 | - | - | - |
| Total number of
dissected lymph nodes (>12) | 0.29 | 0.06–1.33 | 0.11 | - | - | - |
| T4 tumor | 4.00 | 1.21–13.30 | 0.024 | 2.55 | 0.73–8.93 | 0.15 |
| Lymphatic invasion
(positive) | ns | ns | 0.40 | - | - | - |
| Vessel invasion
(positive) | 2.25 | 0.61–8.29 | 0.23 | - | - | - |
| Pathology
(undifferentiated type) | 2.86 | 0.60–12.97 | 0.18 | - | - | - |
Correlations between survival and
clinicopathological findings, including number of lymph nodes
examined, pN stage, LNR and CRP status in stage III colorectal
cancer
In the patients with stage III colorectal cancer, we
determined an optimal cut-off value for the LNR as it was not
previously defined. Fig. 1B shows
that the optimal LNR cut-off value was 0.15 by ROC analysis. By
contrast, optimal cut-off values for the number of lymph nodes
examined and pathological N stage were 12 and 4, respectively.
These values were recommended or stated in the AJCC guidelines
(8) and TNM classification
(27).
The cut-off value for the total number of lymph
nodes retrieved and the pN did not alter the cancer-specific
survival rate significantly (log-rank test: total number of lymph
nodes retrieved, p=0.1008; pN, p=0.784). By contrast, CRP-positive
patients had a significantly poorer prognosis than patients whose
levels were below the cut-off value (log-rank test, p<0.01;
Fig. 2C). Using a cut-off value for
LNR of 0.15 also maintained significance for cancer-specific
survival (log-rank test, p<0.05; Fig. 2D). The results of the univariate and
multivariate analyses of postoperative mortality in stage II are
shown in Table IV. Based on Cox’s
univariate proportional hazard model, LNR >0.15 (p=0.033), CEA
>6 (p=0.01) and CRP positivity (p=0.008) were significant
prognostic factors for poor cancer-specific survival. Multivariate
analysis revealed that CRP levels (p=0.028) were the only
independent risk factor for predicting poor prognosis.
 | Table IVUni- and multivariate Cox’s
proportional hazard model for cancer-specific survival in stage III
colorectal cancer. |
Table IV
Uni- and multivariate Cox’s
proportional hazard model for cancer-specific survival in stage III
