Introduction
Basaloid squamous cell carcinoma (BSCC) is a rare
variant of squamous cell carcinoma, which is characterized
clinically by highly aggressive behavior and is histopathologically
composed of basaloid and squamous components (1). BSCC was first reported by Wain et
al in 1986 as a highly aggressive histopathological variant of
squamous cell carcinoma occurring in the tongue, pharynx and larynx
(2). Since this initial report,
BSCC has been reported in various organs, including the esophagus,
with the most common sites in the head and neck region being the
oral cavity and larynx (1,3). The occurrence of BSCC in the nasal
cavity is extremely rare and only 26 cases have been reported
(3–9). In the present study, two additional
cases of BSCC occurring in the maxillary sinus are reported and the
clinicopathological features and immunohistochemical
characteristics of this rare tumor are discussed. This study was
approved by the ethics committee of Shiga University of Medical
Science. Written informed consent was obtained from the
patients.
Case reports
Case 1
An 85-year-old Japanese female patient presented
with a nasal tumor and right-sided exophthalmos. Computed
tomography (CT) and magnetic resonance imaging (MRI) scans revealed
a right maxillary sinus tumor invading into the right ethmoid
sinus, orbit and dura mater (Fig.
1). A biopsy specimen from the right maxillary sinus tumor
indicated a diagnosis of BSCC (T4, N0, M0), and radiation therapy
(58 Gy) with TS-1 administration was subsequently administered. CT
and MRI scans following therapy showed that the tumor had
contracted and a biopsy from the tumor showed a few residual
degenerative carcinoma cells. The patient has survived with the
disease for 10 months since the initial diagnosis.
Case 2
A 60-year-old Japanese male patient presented with
an approximate three-month history of persistent nasal obstruction.
CT revealed a right-sided maxillary sinus tumor with destruction of
the surrounding bone tissues and dural invasion. A biopsy specimen
from the maxillary sinus tumor revealed a poorly-differentiated
carcinoma. Surgical resection of the maxillary sinus tumor was
subsequently performed. Following surgery, the patient received
chemotherapy. The post-operative course was uneventful, although
local recurrence occurred one and a half years subsequent to the
surgery. This was followed by the development of multiple liver and
lung metastases to which the patient succumbed.
Materials and methods
The formalin-fixed, paraffin-embedded tissue blocks
of the maxillary sinus specimens were cut into 3-μm thick
sections, then deparaffinized and rehydrated. Each section was
stained with hematoxylin and eosin, then used for immunostaining.
Immunohistochemical analyses were performed using an autostainer
(Benchmark XT system, Ventana Medical Systems, Tucson, AZ, USA)
according to the manufacturer’s instructions. The following primary
antibodies were used: mouse monoclonal antibody against α-smooth
muscle actin (alphasm-1; Novocastra Laboratories, Ltd., Newcastle
upon Tyne, UK), mouse monoclonal antibody against cathepsin K (3F9;
Abcam, Cambridge, UK), mouse monoclonal antibody against
cytokeratin (AE1/AE3; DAKO Cytomation, Glostrup, Denmark), mouse
monoclonal antibody against high molecular weight cytokeratin
(34betaE12; DAKO Cytomation), mouse monoclonal antibody against
epithelial membrane antigen (GP1.4; Novocastra), mouse monoclonal
antibody against p63 (7JUL; Novocastra) and mouse monoclonal
antibody against vimentin (VIM3B4; Novocastra).
