Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common type of cancer worldwide and the third most common cause of
cancer mortality (1,2). The prognosis of HCC is generally poor.
It represents a major public health problem in the Asia-Pacific
region, where the incidence of viral hepatitis is high. The
incidence of HCC in China alone accounts for 55% of all cases
worldwide (3). By emphasizing the
importance of HCC surveillance in patients with chronic liver
disease in endemic Asian countries, the treatment of small HCC has
become a focus in hepatobiliary surgery. Surgical resection (SR) is
widely accepted as a curative treatment for the majority of
patients with small HCC who are unwilling to receive liver
transplantations (4,5). SR remains the best hope for a cure,
but is suitable for only 9–27% of patients (6,7). The
presence of significant background liver cirrhosis (LC) often
precludes hepatic resection in patients with HCC. Recurrence in the
liver remnant is also common in patients who have undergone radical
hepatic resection (8).
Local ablative techniques, including percutaneous
ethanol injection, microwave coagulation therapy, radiofrequency
ablation and percutaneous cryosurgery (PC) have become increasingly
popular in the treatment of small HCC due to the severity of the
underlying liver disease. PC, also known as cryosurgery, is a
technique based on the in situ freezing and devitalization
of tissues, which may be applied and controlled precisely to
produce a predictable zone of necrosis that destroys the target
lesion, as well as an appropriate margin of surrounding tissue
(9). In China, PC has been widely
used to ablate lung, liver and kidney cancer due to its ease of
use, safety, cost-effectiveness and minimal invasiveness. Previous
studies have shown PC to give good results from the perspective of
tumor control, and PC with ultrasound (US) guidance or computed
tomography (CT) monitoring is feasible, safe and effective for the
treatment of HCC (9,10). However, debate continues with regard
to whether PC or SR is the most suitable therapy for small HCC. In
the present study, a retrospective cohort study was conducted to
compare the results of PC and SR in the treatment of small HCC.
Materials and methods
Characteristics of study
population
Between June 2005 and July 2011, 82 patients with
solitary HCCs ≤3 cm in diameter received curative treatment using
PC or SR at the Third Affiliated Hospital of Harbin Medical
University (Harbin, Heilongjiang, China). Prior to performing PC or
SR, a full discussion was held between the physician and surgeon.
Subsequent to being provided with enough information, including the
contents of the discussion between the physician and surgeon, the
patients themselves made the decision as to whether they received
PC or SR. PC was administered to 24 patients and SR to 58 patients.
Written informed consent was obtained from all patients. The
protocols for PC and SR were approved by the ethics committee of
the Third Affiliated Hospital of Harbin Medical University. The
present study consisted of a retrospective analysis of patient
records. All treatments were performed in an open-label manner. The
primary end point was overall survival (OS) and the secondary end
points were recurrence-free survival (RFS) and adverse events. The
characteristics of the study population are shown in Table I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variable | PC group (n=24) | SR group (n=58) | P-value, T or
χ2 |
|---|
| Mean age, years
(range) | 514±10 | 58±9 | 0.08a |
| Gender | | | 0.68b |
| Male | 16 (66.67) | 33 (56.90) | |
| Female | 8 (33.33) | 25 (43.10) | |
| AFP (ng/ml) | 321±661.78 | 127±370.62 | 0.36a |
| Liver cirrhosis | | | 0.21b |
| With cirrhosis | 16 (66.67) | 39 (67.24) | |
| Without
cirrhosis | 8 (33.33) | 19 (32.76) | |
| Liver function
(Child-Pugh classification) | | | 0.30b |
| Class A | 19 (79.16) | 45 (77.59) | |
| Class B | 5 (20.83) | 13 (22.41) | |
| Tumor size (cm) | 2.25±0.56 | 2.35±0.49 | 0.09a |
| Serum albumin
(g/dl) | 3.89±0.72 | 3.78±0.51 | 0.13a |
| Total bilirubin
(mg/dl) | 0.75±0.41 | 0.87±0.54 | 0.12a |
| Platelet count
(×104/mm3) | 10.92±5.74 | 13.11±3.89 | 0.02a |
| ALT (U/l) | | | |
| >40 | 14 (58.33) | 42 (72.41) | 1.60b |
| ≤40 | 10 (41.67) | 16 (27.59) | |
| AST (U/l) | | | 0.76b |
| >40 | 2 (50.00) | 36 (62.07) | |
| ≤40 | 12 (50.00) | 22 (37.93) | |
HCC diagnosis
HCC was diagnosed using abdominal US and dynamic CT
scans (hyper-attenuation during the arterial phase in all or
certain areas of the tumor and hypo-attenuation in the
portal-venous phase) and/or magnetic resonance imaging (MRI),
mainly based on the recommendations of the American Association for
the Study of Liver Diseases (11).
Arterial and portal phase dynamic CT images were obtained at ∼30
and 120 sec, respectively, following the injection of the contrast
material. With regard to the diagnosis of LC, a specimen resected
at surgery was used for the SR group and a biopsy specimen was used
for the PC group. The baseline characteristics of the two groups
are shown in Table I. There were no
significant differences between the two groups, with the exception
of platelet count (P=0.02).
Equipment
The cryosurgery equipment was an 8-cryoprobe surgery
system manufactured by Endocare Corporation (Irvine, CA, USA), with
superconducting cryoprobes 2, 3 and 5 mm in diameter. US diagnostic
apparatus (GE Healthcare, Milwaukee, WI, USA) and high-speed
16-slice spiral CT (Philips, Amsterdam, The Netherlands) were used
for probe guidance and localization.
Treatment
Cryosurgery
Cryosurgery was performed in a CT room. Following
routine disinfection and local anesthesia, a 0.5-cm incision was
created through the planned puncture spot into the skin and
subcutaneous tissue. US and CT were used to place the guide needle
into the tumor tissue in the liver. The cryoprobes were then
introduced into the tumor using a sheath-and-guidewire technique.