colorectal cancer.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|---|
| Factors | HR | 95% CI | p-value | HR | 95% CI | p-value |
|---|
| CRP (>0.5) | 3.80 | 1.41–10.21 | 0.008 | 3.11 | 1.13–8.55 | 0.028 |
| CEA (>5) | 4.76 | 1.36–16.63 | 0.015 | 3.49 | 0.97–12.55 | 0.057 |
| Total number of
lymph nodes examined (>12) | 2.28 | 0.83–6.27 | 0.11 | - | - | - |
| LNR (>0.15) | 2.32 | 1.07–5.02 | 0.033 | 2.26 | 0.78–6.54 | 0.2262 |
| pN2 vs. pN1 | 0.73 | 0.21–2.56 | 0.63 | - | - | - |
| Pathology
(undifferentiated type) | 2.46 | 0.70–8.64 | 0.16 | - | - | - |
| T4 tumor
(positive) | 2.48 | 0.91–6.80 | 0.079 | - | - | - |
Evaluation of preoperative CRP predicts
understaging in patients is correlated with poor prognosis and
occurs due to sub-optimal lymph node assessment in stage II or III
colorectal cancer
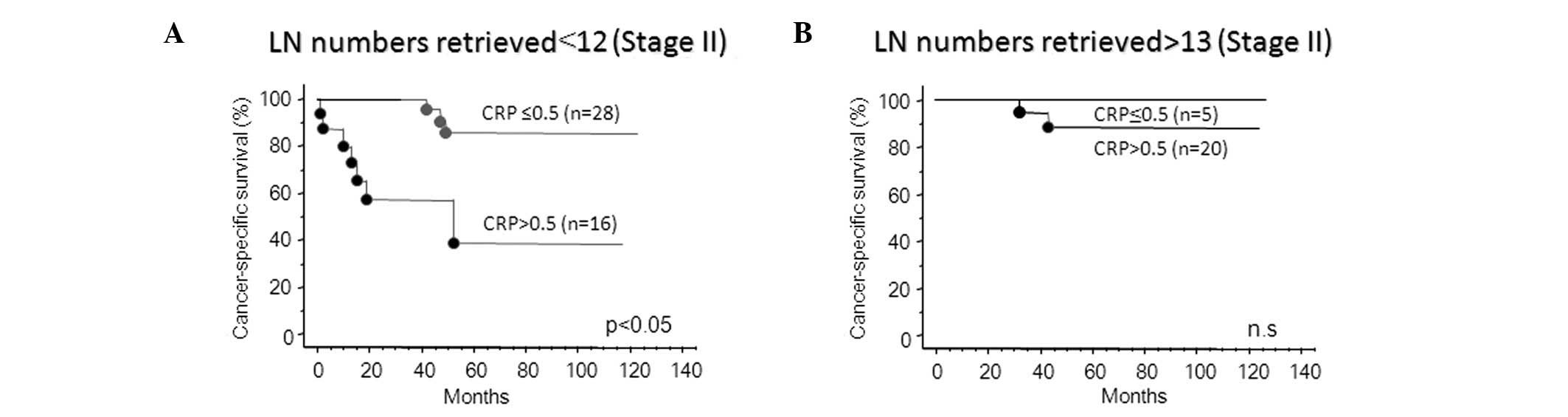
Fig. 3A shows that,
in stage II colorectal cancer, an adequate number of lymph nodes
examined resulted in a relatively good prognosis. Therefore,
recurrent patients were not predicted using CRP status. By
contrast, CRP positivity significantly predicts the risk of
recurrence if an inadequate number of lymph node are examined in
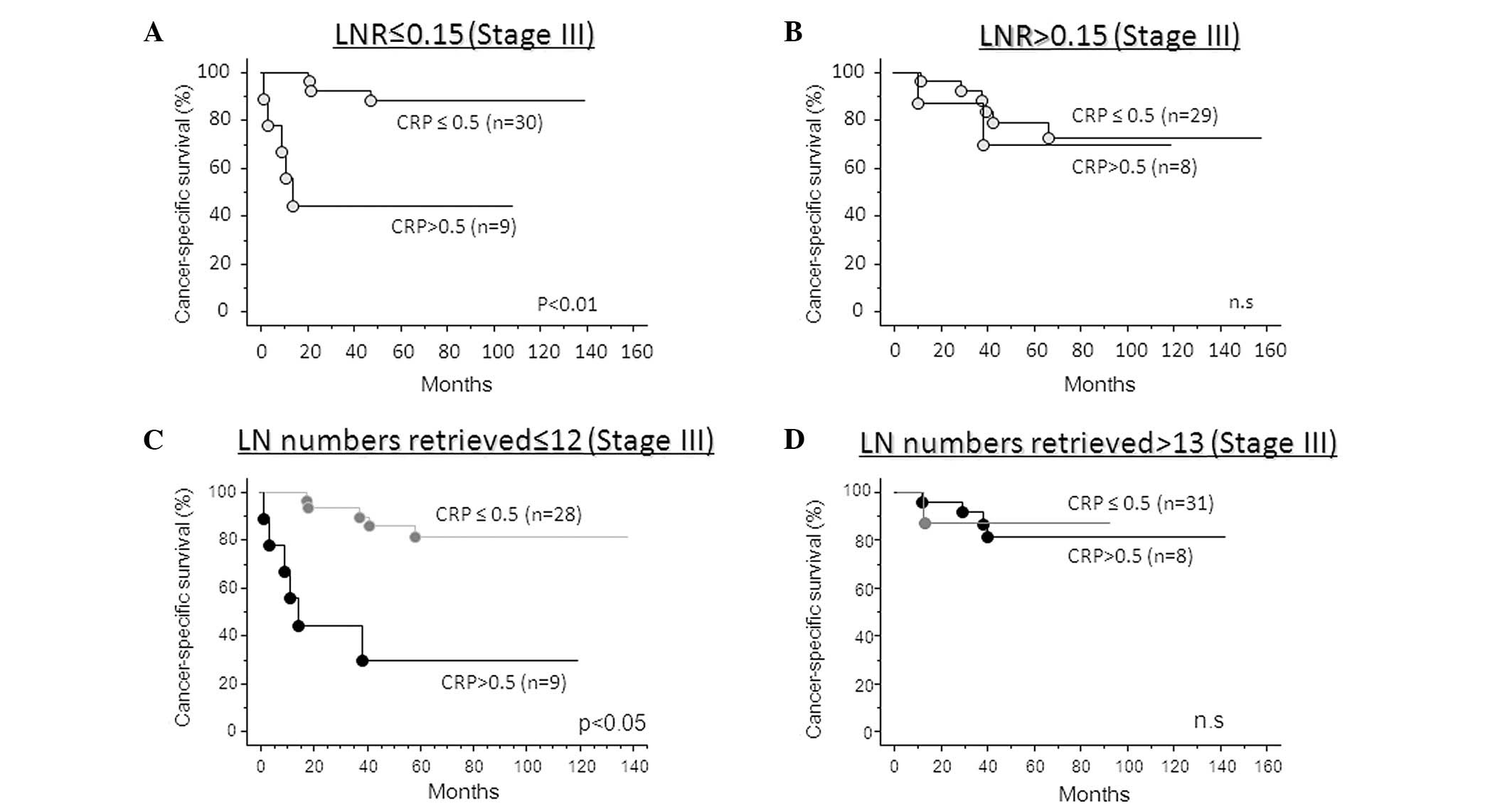
patients that demonstrate poor prognosis (Fig. 3B). In addition, the CRP-positive
group had significantly poorer cancer-specific survival compared
with the CRP-negative group in stage III colorectal cancer patients
with an inadequate number of examined lymph nodes (<12) or LNR
<0.15 (log-rank test; lymph node number examined, p<0.05;
LNR, p<0.01; Fig. 4A and C). By
contrast, the incorporation of CRP status was not required when
adequate lymph node numbers were examined (>13) or when LNR was
>0.15 (Fig. 4B and D).
Discussion
The TNM staging system provides the most reliable
information on prognosis and aids in the discrimination of patients
with early stage versus advanced stage disease. However, it is less
accurate in predicting the prognosis of patients with an
intermediate extent of tumor invasion. CEA is a complex
glycoprotein that is upregulated in approximately 90% of advanced
colorectal cancers and contributes to the malignant characteristics