Histopathological results
Case 1
The biopsy specimen from the maxillary sinus tumor
exhibited an infiltrative proliferation of solid epithelial nests
composed of basaloid cells and the surface epithelium was eroded
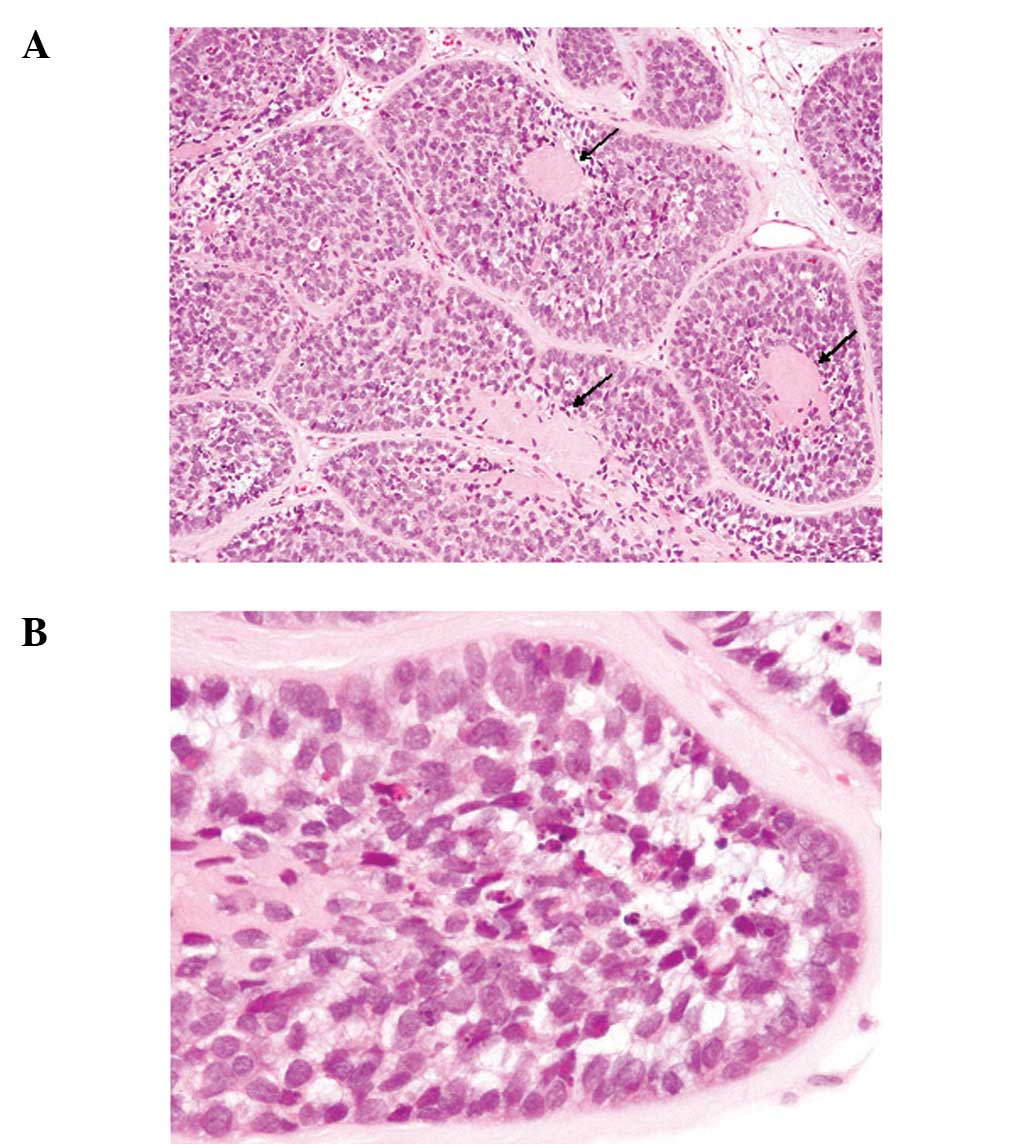
(Fig. 2A). These basaloid cells had
a high nuclear/cytoplasmic ratio, hyperchromatic nuclei without
conspicuous nucleoli and scant cytoplasm (Figs. 2A and B). Mitotic figures were
scattered and apoptotic bodies were frequently observed (Fig. 2B). The characteristic
histopathological finding was the presence of spherical hyalinized
materials within the tumor nests (Fig.
