Introduction
Krüppel-associated box (KRAB)-containing zinc finger
(KRAB-ZNF) proteins, which represent the largest single family of
transcriptional regulators in mammals (1), have been shown to regulate gene
expression by binding to target DNA sequences through the zinc
finger domain, thereby allowing KRAB to repress transcription
(2,3). However, little is known concerning the
biological functions of KRAB-ZNF proteins (4). ZNF268, which was isolated from a human
embryo cDNA library (5), is a
typical KRAB-containing zinc finger protein that has been observed
to produce eight splice variants and is translated into two
proteins, ZNF268a and ZNF268b2 (6).
ZNF268a contains a KRAB domain and up to 24 zinc fingers and may
function as a transcriptional repressor (7), while ZNF268b2 consists of only the
zinc finger domain and contributes to human cervical cancer via the
NF-κB signalling pathway (8). The
ZNF268 promoter is located in the first exon of the gene and is
regulated by cAMP response element binding protein 2 (CREB-2)
(9). Previous studies have
suggested that ZNF268 may be involved in human foetal liver
development (10), haematological
diseases (11–13) and cervical cancer development
(8). Based on tissue microarray
results, ZNF268 may be a multifunctional molecule that functions as
either a promoter or a suppressor, depending on the cancer subtype
(8). This hypothesis is supported
by findings indicating that ZNF268-knockdown promotes the
proliferation of erythroleukemia K562 cells (14), while inhibiting the growth of
cervical cancer HeLa cells (8).
However, the function of ZNF268 in ovarian tissues remains to be
determined.
Ovarian cancer is one of the most lethal types of
gynaecological cancer and the seventh leading cause of cancer
mortality among females worldwide (15). Its incidence in Asian countries is
increasing (16). The high
mortality associated with ovarian cancer is largely due to the
asymptomatic nature of early stages of the disease (prior to the
development of widespread metastases) and the significant failure
rate of chemotherapy for curing the advanced disease (17,18).
At present, the pathogenic mechanisms underlying the development of
human ovarian cancers are too complicated to be fully understood
(19). Therefore, there is an
urgent requirement to investigate the cellular and molecular
mechanisms underlying ovarian carcinogenesis and identify novel
therapeutic targets that improve the survival of patients with
ovarian cancer.
The present study demonstrated that ZNF268 was
overexpressed in human ovarian cancer tissues and that
ZNF268-knockdown increased the proliferation, while simultaneously
decreasing the migration, of SKOV-3 ovarian cancer cells. The
effects of ZNF268 on SKOV-3 cell growth may be mediated by altering
cell cycle progression. The results obtained from the current study
are likely to provide an improved understanding of the molecular
mechanisms underlying ovarian cancer progression and demonstrate
the potential of ZNF268 as a novel therapeutic target for ovarian
cancer.
Materials and methods
Cell culture, tissue specimens and
immunohistochemistry
SKOV-3 cells (CCTCC, Wuhan, China) were grown in
McCoy’s 5A medium supplemented with 10% fetal bovine serum (FBS;
Invitrogen, Carlsbad, CA, USA), penicillin (100 units/ml) and
streptomycin (100 μg/ml) at 37°C in a 5% CO2
incubator. Four paraffin-embedded normal ovarian specimens and 20
paraffin-embedded ovarian carcinoma specimens were obtained during
routine clinical practice from female patients who were undergoing
either biopsy or surgery at the Department of Pathology, Zhongnan
Hospital, Wuhan University (Wuhan, Hebei, China). The study was
approved by the Regional Committee of Medical Research Ethics in
China and informed consent was obtained from each patient.
All immunohistochemical staining assays were
performed by the Jiayuan Quantum Dots Company (Wuhan, China)
according to the standard procedure. The expression levels of the
examined proteins were scored by two independent pathologists who
had no knowledge of the clinical or histopathological data. The
expression levels were scored as follows: 0 points (−, no
detectable staining), one point (+, weak staining), two points (++,
clear but not strong staining) and three points (+++, marked
staining) (20).
Establishment of ZNF268 stable knockdown
SKOV-3 cells
SKOV-3 cells were seeded in 60-mm dishes at a
density of 3.0×104 cells/dish. When the cells had
reached ∼70% confluence, they were infected with shZNF268 or sh
control lentiviral particles as described previously (14). Flow cytometry was used to sort the
GFP-positive cells. Reduced ZNF268 expression was confirmed at the
mRNA and protein levels (14).