of tumors (18). However, it is not
useful in detecting asymptomatic cancer, as the sensitivity of CEA
determination for early colorectal cancer is as low as 30–40%
(19). Moreover, CEA is not
significantly associated with survival in patients with stage I and
II lesions, and CEA testing is relatively insensitive to tumors
with local or peritoneal involvement (20). Therefore, the identification of
sensitive prognostic markers in this subgroup would allow for the
use of postoperative adjuvant therapy in a subset of patients with
poor prognosis and ultimately improve survival.
In this study, we aimed to determine whether CRP
provides more accurate prognostic information than that offered by
the existing staging systems or tumor markers in stages I–III, or
in subgroups of stages II or III colorectal cancer patients. We
revealed that CRP was significantly associated with serosal
invasion, preoperative CEA levels and TNM classification, which are
established conventional prognostic factors. Furthermore, CRP
positivity was found to have independent prognostic value in stages
I–III, whereas the prognostic values of CEA or TNM classification
were affected by other clinical factors.
Studies on various types of malignancies have
emphasized the importance of examining multiple lymph nodes in
determining prognosis. In colon and rectal cancer, staging accuracy
and survival are improved by increasing the number of nodes
examined and analyzed (2,21,22).
In addition, failure to examine a sufficient number of lymph nodes
may result in the inability to identify patients in whom lymph
nodes are affected by cancer, thus resulting in understaging
(23). However, the number of lymph
nodes reported with colectomy varies widely and may be due to
variations in surgical technique, the thoroughness of the
pathologist in finding nodes in the specimen, or the actual number
of regional lymph nodes. Therefore, it is crucial to establish the
minimum number of lymph nodes required for an acceptable accuracy
in classifying a tumor as LN-negative. Current guidelines
established by the AJCC recommend the assessment of 12 or more
nodes for accurate staging (8).
