Introduction
Osteosarcoma (OS) is the most common primary
mesenchymal malignant tumor of the bone tissue in humans,
particularly children and adolescents (1). It was not until the early 1970’s that
the introduction of doxorubicin and methotrexate with leucovorin
rescue showed promise for improving survival (2). Following the advent of effective
chemotherapy, the five-year survival rate of patients treated with
intensive multidrug chemotherapy and aggressive local control has
been reported at 55–80% (1–3), although this figure has not noticeably
improved in several years. Investigations into the reason for this
are multifaceted and the main aspect is considered to be
drug-resistance during chemotherapy (2).
Gynura procumbens is a decumbent perennial
herb belonging to the Asteraceae family and is widely distributed
in Southeast Asian countries, including China, Indonesia, Thailand
and Malaysia. The stem and leaves of Gynura procumbens have
been used as a food and traditional medicine, particularly in
treating cancer, inflammation, rheumatism and viral infections
(4,5). Pharmacological studies have shown that
Gynura procumbens has anti-inflammatory (6), anti-hypertensive (7,8),
anti-hyperglycemic (9,10), anti-herpes simplex virus (5), anti-oxidative (11) and anti-hyperlipidemic (12) effects. However, it is unclear
whether Gynura procumbens has antitumor activities against
OS, and its potential molecular mechanism is unknown.
Kim et al (13) revealed that the ethanolic extract of
Gynura procumbens inhibited matrix metalloproteinase (MMP)-1
and MMP-9 expression induced by UV-B irradiation via the inhibition
of pro-inflammatory cytokine mediator release and reactive oxygen
species (ROS) production. It has been demonstrated that MMPs are
essential in the degradation of the basement membrane and
epimatrix, among which MMP-2 and MMP-9 are the most markedly
correlated with tumor invasion and metastasis (14–19).
MMP-2 and MMP-9 are overexpressed in osteosarcoma and promote
osteosarcoma cell migration and invasion by degrading certain
components of the basement membrane and epimatrix (20). A large number of studies have
indicated that the activation and transposition of the NF-κB gene,
an upstream gene of MMPs, are closely associated with tumor
invasion and migration (19,21–23).
In the present study, the effect of Gynura
procumbens ethanolic extract (GPE) on cell proliferation,
apoptosis and metastais was analyzed in the OS cell line, U2-OS.
Subsequently, the effect of GPE on the inhibition of the nuclear
transfer of NF-κB in U2-OS cells was investigated.
Materials and methods
Plant material
Whole Gynura procumbens (Lour.) Merr. plants,
excluding the roots, were collected from the Yifeng county of
Jiangxi, China and authenticated at the Jiangxi University of
Traditional Chinese medicine, China, by Professor Luo
Guangming.
Preparation of GPE
The leaves and stems from the fresh plant were
cleaned and dried in an oven at 40°C, then ground into powdered
form at 100 mesh size. A crude ethanolic extract was created by
macerating the powder with 95% ethanol at 85°C for 12 h. The
extract was concentrated until dry in vacuo, with a yield of
1.4%. The extract was then reconstituted in 95% ethanol and vacuum
filtered. The resulting filtrate was subjected to evaporation in
vacuo, which removed the ethanol and left an aqueous solution
containing an ethanol-soluble precipitate. The GPE was dissolved in
dimethyl sulfoxide (DMSO; 100 mg/ml) and the final concentration of
DMSO in the culture medium was controlled at 0.1% (v/v).
Cell lines and cell culture
The human OS cell line, U2-OS, was obtained from the
American Type Culture Collection (Manassas, VA, USA) and routinely
cultured in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Sigma, St. Louis, MO, USA) in a humidified 37°C incubator
containing 5% CO2.
Cell growth assay
The U2-OS cell line was cultured in 96-well tissue
culture plates at a cell density of 5,000 cells per well, in DMEM
containing 10% FBS and 2 mM L-glutamine. Following adherence
overnight, the medium was replaced and the cells were incubated
with increasing concentrations (0, 5, 10, 20, 40, 80 and 160
μg/ml) of GPE. Subsequent to treatment for 24 h,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assays were performed in triplicate at a wave-length of 490 nm.