The cryoprobes were stabilized and the sheath was withdrawn by 3–5
cm. The procedure was repeated as additional cryoprobes were
required. Once all the cryoprobes had been placed, the cryosurgery
system was initiated to begin rapid freezing. The temperature of
the cryoprobes was decreased to −100°C within 1 min. The
temperature was then gradually decreased and maintained between
−150°C and −160°C for 20 min. The ice-ball size was monitored
during the freezing process and technical success was defined at
the point when the extension of a visible ice ball was beyond 5 mm
from the tumor margin. Once the freezing cycle was complete, the
heating system was initiated to re-warm the cryoprobes and the
secondary cycle was repeated. Once the freezing and re-warming
cycles were complete, the cryoprobes were withdrawn and a
hemostatic gelatin sponge was used to stop bleeding and fill the
sinuses. Sterile gauze was used to cover and dress the wounds and
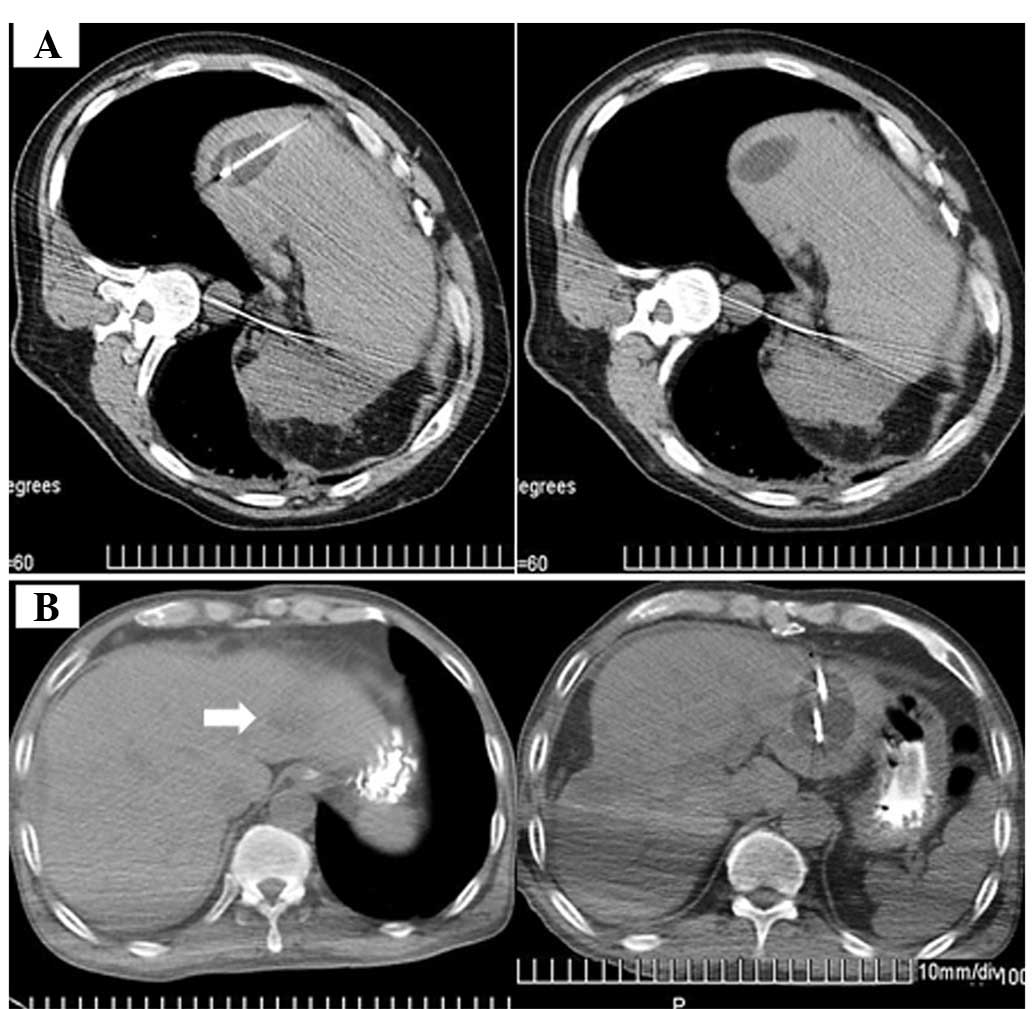
an abdominal bandage was used to fix the gauze. Representative
cryosurgery images are shown in Fig.
1.
SR. SR was performed under general anesthesia
using a right subcostal incision with a midline extension. An
anatomical partial hepatectomy was performed with a resection
margin of ≥1 cm over the tumor, based on intraoperative
ultra-sonography (IOUS) guidance. IOUS was routinely performed to
estimate the location, size and number of vessels feeding the
tumor, as well as to produce an exact vascular map of the liver
anatomy. When liver function approached normal and any adverse
events had disappeared following surgical resection, patient
discharge was permitted.
Evaluation and follow-up
All patients were evaluated by examining serum tumor
markers (α-fetoprotein, AFP), liver function markers [alanine
aminotransferase (ALT) and aspartate aminotransferase, (AST)] and
total bilirubin (T-Bil), as well as tumor changes as visualized
with CT or MRI prior to and following surgery. The primary endpoint
was OS. The secondary endpoints were those of RFS and adverse
events.
Statistical analysis
The levels of serum tumor and liver function markers
were compared prior to and following treatment using a t-test and
χ2 tests. OS was calculated from the date of enrollment
to the date of mortality or last follow-up. The OS and RFS curves
were generated using the Kaplan-Meier method and compared using the
log-rank test. The relative prognostic significance of the
variables for predicting OS was assessed using univariate and
multivariate Cox proportional hazards regression models. All
variables with P<0.05 as evaluated using univariate analysis
were subjected to multivariate analysis. The results of the
multivariate analysis are presented as the hazard ratio (HR) with
the corresponding 95% confidence interval (CI). All statistical
analyses were performed using SPSS software (version 17.0, SPSS
Inc., Chicago, IL, USA). All tests were two-sided and P<0.05 was
considered to indicate a statistically significant difference.
Results
Patients
All patients completed the treatment and follow-up.
There were no surgery-related mortalities. Ultimately, 24 patients
were included in the PC group and 58 patients were included in the
SR group. In addition, no patients succumbed within the
hospitalization period, making the mortality rate 0% for the two
groups.
Patient OS and RFS
The median follow-up periods were 4.7 years (range,
1.7–6.4 years) in the PC group and 4.9 years (range, 2.4–6.5 years)
in the SR group. In the PC group, eight patients (33.33%) succumbed
during the follow-up period. The causes of mortality were all due
to HCC recurrence. In the SR group, 17 patients (29.31%) succumbed
during the follow-up period. The causes of mortality were HCC
recurrence (15 patients) and miscellaneous causes (two patients).
The one-, three- and five-year OS rates following PC and SR were
100, 75.00 and 66.67%, respectively, in the PC group and 100, 77.59
and 70.69%, respectively, in the SR group (Fig. 2). The corresponding RFS rates at
one, three and five years after PC and SR were 83.33, 45.83 and
29.17%, respectively, in the PC group and 84.48, 48.28 and 32.76%,
respectively, in the SR group (Fig.
3). In terms of OS (P=0.584) and RFS (P=0.875), no significant
differences were observed between the two groups.