Nonetheless, the agenda for adequate lymph node
evaluation remains debatable. Recently, published studies assessing
the number of lymph nodes resected in colorectal cancer have
reported wide variation in the extent of resection. Although these
studies demonstrate a prognostic association between the number of
lymph nodes examined and survival, the cut-off values vary widely,
ranging from 6 to 40 (11). These
differences can be attributed to the fact that the right side of
the colon is associated with a higher number of lymph nodes
retrieved compared with the left (3,9,24). In
addition, older age and obesity may reduce the number of lymph
nodes retrieved (3,9). The number of lymph nodes that can be
retrieved may depend on the immune response of the patient as the
size and morphology of lymph nodes are modified by immune response
(25,26).
Our results revealed that the optimal cut-off value
for the lymph node number retrieval is 12 in stage II, but no
statistical difference was observed between the number of patients
in the ≤12 and the >13 node groups. However, in patients with
≤12 nodes retrieved, CRP-positive patients had a significantly
poorer prognosis than CRP-negative patients. By contrast, the
cancer-specific survival rate of CRP-positive patients was the same
as that of CRP-negative patients in those with >13 nodes
counted. Therefore, CRP evaluation may be a useful approach for the
detection of patients with the possibility of stage migration
caused by sub-optimal lymph node examination.
The most widely used staging system is the TNM
staging system, which was proposed by the AJCC/UICC. In the sixth
edition, pN stage was stratified into pN1 (LN 1–3) and pN2 (LN ≥4)
according to the number of positive lymph nodes (27). However, this system is also affected
by the total number of lymph nodes harvested and examined (2,4,28). To
overcome these variables, a ratio-based node staging system has
been proposed. The LNR is defined as the number of positive lymph
nodes divided by the total number of lymph nodes examined. It was
demonstrated that the ratio-based classification was superior to
the traditional categorical pN stage in gastric, pancreatic and
breast cancer (29–31). LNR reflects the probability of
positive lymph nodes in the harvested nodes, which does not
significantly depend on the number of lymph nodes harvested.
The LNR has been demonstrated to have prognostic
value in colon cancer in various studies (33–35).
Data for rectal cancer were limited but Rosenberg et al
reported that, following subgroup analysis, the LNR was an
independent prognostic factor for cause-specific survival in
patients with rectal cancer (36).
In addition, Peng et al found that LNR was the most
significant factor for overall survival, disease-free survival and
local recurrence in patients with rectal cancer (37). However, there is no consensus
concerning LNR, as different techniques for its calculation have
been described. With more studies demonstrating the importance of
LNR, the pooling of data may help to determine the proper
methodology for LNR stratification.
In our study of patients with stage III colorectal
cancer, the LNR (>0.15) and preoperative CRP status were
independent prognostic factors for cancer-specific survival, while
the conventional categorical pN stage (LN ≥4) and the total
harvested lymph node number (>13) were not significant in the
multivariate analysis. This confirmed the stronger prognostic value
of the LNR or preoperative CRP status in patients with stage III
colorectal cancer. Furthermore, combining the LNR or harvested
lymph node number and the preoperative CRP status may provide more
accurate data for predicting poor prognosis and identify patients
requiring further, intense chemotherapy in stage III colorectal
cancer. In the patients with LNR ≤0.15 or ≤12 lymph nodes
retrieved, CRP-positive patients had a significantly poorer
prognosis than CRP-negative patients. By contrast, cancer-specific
survival in CRP-positive patients was the same as that of
CRP-negative patients in those with LNR >0.15 or >13 nodes
retrieved.
In conclusion, preoperative CRP provides an
independent prognostic value for colorectal cancer in stages I–III
and is superior to the currently used number of lymph nodes
harvested or pN stage. Although the sample size included in this
study is small when compared with other multicenter or
population-based studies, its prognostic power does not depend on
the number of lymph nodes harvested. With the combination of CRP
status and total harvested number of lymph nodes in stages II or
III colorectal cancer, better stratification may aid in identifying
high-risk patients in order that adjuvant therapy be tailored to
increase the number of positive patient outcomes.