2A, arrows). No keratinization was observed in the tumor
cells.
Case 2
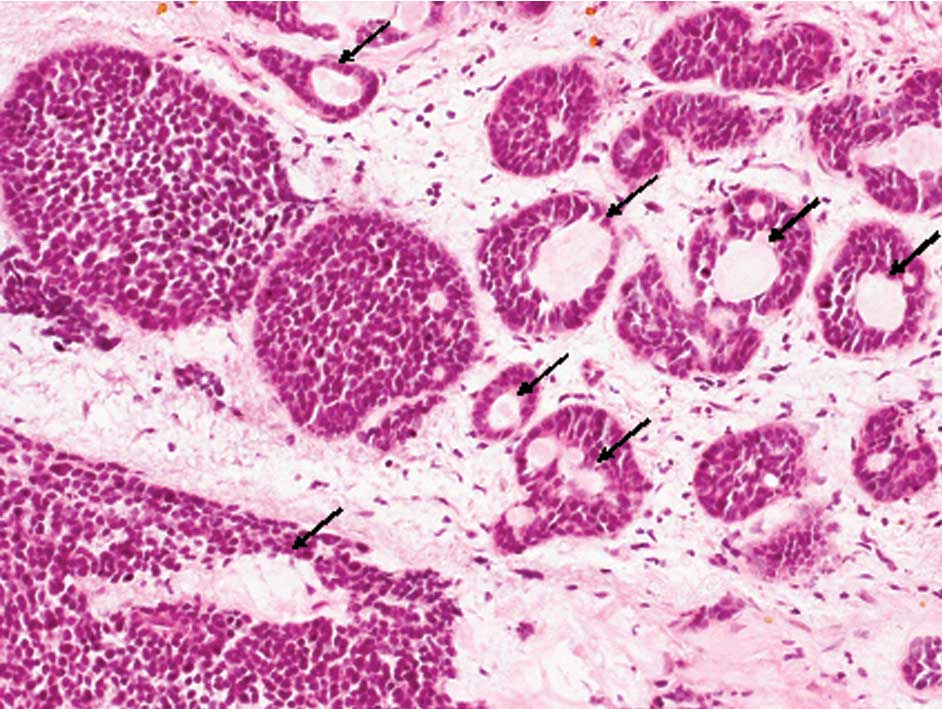
The surgically resected specimen of the maxillary
sinus showed an infiltrative proliferation of solid epithelial
nests composed of the basaloid cells that had scant cytoplasm and
hyperchromatic nuclei without conspicuous nucleoli (Fig. 3). Spherical hyalinized materials
were present within the tumor nests (Fig. 3, arrows) and mitotic figures were
scattered. Focal squamous differentiation, including individual
keratinization and intercellular bridging, was also observed.