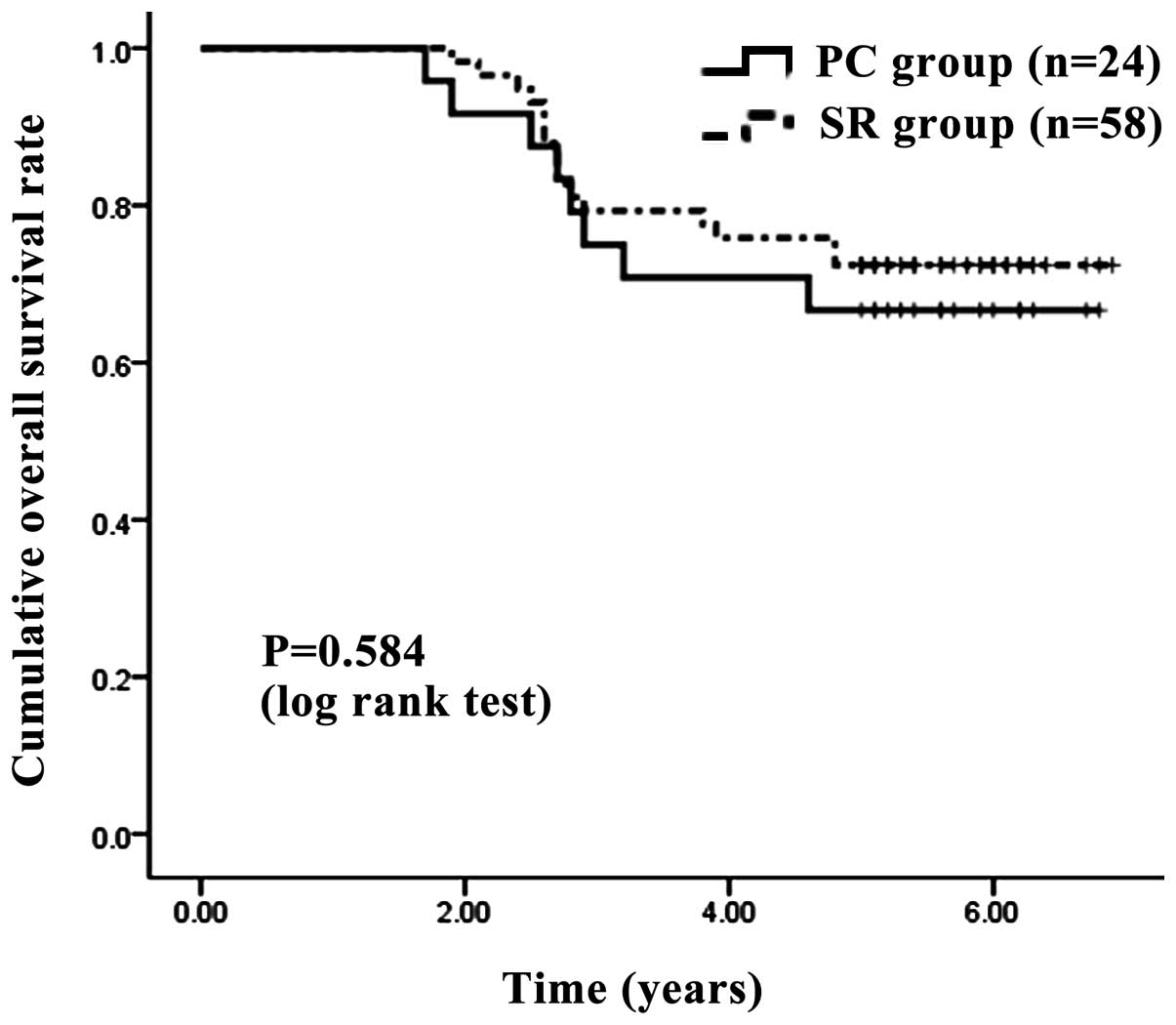 | Figure 2.Cumulative OS rate. The one-, three-
and five-year OS rates after surgery were 100, 75.00 and 66.67%,
respectively, in the PC group and 100, 77.59 and 70.69%,
respectively, in the SR group. No significant difference was
observed between the two groups, as determined using the log-rank
test (P=0.584). OS, overall survival; PC, percutaneous cryosurgery;
SR, surgical resection. |
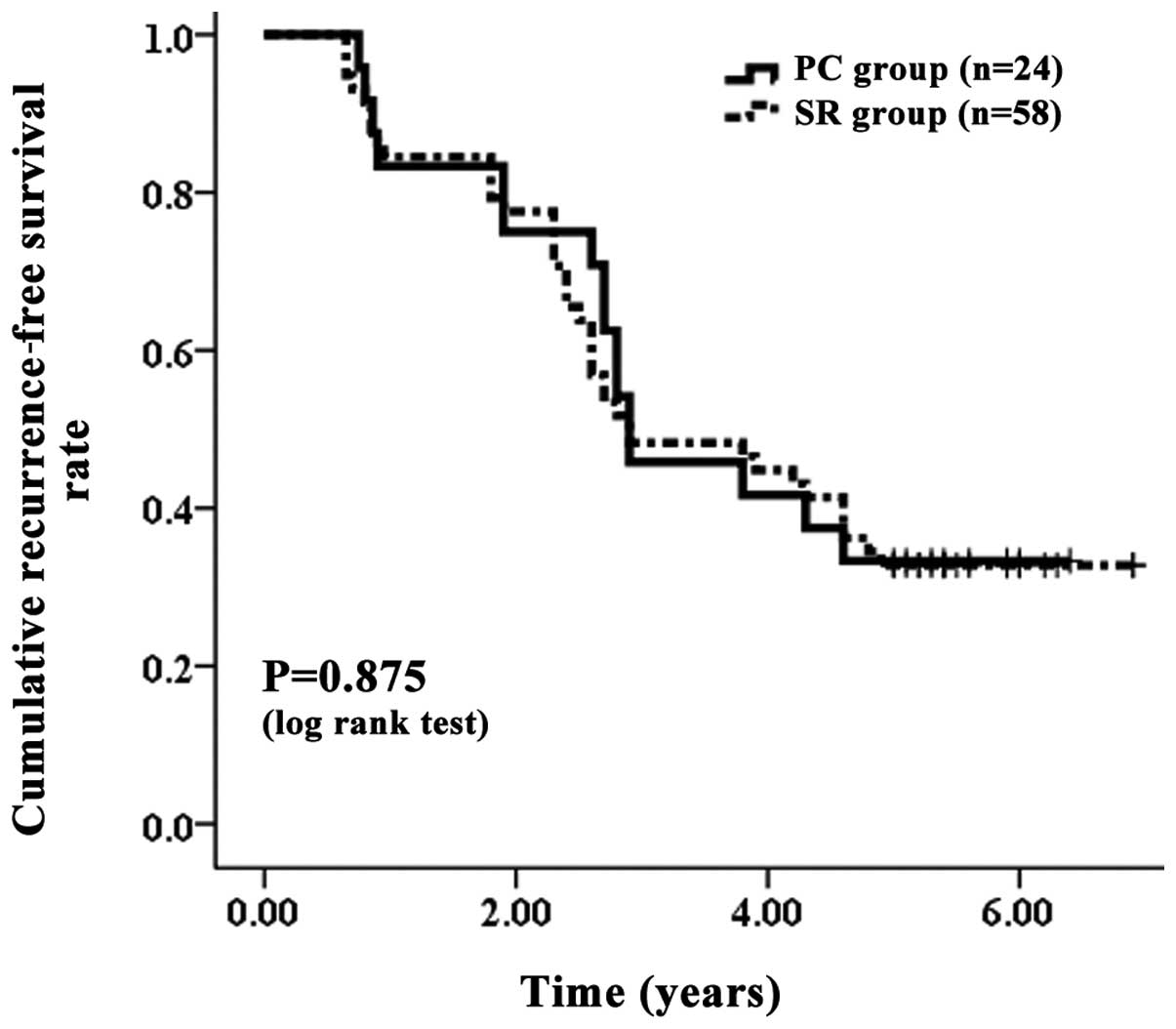 | Figure 3.Cumulative RFS rate. The corresponding
RFS rates at one, three and five years after PC and SR were 83.33,
45.83 and 29.17%, respectively, in the PC group and 84.48, 48.28
and 32.76%, respectively, in the SR group. No significant
differences were observed between the two groups, as determined
using the log-rank test (P=0.875). RFS, recurrence-free survival;
PC, percutaneous cryosurgery; SR, surgical resection. |
Patient OS and RFS with or without
LC
Comparisons were made between PC and SR group
patients with LC. There were 16 PC group patients and 39 SR group
patients with LC. The one-, three- and five-year OS rates were 100,
75.00 and 68.75%, respectively, in the PC group with LC and 100,
76.92 and 66.67%, respectively, in the SR group with LC (Fig. 4). The corresponding RFS rates at