References
|
1
|
Moertel CG: Chemotherapy for colorectal
cancer. N Engl J Med. 330:1136–1142. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Joseph NE, Sigurdson ER, Hanlon AL, et al:
Accuracy of determining nodal negativity in colorectal cancer on
the basis of the number of nodes retrieved on resection. Ann Surg
Oncol. 10:213–218. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baxter NN, Virnig DJ, Rothenberger DA,
Morris AM, Jessurun J and Virnig BA: Lymph node evaluation in
colorectal cancer patients: a population-based study. J Natl Cancer
Inst. 97:219–225. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang GJ, Rodriguez-Bigas MA, Skibber JM
and Moyer VA: Lymph node evaluation and survival after curative
resection of colon cancer: systematic review. J Natl Cancer Inst.
99:433–441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mamounas E, Wieand S, Wolmark N, et al:
Comparative efficacy of adjuvant chemotherapy in patients with
Dukes’ B versus Dukes’ C colon cancer: results from four National
Surgical Adjuvant Breast and Bowel Project adjuvant studies (C-01,
C-02, C-03, and C-04). J Clin Oncol. 17:1349–1355. 1999.
|
|
6
|
Ratto C, Sofo L, Ippoliti M, et al:
Accurate lymph-node detection in colorectal specimens resected for
cancer is of prognostic significance. Dis Colon Rectum. 42:143–158.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Caplin S, Cerottini JP, Bosman FT,
Constanda MT and Givel JC: For patients with Dukes’ B (TNM stage
II) colorectal carcinoma, examination of six or fewer lymph nodes
is related to poor prognosis. Cancer. 83:666–672. 1998.
|
|
8
|
Nelson H, Petrelli N, Carlin A, et al:
Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer
Inst. 93:583–596. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prandi M, Lionetto R, Bini A, et al:
Prognostic evaluation of stage B colon cancer patients is improved
by an adequate lymphadenectomy: results of a secondary analysis of
a large scale adjuvant trial. Ann Surg. 235:458–463. 2002.
View Article : Google Scholar
|
|
10
|
Wong SL, Ji H, Hollenbeck BK, Morris AM,
Baser O and Birkmeyer JD: Hospital lymph node examination rates and
survival after resection for colon cancer. JAMA. 298:2149–2154.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noura S, Ohue M, Kano S, et al: Impact of
metastatic lymph node ratio in node-positive colorectal cancer.
World J Gastrointest Surg. 2:70–77. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakazaki H: Preoperative and postoperative
cytokines in patients with cancer. Cancer. 70:709–713. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nozoe T, Matsumata T, Kitamura M and
Sugimachi K: Significance of preoperative elevation in serum
C-reactive protein as an indicator for prognosis in colorectal
cancer. Am J Surg. 176:335–338. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nozoe T, Matsumata T and Sugimachi K:
Preoperative elevation of serum C-reactive protein is related to
impaired immunity in patients with colorectal cancer. Am J Clin
Oncol. 23:263–266. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gunter MJ, Stolzenberg-Solomon R, Cross
AJ, et al: A prospective study of serum C-reactive protein and
colorectal cancer risk in men. Cancer Res. 66:2483–2487. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miki C, Konishi N, Ojima E, Hatada T,
Inoue Y and Kusunoki M: C-reactive protein as a prognostic variable
that reflects uncontrolled up-regulation of the IL-1-IL-6 network
system in colorectal carcinoma. Dig Dis Sci. 49:970–976. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koike Y, Miki C, Okugawa Y, et al:
Preoperative C-reactive protein as a prognostic and therapeutic
marker for colorectal cancer. J Surg Oncol. 98:540–544. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wiggers T, Arends JW, Schutte B, Volovics
L and Bosman FT: A multivariate analysis of pathologic prognostic