Immunohistochemical results
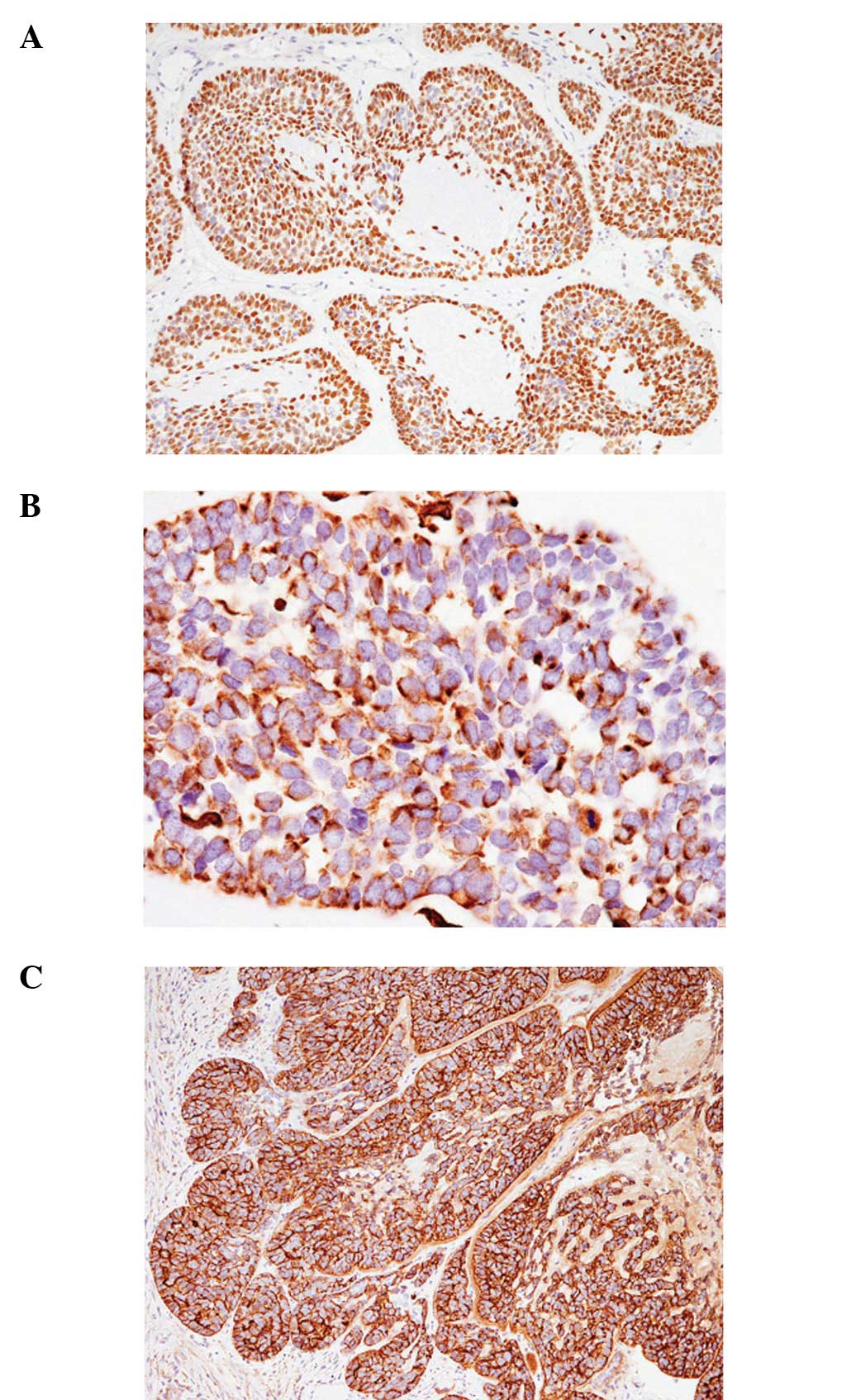
Table I shows the
immunohistochemical findings of cases 1 and 2, each revealing
similar results. The characteristic findings were diffuse positive
immunoreactivity for p63 (Fig. 4A),
negative immunoreactivity for α-smooth muscle actin and perinuclear
dot-like positivity for vimentin (Fig.
4B). In addition, cathepsin K was diffusely expressed in each
of the two cases (Fig. 4C).
 | Table IImmunohistochemical findings. |
Table I
Immunohistochemical findings.
| Target | Case 1 | Case 2 |
|---|
| Cytokeratin
(AE1/AE3) | + | + |
| Cytokeratin
(34betaE12) | + | + |
| Epithelial membrane
antigen | + | + |
| p63 | + | + |
| α-smooth muscle
actin | - | - |
| Vimentin | + (dot) | + (dot) |
| Cathepsin K | + | + |
Discussion
In the present study, two cases of BSCC of the
maxillary sinus are described. The clinicopathological features of
the 26 previously reported cases of BSCC of the nasal cavity, as
well as the 2 present cases, are shown in Table II. BSCC mainly affects the elderly
(particularly individuals between 60 and 80 years of age), although
it may occur, albeit rarely, in young adults. A comparison of all
28 cases showed that males are more commonly affected (male/female
18:10) and that the most common clinical symptoms are nasal
obstruction, epistaxis and nasal tumors. The prognosis of BSCC of
the nasal cavity is poor; 14 of the 28 reported cases succumbed to
the disease and only seven were free of tumors following treatment.