one, three and five years after PC and SR were 81.25, 43.75 and
31.25%, respectively, in the PC group with LC and 84.62, 43.59 and
30.77%, respectively, in the SR group with LC (Fig. 5). In terms of OS (P=0.922) and RFS
(P=0.998) with LC, no significant differences were observed between
the two groups. The PC and SR group patients without LC were also
compared. There were 8 PC group patients and 19 SR group patients
without LC. The one-, three- and five-year OS rates were 100, 75.00
and 62.50%, respectively, in the PC group without LC and 100, 78.95
and 78.95%, respectively, in the SR group without LC (Fig. 6). The corresponding RFS rates at
one, three and five years were 87.50, 50.00 and 37.50%,
respectively, in the PC group without LC and 84.21, 57.89 and
36.84%, respectively, in the SR group without LC (Fig. 7). In terms of the OS (P=0.351) and
RFS (P=0.819) of the patients without LC, no significant
differences were observed between the two groups.
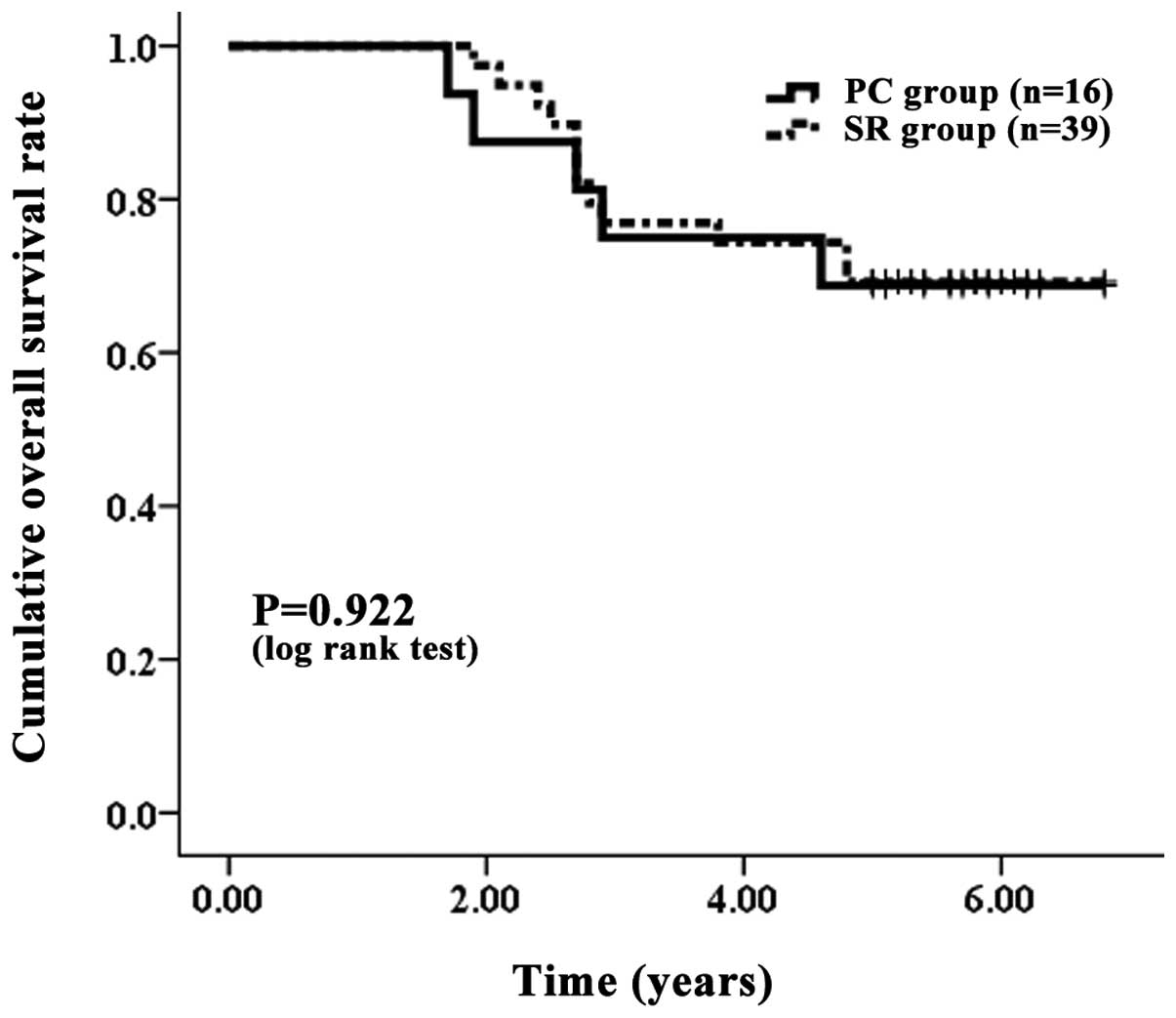 | Figure 4.Cumulative OS rate with LC. The PC
group contained 16 patients with LC and the SR group contained 39
patients with LC. The one-, three- and five-year OS rates were 100,
75.00 and 68.75%, respectively, in the PC group with LC and 100,
76.92 and 66.67%, respectively, in the SR group with LC. No