Flow cytometry (FCM)
Human OS U2-OS cells were seeded at 5×105
cells/ml into T25 culture flasks for 24 h. The cells were then
treated with 0, 10, 20, 40 and 80 μg/ml GPE. Following
incubation, the cells were trypsinized, washed with
phosphate-buffered saline (PBS) and fixed overnight in ice-cold 70%
ethanol. Subsequent to fixation, the cells were washed twice with
1% bovine serum albumin (BSA) in PBS, then resuspended in 1 ml
DNA-binding propidium iodide (PI) solution (10 mg/ml in PBS,
containing 0.05 mg/ml RNase A), incubated at room temperature in
the dark for 15 min and analyzed with an EPICS XL flow cytometer
(Beckman Coulter, Miami, FL, USA). The number of apoptotic cells
were measured using the control software of the flow cytometer.
Western blot analysis
U2-OS cells in the exponential growth phase were
treated with various concentrations of GPE (0, 20 and 40
μg/ml) for 24 h. The cells were then washed with cold PBS.
Total protein from the cells was extracted using
radio-immunoprecipitation assay (RIPA) lysis buffer containing 60
μg/ml phenylmethanesulfonylfluoride (PMSF) and the protein
concentration was determined using a Bradford assay. Equal amounts
of protein were electrophoresed by 10% SDS-PAGE and transferred
onto a pure nitrocellulose blotting membrane (0.22-μm
pores). The membranes were blocked with 5% Difco skimmed milk for 1
h at room temperature (RT), then blocked with primary antibody
(rabbit anti-NF-κBp65 IgG; 1:2,000; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) overnight at 4°C. The membranes were then
washed prior to incubation with the appropriate
peroxidase-conjugated secondary antibodies (anti-rabbit, 1:5,000;
Santa Cruz Biotechnology, Inc.). The immune complexes were detected
with a Pro-light HRP kit (Tiangen, Beijing, China). All experiments
were repeated six times.
Invasion assay
The invasiveness of the U2-OS cells was measured
using BD BioCoat™ BD Matrigel TM Invasion Chambers (BD Bioscience,
Franklin Lakes, NJ, USA) according to the manufacturer’s
instructions. The medium in the lower chamber contained 5% fetal
calf serum as a source of chemoattractant. The cells were suspended
in serum-free medium containing various concentrations of GPE (0 or
40 μg/ml) and added to the upper chambers simultaneously
(2×104 cells/ml in 0.1 ml). The cells that passed
through the Matrigel-coated membrane were stained with Diff-Quik
(Sysmex, Kobe, Japan) and images were captured. Cell migration was
quantified by direct microscopic visualization and counting. The
values for invasion were obtained by counting three fields per
membrane and the results are presented as the average of six
independent experiments performed over multiple days.
Migration assay
Cell migration was assessed by determining the
ability of the cells to move into a cellular space in a
two-dimensional in vitro wound healing assay. In brief, the
cells were grown to confluence in six-well tissue culture plastic
dishes to a density of ∼5×106 cells/well. Subsequent to
being treated with various concentrations of GPE (0 or 40.0
μg/ml) for 24 h, the cells were denuded by dragging a rubber
policeman (Fisher Scientific, Hampton, NH, USA) through the center
of the plate. The cultures were rinsed with PBS and fresh quiescent
medium alone or with 10% FBS was added, after which the cells were
incubated at 37°C for 24 h. The cells were photographed at 0 and 24
h and the migrated distance was measured. The cell migration rate
was obtained by counting three fields per area and the results
presented as the average of six independent experiments performed
over multiple days.
Statistical analysis
Data are expressed as the mean ± SD. The differences
in invasion and migration between cells treated with GPE and the
control group were evaluated using independent-sample t-tests.
P<0.05 was considered to indicate a statistically significant
difference. All analyses were performed using SPSS Version 13.0
(SPSS Inc., Chicago, IL, USA).