BSCC of the head and neck region shows aggressive clinical behavior
[frequent lymph node metastases (62.5%), high mortality rate
(47.5%) and poor three- and five-year overall survival rates (50%
and 38.5%, respectively)] (10).
Additionally, the present survey of the clinicopathological
features of BSCC of the nasal cavity in all reported cases revealed
that it has an aggressive clinical course, which corresponds to
that of BSCC of the other head and neck regions.
 | Table IIClinicopathological features of
basaloid squamous cell carcinoma of the nasal cavity. |
Table II
Clinicopathological features of
basaloid squamous cell carcinoma of the nasal cavity.
| Case No. | Age/Gender | Location | Clinical symptom |
Metastases/invasion | Outcome | Ref. |
|---|
| 1 | 78/M | Maxillary sinus | Cheek swelling,
pain | None | NED, 25 months | 3 |
| 2 | 60/M | Maxillary sinus | Cheek swelling,
diplopia | Orbit, skull base,
lung | STD, 6 months | 3 |
| 3 | 50/F | Nose | Dyspnea | None | AWD, 1 year | 4 |
| 4 | 59/M | Nose | Epistaxis | Not available | NED, 1 year | 4 |
| 5 | 67/M | Nasal cavity | Epistaxis | None | NED, 4 months | 5 |
| 6 | 53/F | Nasal septum | Epistaxis | None | STD, 8 years | 6 |
| 7 | 81/F | Nasal cavity | Obstruction | None | Alive, 3 years | 6 |
| 8 | 69/M | Nasal cavity | Blurred vision | None | STD, 1 years | 6 |
| 9 | 32/M | Nasal cavity | Obstruction | Brain | STD, 7 years | 6 |
| 10 | 72/M | Nasal cavity | Obstruction | Brain | STD, 1 years | 6 |
| 11 | 33/F | Sinus | Obstruction,
diplopia | Bone, lung | STD, 1 years | 6 |
| 12 | 41/F | Nasal cavity | Obstruction | Lung | AWD, 5 years | 6 |
| 13 | 75/F | Sinus | Obstruction | Dura | AWD, 2 years | 6 |
| 14 | 64/M | Nasal cavity | Nasal mass | None | STD, 1 years | 6 |
| 15 | 79/F | Sinus | Sinusitis,
headache | Bone, lung | STD, 1 years | 6 |
| 16 | 56/M | Nasal septum | Nasal mass | Cervical lymph
node | NED, 2 years | 6 |
| 17 | 46/M | Nasal septum | Obstruction,
epistaxis | None | AWD, 8 months | 6 |
| 18 | 86/F | Nasal septum | Nasal mass | None | STD, 6 months | 6 |
| 19 | 79/M | Nasal cavity | Nasal mass | None | NED, 1 months | 6 |
| 20 | 86/M | Nasal cavity | Epistaxis | None | STD, 2 years | 7 |
| 21 | 36/M | Nasal cavity | Epistaxis | None | AWD, 1.5 years | 7 |
| 22 | 59/M | Maxillary sinus | Not available | Not available | STD, 1 years | 8 |
| 23 | 47/M | Maxillary sinus | Not available | Not available | STD, 1 years | 8 |
| 24 | 69/M | Maxillary
sinus | Not available | Not available | STD, 2.5 years | 8 |
| 25 | 48/M | Maxillary
sinus | Not available | Not available | Alive, 3.5
years | 8 |
| 26 | 58/F | Nasal cavity | Epistaxis,
obstruction | None | AWD, 17 months | 9 |
| Present case 1 | 85/F | Maxillary
sinus | Nasal tumor,
exophthalmus | Orbit, dura | AWD, 10 months | |
| Present case 2 | 60/M | Maxillary
sinus | Obstruction | Dura, lung,
liver | STD, 18 months | |
The main histopathological differential diagnostic
consideration for BSCC is adenoid cystic carcinoma (particularly
the solid variant) since these tumors are also composed of basaloid
cells and may have areas with a cribriform growth pattern (11). The main histopathological
characteristics of BSCC that aid in distinguishing it from adenoid
cystic carcinoma are greater nuclear pleomorphism, evidence of
squamous differentiation, presence of necrosis and abundant mitotic