significant differences were observed between the two groups, as
determined using the log-rank test (P=0.992). OS, overall survival;
LC, liver cirrhosis; PC, percutaneous cryosurgery; SR, surgical
resection. |
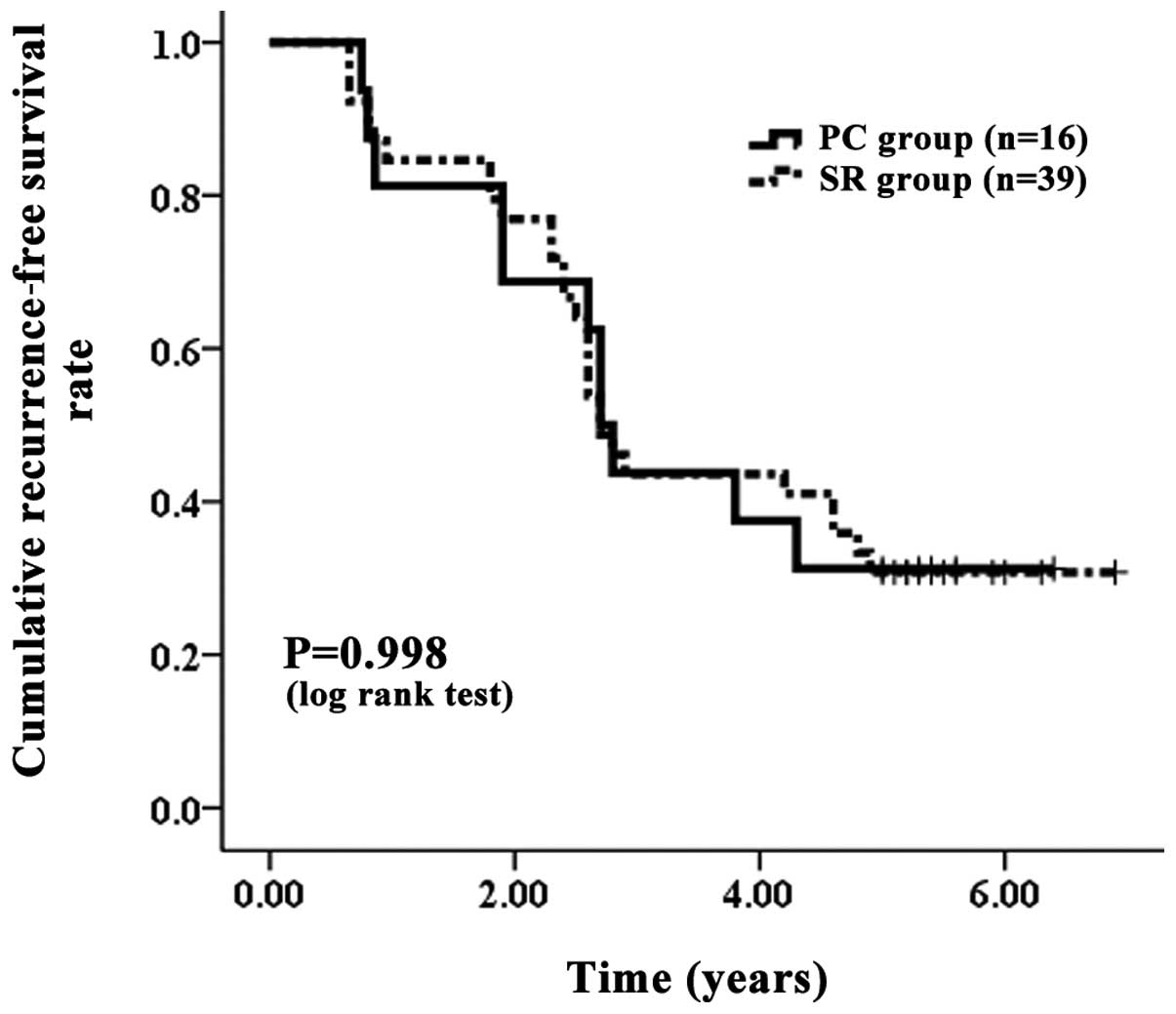 | Figure 5.Cumulative RFS with LC. The PC group
contained 16 patients with LC and the SR group contained 39
patients with LC. The corresponding RFS rates at one, three and
five years after PC and SR were 81.25, 43.75 and 31.25%,
respectively, in the PC group with LC and 84.62, 43.59 and 30.77%,
respectively, in the SR group with LC. No significant differences
were observed between the two groups, as determined using the
log-rank test (P=0.998). RFS, recurrence-free survival; LC, liver
cirrhosis; PC, percutaneous cryosurgery; SR, surgical
resection. |
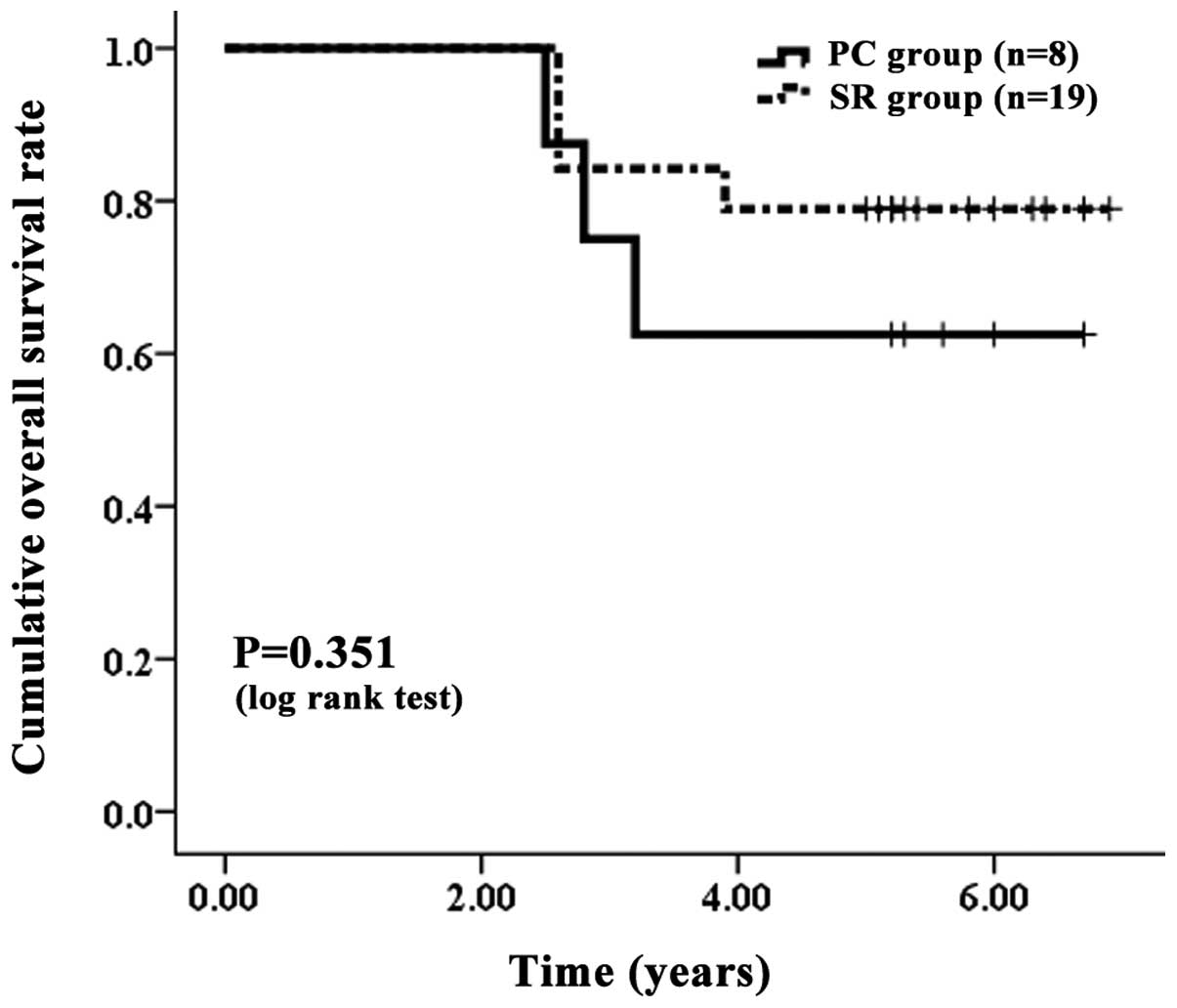 | Figure 6.Cumulative OS rate without LC. The PC
group contained eight patients with LC and the SR group contained
19 patients with LC. The one-, three- and five-year OS rates were
100, 75.00 and 62.50%, respectively, in the PC group without LC and
100, 78.95 and 78.95%, respectively, in the SR group without LC. No
significant differences were observed between the two groups, as
determined using the log-rank test (P=0.351). OS, overall survival;