Results
Effect of GPE on U2-OS cell proliferation
in vitro
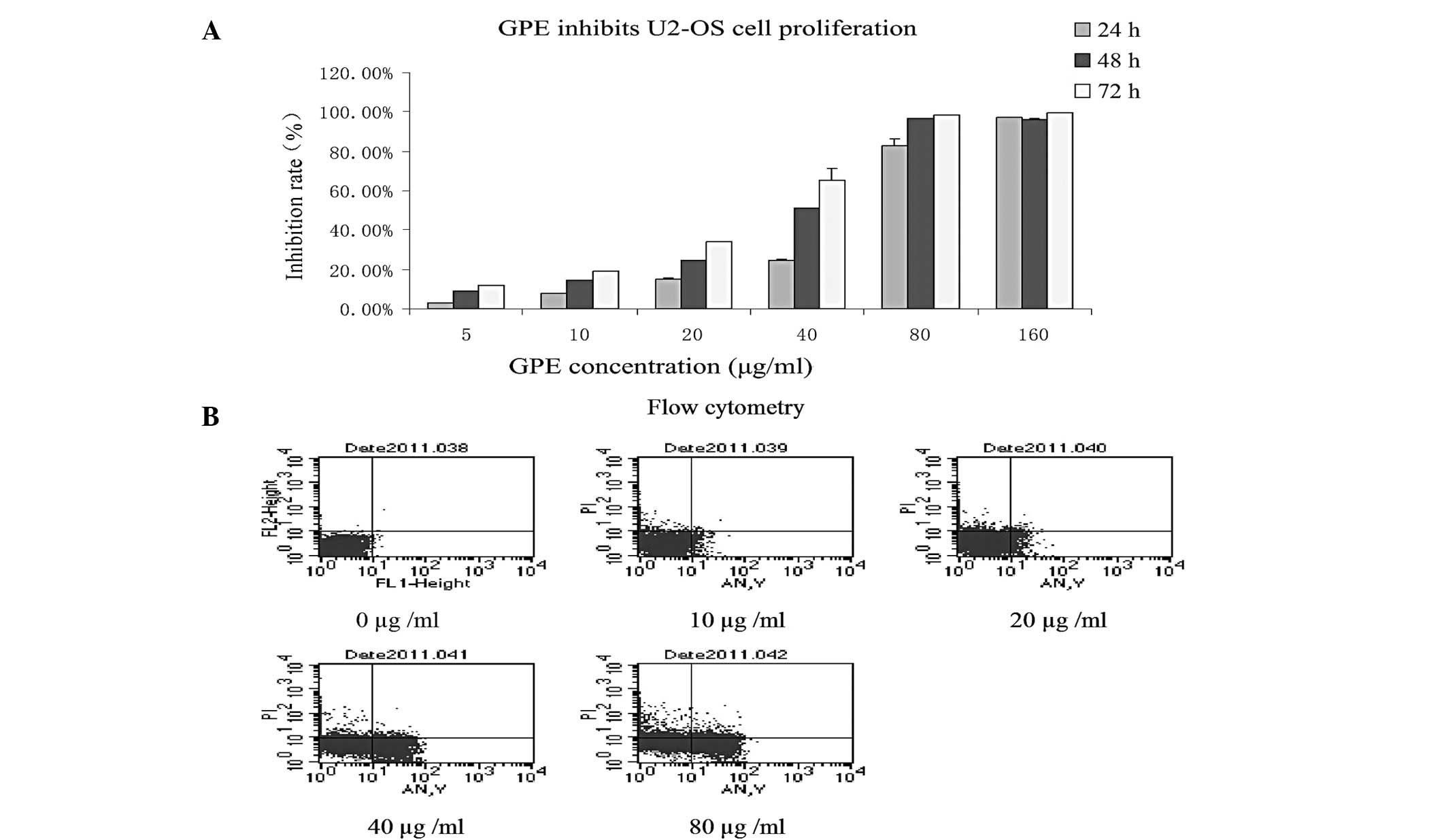
The effect of GPE on the growth of the U2-OS cell
line was investigated using MTT assays. The growth curves indicated
that the U2-OS cells were sensitive to GPE and that growth
inhibition occurred in a dose- and time-dependent manner (Fig. 1A), indicating that GPE was able to
inhibit U2-OS cell proliferation in vitro.