figures (11). Moreover,
immunohistochemical analyses are also useful for differentiating
between these two diseases. The majority of adenoid cystic
carcinomas show positive immunoreactivity for smooth muscle actin,
but this marker is negative in BSCC (11). Moreover, p63 is diffusely expressed
in the basaloid cells of BSCC, while this protein is only observed
in the peripheral cells of adenoid cystic carcinoma (12). In addition, perinuclear dot-like
vimentin expression is characteristic of BSCC, in contrast to the
diffuse cytoplasmic expression of adenoid cystic carcinoma
(11). In the present two cases,
the histopathological features, including nuclear pleomorphism,
evidence of squamous differentiation and frequent mitotic figures,
and the immunohistochemical characteristics (diffuse p63
positivity, perinuclear dot-like vimentin expression and smooth
muscle actin negativity) led to the final diagnosis of BSCC of the
maxillary sinus.
To further identify the markers of this devastating
disease, the expression of cathepsin K, a cysteine protease with
marked collagenolytic and elastolytic activities, was investigated.
Cathepsin K cleaves multiple sites within the triple helix of
collagen types I and III, as well as at extracellular regions,
whereas other proteases are more limited in their proteolytic
activities. Cathepsin K was first demonstrated to play a
significant role in osteoclast-mediated bone resorption (13), and it has also been recognized that
this protein is involved in the extracellular matrix turnover in
certain organs (13). Previous
studies have demonstrated a role for cathepsin K in malignant
tumors in certain organs, including the breast, skin and lungs
(14–18). The majority of malignant melanoma
cases (10/12) showed marked cathepsin K expression in the tumor
cells (15), while all 50 cases of
cutaneous basal cell carcinoma also showed expression of this
protease (16). These results
suggest that cathepsin K expression in tumor cells contributes to
tumor invasion (15,16). By contrast, the majority of squamous
cell carcinomas of the skin exhibited no positive immunoreactivity
for cathepsin K in the tumor cells (only 2/38 cases were weakly
positive), while the peritumoral stromal cells were markedly
positive for cathepsin K (19).
Moreover, cathepsin K expression was noted in only 31% of the cases
of esophageal invasive squamous cell carcinoma (being particularly
confined to the relatively sparse cancer cells located externally
in the tumor foci) (20). Diffuse
cathepsin K expression was observed in the two present BSCC cases.
Therefore, although only two cases were examined in the present
study, diffuse cathepsin K expression may be a characteristic
immunohistochemical feature of BSCC. Moreover, cathepsin K
expression in BSCC may contribute to tumor invasion and its highly
aggressive clinical course since this protein has marked
collagenolytic and elastolytic activities. Additional
clinicopathological analyses are consequently required to clarify
these issues and potentially aid in the future treatment of
BSCC.
References
|
1
|
Cardesa A, Zidar N and Ereño C: Basaloid
squamous cell carcinoma. World Health Organization Classification
of Tumours. Pathology and Genetics of Head and Neck Tumours. Barnes
L, Eveson JW, Reichart P and Sidransky D: IARC Press; Lyon: pp.