LC, liver cirrhosis; PC, percutaneous cryosurgery; SR, surgical
resection. |
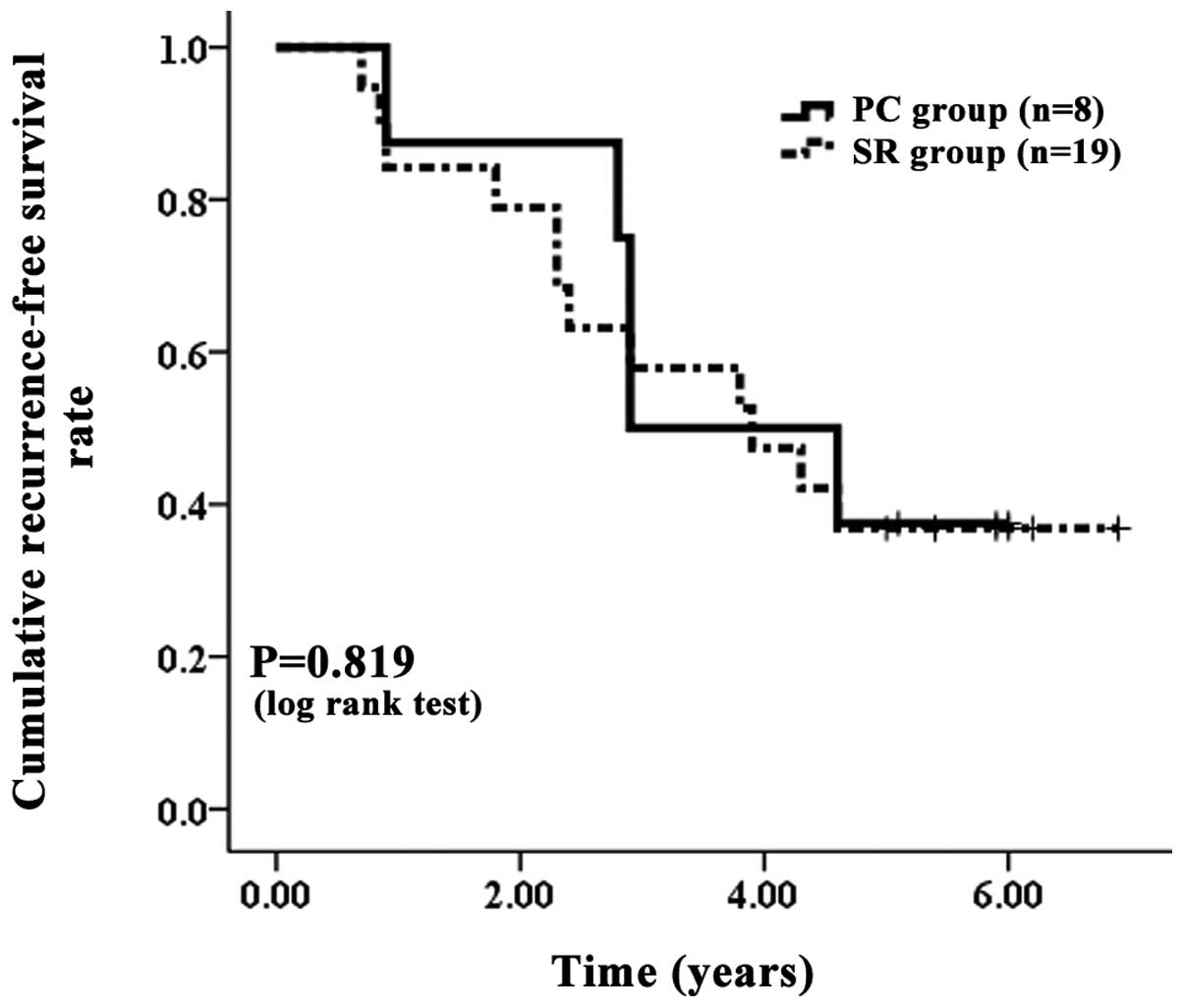 | Figure 7.Cumulative RFS rate without LC. The PC
group contained eight patients with LC and the SR group contained
19 patients with LC. The corresponding RFS rates at one, three and
five years after PC and SR were 87.50, 50.00 and 37.50%,
respectively, in the PC group without LC and 84.2, 57.89 and
36.84%, respectively, in the SR group without LC. There were no
significant differences between these two groups, as determined
using the log-rank test (P=0.819). RFS, recurrence-free survival;
LC, liver cirrhosis; PC, percutaneous cryosurgery; SR, surgical
resection. |
Univariate and multivariate analysis
of prognostic factors contributing to OS and RFS
In the univariate analysis of factors contributing
to OS, serum albumin (P=0.047) and platelet count (P=0.041) were
observed to be significant factors (Table II). However, in the multivariate
analyses involving these two factors, no factors were significant
factors contributing to OS. Similarly, in the univariate analysis
of factors contributing to RFS, only platelet count (P=0.014) was
identified as a significant factor (Table III). However, in the multivariate
analyses involving this factor, it was also not a significant
factor contributing to RFS.