GPE induces U2-OS cell apoptosis
FCM analysis was used to investigate the effect of
GPE in inducing U2-OS cell apoptosis in vitro. GPE at
various concentrations was added to the U2-OS cell cultures in the
exponential growth phase. Subsequent to treatment for 24 h, treated
and untreated cell samples were obtained and fixed for FCM
analysis. The FCM analysis demonstrated that the percentages of
apoptotic cells were 0, 5.5, 7.6, 24.7 and 37.94% at 0, 10, 20, 40
and 80 μg/ml GPE. This indicates that apoptosis occurred in
a dose-dependent manner in cells treated with GPE (Fig. 1B) and GPE was able to induce U2-OS
cell apoptosis in vitro.
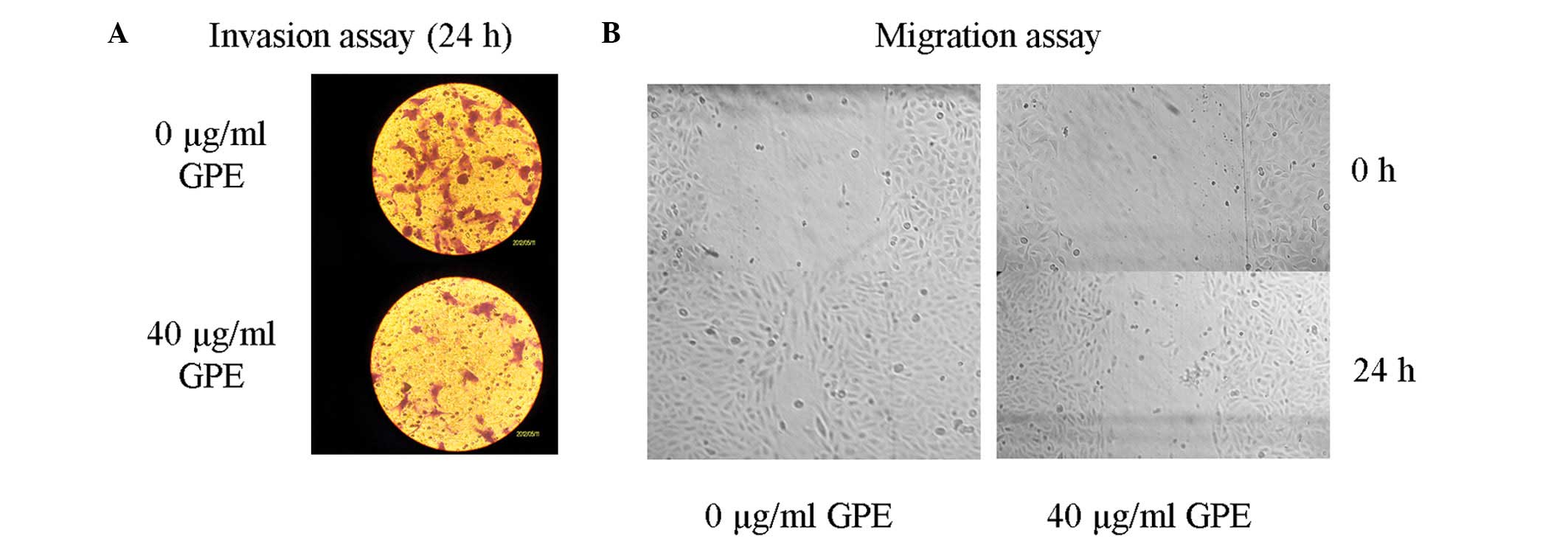
GPE inhibits U2-OS cell invasion in
vitro
The appropriate GPE concentration for the transwell
invasion cell assay was determined according to the IC50
value. Invasion was measured using a transwell assay to analyze the
effect of GPE on the invasiveness of the U2-OS cells (Fig. 2A). The cells were treated with 40
μg/ml GPE for 24 h. The results showed that the invasion of
the cells treated with GPE was significantly inhibited compared
with the untreated cells, suggesting that GPE was able to suppress
U2-OS cell invasion.