indicators in large bowel cancer. Cancer. 61:386–395. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fletcher RH: Carcinoembryonic antigen. Ann
Int Med. 104:66–73. 1986. View Article : Google Scholar
|
|
20
|
Moertel CG, Fleming TR, Macdonald JS,
Haller DG, Laurie JA and Tangen C: An evaluation of the
carcinoembryonic antigen (CEA) test for monitoring patients with
resected colon cancer. JAMA. 270:943–947. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tepper JE, O’Connell MJ, Niedzwiecki D, et
al: Impact of number of nodes retrieved on outcome in patients with
rectal cancer. J Clin Oncol. 19:157–163. 2001.PubMed/NCBI
|
|
22
|
Joseph NE, Sigurdson ER, Hanlon AL, et al:
Colon cancer survival is associated with increasing number of lymph
nodes analyzed: A secondary survey of intergroup trial INT-0089. J
Clin Oncol. 21:2912–2919. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Swanson RS, Compton CC, Stewart AK and
Bland KI: The prognosis of T3N0 colon cancer is dependent on the
number of lymph nodes examined. Ann Surg Oncol. 10:65–71. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bilimoria KY, Palis B, Stewart AK, et al:
Impact of tumor location on nodal evaluation for colon cancer. Dis
Colon Rectum. 51:154–161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leibl S, Tsybrovskyy O and Denk H: How
many lymph nodes are necessary to stage early and advanced
adenocarcinoma of the sigmoid colon and upper rectum? Virchows
Arch. 443:133–138. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Horzic M and Kopljar M: Minimal number of
lymph nodes that need to be examined for adequate staging of
colorectal cancer – factors influencing lymph node harvest.
Hepatogastroenterology. 52:86–89. 2005.PubMed/NCBI
|
|
27
|
AJCC Cancer Staging Manual. 6th edition.
Springer; New York: 2002
|
|
28
|
Le Voyer TE, Sigurdson ER, Hanlon AL, et
al: Colon cancer survival is associated with increasing number of
lymph nodes analyzed: a secondary survey of intergroup trial
INT-0089. J Clin Oncol. 21:2912–2919. 2003.PubMed/NCBI
|
|
29
|
Nitti D, Marchet A, Olivieri M, et al:
Ratio between metastatic and examined lymph nodes is an independent
prognostic factor after D2 resection for gastric cancer: analysis
of a large European monoinstitutional experience. Ann Surg Oncol.
10:1077–1085. 2003. View Article : Google Scholar
|
|
30
|
Voordeckers M, Vinh-Hung V, Van de Steene
J, Lamote J and Storme G: The lymph node ratio as prognostic factor
in node-positive breast cancer. Radiother Oncol. 70:225–230. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Berger AC, Watson JC, Ross EA and Hoffman
JP: The metastatic/examined lymph node ratio is an important
prognostic factor after pancreaticoduodenectomy for pancreatic
adenocarcinoma. Am Surg. 70:235–240. 2004.
|
|
32
|
Inoue K, Nakane Y, Iiyama H, et al: The
superiority of ratio-based lymph node staging in gastric carcinoma.
Ann Surg Oncol. 9:27–34. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Berger AC, Sigurdson ER, LeVoyer T, et al:
Colon cancer survival is associated with decreasing ratio of
metastatic to examined lymph nodes. J Clin Oncol. 23:8706–8712.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee HY, Choi HJ, Park KJ, et al:
Prognostic significance of metastatic lymph node ratio in
node-positive colon carcinoma. Ann Surg Oncol. 14:1712–1717. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang J, Hassett JM, Dayton MT and Kulaylat
MN: Lymph node ratio: role in the staging of node-positive colon
cancer. Ann Surg Oncol. 15:1600–1608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rosenberg R, Friederichs J, Schuster T, et
al: Prognosis of patients with colorectal cancer is associated with
lymph node ratio: a single-center analysis of 3,026 patients over a
25-year time period. Ann Surg. 248:968–978. 2008.PubMed/NCBI
|
|
37
|
Peng J, Xu Y, Guan Z, et al: Prognostic
significance of the metastatic lymph node ratio in node-positive
rectal cancer. Ann Surg Oncol. 15:3118–3123. 2008. View Article : Google Scholar : PubMed/NCBI
|