 | Table II.Univariate and multivariate analysis
of the prognostic factors contributing to OS. |
Table II.
Univariate and multivariate analysis
of the prognostic factors contributing to OS.
| Variables | Univariate analysis
| Multivariate analysis
|
|---|
| P-value | Hazard ratio (95%
CI) | P-value |
|---|
| PC vs. SR | 0.370 | 0.927
(0.82–1.273) | 0.685 |
| Gender (male vs.
female) | 0.750 | | |
| Age (>65 vs. ≤65
years) | 0.520 | | |
| LC vs. non-LC | 0.063 | | |
| Tumor size (>2
vs. ≤2 cm) | 0.740 | | |
| Serum AFP (>100
vs. ≤100 ng/ml) | 0.490 | | |
| Serum albumin
(>100 vs. ≤100 g/dl) | 0.047 | 1.223
(0.675–2.334) | 0.263 |
| Platelet count
(>10 vs. ≤10×104/mm3) | 0.041 | 1.117
(0.539–2.183) | 0.588 |
| T-Bil (>1.0 vs.
≤1.0 mg/dl) | 0.260 | | |
 | Table III.Univariate and multivariate analysis
of the prognostic factors contributing to RFS. |
Table III.
Univariate and multivariate analysis
of the prognostic factors contributing to RFS.
| Variables | Univariate analysis
| Multivariate
analysis
|
|---|
| P-value | Hazard ratio (95%
CI) | P-value |
|---|
| PC vs. SR | 0.312 | 0.964
(0.873–1.153) | 0.738 |
| Gender (male vs.
female) | 0.525 | | |
| Age (>65 vs. ≤65
years) | 0.415 | | |
| LC vs. non-LC | 0.023 | 0.766
(0.412–1.168) | 0.157 |
| Tumor size (>2
vs. ≤2 cm) | 0.461 | | |
| Serum AFP (>100
vs. ≤100 ng/ml) | 0.697 | | |
| Serum albumin
(>100 vs. ≤100 g/dl) | 0.097 | | |
| Platelet count
(>10 vs. ≤10×104/mm3) | 0.014 | 1.238
(0.783–1.918) | 0.295 |
| T-Bil (>1.0 vs.
≤1.0 mg/dl) | 0.573 | | |
Serious adverse events
Serious adverse events were more frequent in the SR
group (5/58, 8.62%) compared with the PC group (1/24, 4.17%),
although no statistically significant differences were identified
between these two groups (P=0.820; Table IV). The serious adverse events in
the SR group were as follows: bile leakage (two patients);
refractory ascites (one patient); acute pulmonary embolism (one
patient); and intra-abdominal bleeding (one patient). The only
serious adverse event in the PC group was intra-abdominal bleeding
(one patient).
 | Table IV.Hospitalization duration and serious
adverse events. |
Table IV.
Hospitalization duration and serious
adverse events.
| Variables | PC group
(n=24) | SR group
(n=58) | P-value, T or
χ2 |
|---|
| Duration of
hospitalization (days) | 8.6±4.3 | 15.2±9.7 | <0.01a |
| Serious adverse
events, n (%) | 1 (4.17) | 5 (8.62) | 0.82b |
Duration of hospitalization
The duration of hospitalization was significantly
longer in the SR group (15.2±9.7 days) compared with the PC group
(8.6±4.3 days; P<0.01; Table
IV).
Discussion
The present study demonstrated that PC is a
reliable, safe and minimally invasive method that may be used to
treat small HCC. Firstly, the OS and RFS rates were the same for
patients with solitary HCCs ≤3 cm in diameter treated with either
PC or SR. Secondly, the results showed that PC had certain
advantages over SR, being less invasive, causing less serious
adverse events and resulting in a shorter hospitalization period.
Thirdly, ultrasound and CT were simultaneously applied to guide the
cryoprobes in the present study, which greatly improved the
precision of probe localization, reducing the damage to the normal
liver tissue and vasculature, and thus enhancing the safety of the
therapy.
Patients with cirrhosis are at high risk of
developing malignant disease and US is recommended every six
months. Surveillance with US allows a diagnosis at the early stages
when the tumor may be curable by resection, liver transplantation
or ablation and five-year survival rates >50% may be achieved
(12). LC is an important
prognostic factor of HCC. However, with regard to the OS and RFS of
patients with LC, no differences were observed between the two
groups in the present study. Moreover, in the univariate and
multivariate analysis of factors contributing to OS and RFS, LC was
not identified as a significant prognostic factor. A possible
reason for this may be that all patients who underwent surgery had
improved liver function (the Child-Pugh classification of each
patient was A or B).