GPE inhibits U2-OS cell migration in
vitro
The appropriate GPE concentration for the migration
assay was determined according to the IC50 value.
Migration was measured using a migration assay to investigate the
effect of GPE on the invasion of the U2-OS cells. The cells were
treated with 40 μg/ml GPE for 24 h. The results showed that
the cell migration of the cells treated with GPE was significantly
inhibited compared with the untreated cells, indicating that GPE
was able to suppress U2-OS cell migration (Fig. 2B).
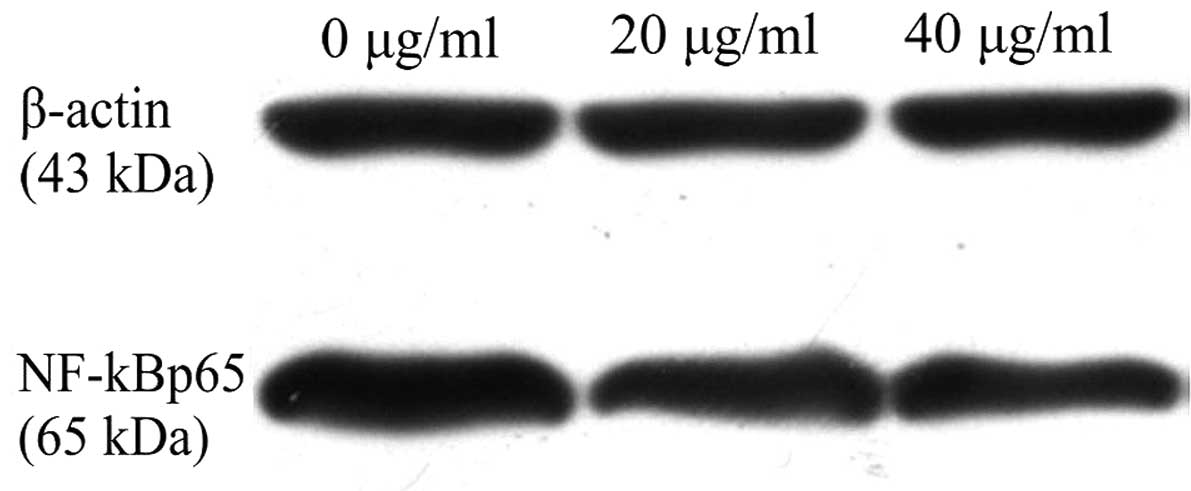
GPE suppresses the nuclear transfer of
NF-κB
To investigate the effect of GPE on the nuclear
transfer of NF-κB, the protein expression level of NF-κBp65 was
detected. The results showed that NF-κBp65 protein expression was
decreased significantly in the cells treated with GPE for 24 h
compared with the untreated cells (Fig.
3), suggesting that GPE was able to inhibit the nuclear
transfer of NF-κB in the U2-OS cells.
Discussion
OS is the most common primary bone tumor in children
and adolescents, with a five-year disease free survival rate of
70%. However, this figure has not noticeably improved over the past
several years, mainly due to drug-resistance during chemotherapy
and metastasis of the disease. Hence, it is necessary to develop
novel therapeutic agents.
Herbal medicine is gaining popularity in developing
countries. Gynura procumbens (Lour.) Merr., which is also
known as ‘Sambung nyawa’, is widely used in Southeast Asian
countries as a herbal medicine in the traditional treatment of
numerous ailments, including eruptive fevers, rashes, kidney
disease, migraines, constipation, hypertension and diabetes
mellitus. The benefits of the traditional use of Gynura
procumbens have also been supported by the isolation and
identification of several possible active chemical components from
this plant, including flavonoids, saponins, tannins and terpenoids
(10). However, there is little
evidence with regard to the anticancer activity of Gynura
procumbens. In the present study, U2-OS cells were treated with
GPE at various concentrations and the proliferation and apoptosis
were measured by MTT and FCM analysis to investigate the effects of
Gynura procumbens on tumor cell proliferation and apoptosis,
respectively. The results demonstrated that cell proliferation was
inhibited by GPE in a dose- and time-dependent manner and the rate
of apoptosis was increased in cells treated with GPE, indicating
that GPE was able to induce apoptosis and inhibit proliferation in
U2-OS cells. Additionally, transwell invasion and migration assays
were performed to evaluate the effect of GPE on U2-OS cell invasion
and migration. The results revealed that the invasion and migration
abilities of cells treated with GPE were significantly lower
compared with cells that were not treated with GPE. This suggested
that GPE was able to inhibit U2-OS cell invasion and migration
in vitro.