124–125. 2005
|
|
2
|
Wain SL, Kier R, Vollmer RT and Bossen EH:
Basaloid-squamous cell carcinoma of the tongue, hypopharynx, and
larynx: report of 10 cases. Hum Pathol. 17:1158–1166. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oikawa K, Tabuchi K, Nomura M, et al:
Basaloid squamous cell carcinoma of the maxillary sinus: a report
of two cases. Auris Nasus Larynx. 34:119–123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paulino AF, Singh B, Shah JP and Huvos AG:
Basaloid squamous cell carcinoma of the head and neck.
Laryngoscope. 110:1479–1482. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wan SK, Chan JK and Tse KC:
Basaloid-squamous carcinoma of the nasal cavity. J Laryngol Otol.
106:370–371. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wieneke JA, Thompson LDR and Wenig BM:
Basaloid squamous cell carcinoma of the sinonasal tract. Cancer.
85:841–854. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu SY, Eng HL, Huang CC, Chien CY, Lui CC
and Lin JW: Basaloid squamous cell carcinoma of the sinonasal
tract: report of two cases. Otolaryngol Head Neck Surg.
134:883–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu GY, Gao Y, Peng X, Chen Y, Zhao FY and
Wu MJ: A clinicopathologic study on basaloid squamous cell
carcinoma in the oral and maxillofacial region. Int J Oral
Maxillofac Surg. 37:1003–1008. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee JS, Ko IJ, Jun SY and Kim JY: Basaloid
squamous cell carcinoma in nasal cavity. Clin Exp Otorhinolaryngol.
2:207–210. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ereño C, Gaafar A, Garmendia M,
Etxezarraga C, Bilbao FJ and López JI: Basaloid squamous cell
carcinoma of the head and neck: a clinicopathological and follow-up
study of 40 cases and review of the literature. Head and Neck
Pathol. 2:83–91. 2008.PubMed/NCBI
|
|
11
|
Barnes L, Ferlito A, Altavilla G,
MacMillan C, Rinaldo A and Doglioni C: Basaloid squamous cell
carcinoma of the head and neck: clinicopathological features and
differential diagnosis. Ann Otol Rhinol Laryngol. 105:75–82. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Emanuel P, Wang B, Wu M and Burstein DE:
p63 immunohistochemistry in the distinction of adenoid cystic
carcinoma from basaloid squamous cell carcinoma. Mod Pathol.
18:645–650. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garnero P, Borel O, Byrjalsen I, et al:
The collagenolytic activity of cathepsin K is unique among
mammalian proteinases. J Biol Chem. 273:32347–32352. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mohamed MM and Sloane BF: Cysteine
cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer.
6:764–775. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Quintanilla-Dieck MJ, Codriansky K, Keady
M, et al: Cathepsin K in melanoma invasion. J Invest Dermatol.
128:2281–2288. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishida M, Kojima F and Okabe H: Cathepsin
K expression in basal cell carcinoma. Eur Acad Dermatol Venereol.
27:e128–e130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Littlewood-Evans AJ, Bilbe G, Bowler WB,
et al: The osteoclast-associated protease cathepsin K is expressed
in human breast cancer. Cancer Res. 57:5386–5390. 1997.PubMed/NCBI
|
|
18
|
Rapa I, Volante M, Cappia S, et al:
Cathepsin K is selectively expressed in the stroma of lung
adenocarcinoma but not in bronchioloalveolar carcinoma. A useful
marker of invasive growth. Am J Clin Pathol. 125:847–854. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yan X, Takahara M, Xie L, et al: Stromal
expression of cathepsin K in squamous cell carcinoma. Eur Acad
Dermatol Venereol. 25:362–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szumilo J, Burdan F, Zinkiewicz K, et al:
Expression of syndecan-1 and cathepsin D and K in advanced
esophageal squamous cell carcinoma. Folia Histochem Cytobiol.
47:571–578. 2009.PubMed/NCBI
|