It has been reported that patients with small,
solitary tumors and well-preserved liver function are the most
suitable candidates for surgical resection (12). Liver transplantation is the most
beneficial approach for individuals who are not good candidates for
resection. However, donor shortages greatly limit its
applicability. Percutaneous ablation is frequently used for
treatment but its effectiveness is limited by tumor size and
localization (12). Partial
hepatectomy is considered the gold standard therapy, with the aim
of delivering a cure, in patients with resectable HCC who have
normal liver function and are in a good general condition (13). It has become possible to reduce
perioperative mortality to <5% depending on the extent of
resection and hepatic reserve (14). The improved outcome is primarily as
a result of advances in surgical and radiological techniques,
perioperative care and more cautious patient selection (15).
The present long-term follow-up data suggest that PC
and local SR achieve similar survival rates in patients with small
HCC, although a previous study has reported that PC reduces
mortality rates in cancer patients (16). There is a general consensus that a
complete response to PC therapy in patients is associated with an
improved outcome. Therefore, in the present study, patients with
HCC tumors ≤3 cm in size were selected. Moreover, the study also
observed that there were no significant differences between the two
treatment groups in terms of OS and RFS. One possible reason for
this is that a sufficient ablative margin was made around the tumor
by PC, which may have suppressed invasion by micro-dissemination
(Fig. 1). Consequently, obtaining a
sufficient ablative margin around the tumor appears to be essential
in PC therapy. However, the present study had several limitations.
Firstly, it was a retrospective cohort study. Patients who had a
good hepatic reserve tended to receive SR and this may have led to
bias. Secondly, the present study was limited to patients who had
undergone curative treatment. These problems should be resolved in
future prospective studies.
In summary, PC was demonstrated to be as effective
as SR in the treatment of patients with solitary, small HCC who had
undergone curative treatment, while being less invasive and
resulting in shorter hospitalization periods compared with SR.
Cryosurgery caused minimal damage to the normal liver tissue and
resulted in few serious adverse events. In addition, cryosurgery
has also been shown to reduce the risk of disseminating malignant
cells (17), which may be
attributed to the procedures ability to completely necrotize local
tumor tissue. Therefore, PC should be considered for future
application in the treatment of solitary, small HCC.
The present study has clinical significance,
providing optimized individualized treatment selection for patients
with solitary, small HCC.
Abbreviations:
|
HCC
|
hepatocellular carcinoma;
|
|
PC
|
percutaneous cryosurgery;
|
|
SR
|
surgical resection;
|
|
OS
|
overall survival;
|
|
RFS
|
recurrence-free survival;
|
|
LC
|
liver cirrhosis;
|
|
ALT
|
alanine aminotransferase;
|
|
AST
|
aspartate aminotransferase;
|
|
AFP
|
α-fetoprotein;
|
|
T-Bil
|
total bilirubin;
|
|
US
|
ultrasound;
|
|
CT
|
computed tomography;
|
|
MRI
|
magnetic resonance imaging
|
Acknowledgements
The present study was sponsored by
grants from the Foundation of Educational Commission of
Heilongjiang Province (11541224), the Natural Science Foundation of
Heilongjiang Province (D200941) and the Harbin Key Technology
R&D Program, China (007AA3CS083-4).
References
|
1.
|
Amarapurkar D, Han KH, Chan HL and Ueno Y;
Asia-Pacific Working Party on Prevention of Hepatocellular
Carcinoma: Application of surveillance programs for hepatocellular
carcinoma in the Asia-Pacific region. J Gastroenterol Hepatol.
24:955–961. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Bruix J and Sherman M; Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar
|
|
3.
|
Yuen MF, Hou JL and Chutaputti A;
Asia-Pacific Working Party on Prevention of Hepatocellular
Carcinoma: Hepatocellular carcinoma in the Asia-Pacific region. J
Gastroenterol Hepatol. 24:346–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Llovet JM and Bruix J: Novel advancements
in the management of hepatocellular carcinoma in 2008. J Hepatol.
48(Suppl 1): S20–S37. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Duffy JP, Haitt JR and Busuttil RW:
Surgical resection of hepatocellular carcinoma. Cancer J.
14:100–110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
No authors listed:. Liver Cancer Study
Group of Japan: Primary liver cancers in Japan. Cancer.
45:2663–2669. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lai EC, Fan ST, Lo CM, Chu KM, Liu CL and
Wong J: Hepatic resection for hepatocellular carcinoma. An audit of
343 patients. Ann Surg. 221:291–298. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Nishikawa H, Inuzuka T, Takeda H, Nakajima
J, Matsuda F, Sakamoto A, Henmi S, Hatamaru K, Ishikawa T, Saito S,
Nasu A, Kita R, Kimura T, Arimoto A and Osaki Y: Comparison of
percutaneous radiofrequency thermal ablation and surgical resection
for small hepatocellular carcinoma. BMC Gastroenterol. 11:1432011.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Orlacchio A, Bazzocchi G, Pastorelli D,
Bolacchi F, Angelico M, Almerighi C, Masala S and Simonetti G:
Percutaneous cryoablation of small hepatocellular carcinoma with US
guidance and CT monitoring: initial experience. Cardiovasc
Intervent Radiol. 31:587–594. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Shimizu T, Sakuhara Y, Abo D, Hasegawa Y,
Kodama Y, Endo H, Shirato H and Miyasaka K: Outcome of MR-guided
percutaneous cryoablation for hepatocellular carcinoma. J
Hepatobiliary Pancreat Surg. 16:816–823. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Bruix J and Sherman M; Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar
|
|
12.
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 31:379. 1245–1255. 2012.
View Article : Google Scholar
|
|
13.
|
Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH,
Zhang YQ, Lin XJ and Lau WY: A prospective randomized trial
comparing percutaneous local ablative therapy and partial
hepatectomy for small hepatocellular carcinoma. Ann Surg.
243:321–328. 2006. View Article : Google Scholar
|
|
14.
|
Grazi GL, Ercolani G, Pierangeli F, Del
Gaudio M, Cescon M, Cavallari A and Mazziotti A: Improved results
of liver resection for hepatocellular carcinoma on cirrhosis give
the procedure added value. Ann Surg. 234:71–78. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Rahbari NN, Mehrabi A, Mollberg NM, Müller
SA, Koch M, Büchler MW and Weitz J: Hepatocellular carcinoma:
Current management and perspectives for the future. Ann Surg.
253:453–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kerkar S, Carlin AM, Sohn RL, Steffes C,
Tyburski J, Littrup P and Weaver D: Long-term follow up and
prognostic factors for cryotherapy of malignant liver tumors.
Surgery. 136:770–779. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Souna BS, Belot N, Duval H, Langlais F and
Thomazeau H: No recurrences in selected patients after curettage
with cryotherapy for grade I chondrosarcomas. Clin Orthop Relat
Res. 468:1956–1962. 2010. View Article : Google Scholar : PubMed/NCBI
|