The potential molecular mechanism of the inhibition
of U2-OS cell invasion and migration by GPE remains unclear. Kim
et al (13) reported that
the ethanolic extract of Gynura procumbens inhibited MMP-1
and MMP-9 expression induced by UV-B irradiation via the inhibition
of proinflammatory cytokine mediator release and ROS production.
MMPs are enzymes that are directly responsible for the degradation
of extracellular matrix (ECM) components, such as collagen and
elastin. The degradation of ECM components has a significant role
in tumor cell metastasis (24).
Among all the MMPs, MMP-2 and MMP-9 are recognized as being
particularly involved in the degradation of ECM components
(14–19). The primary form of NF-κB is a
heterodimer of the p50 and p65 subunits, which is localized mainly
in the cytoplasm in an inactive form bound to an inhibitory protein
named IκB (25). It has been shown
previously that NF-κB upregulates MMP-9 (22), while the inhibition of NF-κB
downregulates MMP-2 (23). In the
present study, attempts were made to investigate whether GPE was
able to regulate the nuclear translocation of NF-κB, resulting in
the deregulation of the expression of MMPs in OS cells. The effect
of GPE on the nuclear translocation of NF-κB was measured by
detecting the expression of the NF-κBp65 protein. From the western
blotting analysis, it was observed that the NF-κBp65 protein
expression level was significantly lower in the U2-OS cells treated
with GPE compared with the untreated cells. These results indicate
that Gynura procumbens extract may inhibit the nuclear
translocation of NF-κB.
In conclusion, the present study showed that GPE was
able to significantly inhibit U2-OS cell proliferation and
metastasis in vitro and that the inhibition of the nuclear
translocation of NF-κB appeared to be the potential molecular
mechanism. However, previous studies have shown that the tumor
micro-enviroment may affect tumor cell proliferation, invasion and
migration. Consequently, further in vivo experiments are
necessary to confirm the anticancer activity of Gynura
procumbens extract. Based on the results observed in the
present study, it appears that further advances in the
identification of the active principles of GPE are likely to
provide more solid evidence of Gynura procumbens as an
antitumor agent.
Acknowledgements
The present study was supported by
grants from the Health Department of Jiangxi Province (No.
2011A147).
References
|
1.
|
Longhi A, Errani C, De Paolis M, et al:
Primary bone osteosarcoma in the pediatric age: state of the art.
Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Meyers PA, Schwartz CL, Krailo M, et al:
Osteosarcoma: a randomized, prospective trial of the addition of
ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and
high dose methotrexate. J Clin Oncol. 23:2004–2011. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Jawad MU, Cheung MC, Clarke J, et al:
Osteosarcoma: improvement in survival limited to high-grade
patients only. J Cancer Res Clin Oncol. 137:597–607. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Rosidah, Yam MF, Sadikun A, et al:
Toxicology evaluation of standardized methanol extract of Gynura
procumbens. J Ethnopharmacol. 123:244–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Nawawi A, Nakamura N, Hattori M, et al:
Inhibitory effects of Indonesian medicinal plants on the infection
of herpes simplex virus type 1. Phytother Res. 13:37–41. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Iskander MN, Song Y, Coupar IM and
Jiratchariyakul W: Antiinf lammatory screening of the medicinal
plant Gynura procumbens. Plant Foods Hum Nutr. 57:233–244.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Hoe SZ, Lee CN, Mok SL, et al: Gynura
procumbens Merr. decreases blood pressure in rats by
vasodilatation via inhibition of calcium channels. Clinics (Sao
Paulo). 66:143–150. 2011. View Article : Google Scholar
|
|
8.
|
Kim MJ, Lee HJ, Wiryowidagdo S and Kim HK:
Antihypertensive effects of Gynura procumbens extract in
spontaneously hypertensive rats. J Med Food. 9:587–590. 2006.
|
|
9.
|
Akowuah GA, Amirin S, Mariam A and Aminah
I: Blood sugar lowering activity of Gynura procumbens leaf
extracts. J Trop Med Plants. 2:5–10. 2001.
|
|
10.
|
Akowuah GA, Sadikun A and Mariam A:
Flavonoid identification and hypoglycaemic studies of butanol
fraction from Gynura procumbens. Pharm Biol. 40:405–410.
2002. View Article : Google Scholar
|
|
11.
|
Rosidah, Yam MF, Sadikun A and Asmawi M:
Antioxidant potential of Gynura procumbens. Pharm Biol.
46:616–625. 2008. View Article : Google Scholar
|
|
12.
|
Zhang XF and Tan BK: Effects of an
ethanolic extract of Gynura procumbens on serum glucose,
cholesterol and triglyceride levels in normal and
streptozotocin-induced diabetic rats. Singapore Med J. 41:9–13.
2000.
|
|
13.
|
Kim J, Lee CW, Kim EK, et al: Inhibition
effect of Gynura procumbens extract on UV-B-induced
matrix-metalloproteinase expression in human dermal fibroblasts. J
Ethnopharmacol. 137:427–433. 2011.
|
|
14.
|
Davies B, Miles DW, Happerfield LC, et al:
Activity of type IV collagenases in benign and malignant breast
disease. Br J Cancer. 67:1126–1131. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Davies B, Waxman J, Wasan H, et al: Levels
of matrix metalloproteases in bladder cancer correlate with tumour
grade and invasion. Cancer Res. 53:5365–5369. 1993.PubMed/NCBI
|
|
16.
|
Iwata H, Kobayashi S, Iwase H, et al:
Production of matrix metalloproteinases and tissue inhibitors of
metalloproteinases in human breast carcinomas. Jpn J Cancer Res.
87:602–611. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Tokuraku M, Sato H, Murakami S, et al:
Activation of the precursor of gelatinase A/72 kDa type IV
collagenase/MMP-2 in lung carcinomas correlates with the expression
of membrane-type matrix metalloproteinase (MT-MMP) and with lymph
node metastasis. Int J Cancer. 64:355–359. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Brown PD, Bloxidge RE, Stuart NS, et al:
Association between expression of activated 72-kilodalton
gelatinase and tumour spread in non-small-cell lung carcinoma. J
Natl Cancer Inst. 85:574–578. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Zhang XX, Fu Z, Zhang Z, et al:
Microcystin-LR promotes melanoma cell invasion and enhances matrix
metalloproteinase-2/-9 expression mediated by NF-κB activation.
Environ Sci Technol. 46:11319–11326. 2012.PubMed/NCBI
|
|
20.
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–74. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Tsuruda T, Costello-Boerrigter LC and
Burnett JC Jr: Matrix metalloproteinases: pathways of induction by
bioactive molecules. Heart Fail Rev. 9:53–61. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Andela VB, Gordon AH, Zotalis G, et al:
NF-kappaB: a pivotal transcription factor in prostate cancer
metastasis to bone. Clin Orthop Relat Res. (415 Suppl): S75–S85.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Felx M, Guyot MC, Isler M, et al:
Endothelin-1 (ET-1) promotes MMP-2 and MMP-9 induction involving
the transcription factor NF-kappaB in human osteosarcoma. Clin Sci
(Lond). 110:645–654. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Werb Z: ECM and cell surface proteolysis:
regulating cellular ecology. Cell. 91:439–442. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Finco TS and Baldwin AS: Mechanistic
aspects of NF-kappa B regulation: the emerging role of
phosporylation and proteolysis. Immunity. 3:263–272. 1995.
View Article : Google Scholar : PubMed/NCBI
|