Western blotting
The cells were collected and lysed in RIPA buffer on
ice for 15 min, followed by centrifugation at 16,000 x g at 4°C for
10 min. The supernatants were collected and subjected to western
blotting according to the standard procedure as described
previously (8). The antibodies used
for the western blotting analysis were as follows: Actin antibody
was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA); cyclin D2, cyclin E2 and CDK2 antibodies were purchased from
Cell Signaling Technology (Danvers, MA, USA); and anti-SD antibody
for the detection of ZNF268 (produced in our lab) was used as
described previously (6).
RNA isolation, reverse transcription and
quantitative real-time PCR
RNA was extracted using TRIzol reagent (Invitrogen).
cDNA was prepared according to the manufacturer’s instructions
(Toyobo, Osaka, Japan). Real-time PCR was performed and analysed
using an ABI 7500 detection system. The relative mRNA levels of
ZNF268 in each sample were normalised to GAPDH as described
previously (14).
MTT assay
The SKOV-3 cells were plated on 96-well cell culture
plates at a density of 2.0×103 cells/well. At designated
time-points, the cells were incubated with 20 μl
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide dye
(MTT, 5 mg/ml) for 2 h, followed by solubilisation for 10 min in
DMSO (100 μl/well), with agitation at room temperature. The
absorbance was determined at 570 nm using a microplate reader
(El×800, BioTek Instruments, Inc., Winooski, VT, USA).
Soft agar assay
The cells were trypsinised and suspended in 2 ml top
agar containing 10% FBS and 0.3% agarose. The mixture was then
plated onto 60-mm dishes containing 2 ml bottom agar with 10% FBS
and 0.6% agarose. Subsequent to incubation for 3 weeks, 1 ml of a 1
mg/ml solution of 2-(p-iodophenyl)-3-(p-nitrophenyl)-5-phenyl
tetrazolium chloride was added. After 4 h, images of the plates
were captured and the number of colonies was counted.
Cell cycle progression and apoptosis
assays
Cell cycle progression and apoptosis were assessed
using flow cytometric analysis to measure the DNA content. Briefly,
the SKOV-3 cells (1.0×106) were collected, washed twice
with PBS and resuspended in ice cold 70% ethanol for 2 h at 4°C.
The cells were then washed twice with PBS and digested using RNase
A (1 mg/ml) at 37°C for 30 min. The cells were stained with
propidium iodide (PI, 5 μg/ml) for 1 h at 4°C and analysed
using a Beckman Coulter Epics XL flow cytometer (Beckman Coulter,
Miami, FL, USA).
Nude mouse tumour formation assay
Five-week-old male nude mice (Balb/c nu/nu) were
purchased from SJA Lab Animal Limited Company (Changsha, China).
The animals were housed in a specific pathogen-free facility and
maintained in a temperature-controlled environment on a 12-h
light/dark cycle with free access to sterilised food and autoclaved
water. The SKOV-3 cells in the exponential growth phase were
trypsinised into single-cell suspensions and injected
subcutaneously into the nude mice. All mice were maintained for 30
days prior to being sacrificed. All animal experiments were
approved by the Animal Research Ethics Board of Wuhan University
and were conducted in compliance with the institutional guidelines
for the care of experimental animals.
Wound healing migration assay
Either the SKOV-3 or HeLa cells (1×105)
were plated onto six-well plates and allowed to form a confluent
monolayer. The cell monolayer was then scratched in a straight line
to make a ‘scratch wound’ with a 0.2-ml pipette tip and the cell
debris was removed by washing the cells with phosphate-buffered
saline. McCoy’s 5A medium (for the SKOV-3 cells) or DMEM medium
(for the HeLa cells) supplemented with 1% FBS was added, and images
of the closure of the scratch were captured at 0, 24 and 48 h.
Statistical analysis
The data are represented as the mean ± SD of the
samples. All experiments were repeated three times. A two-tailed
Student’s t-test was used to compare the differences between the
two experimental groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
ZNF268 is overexpressed in human ovarian
cancer tissues
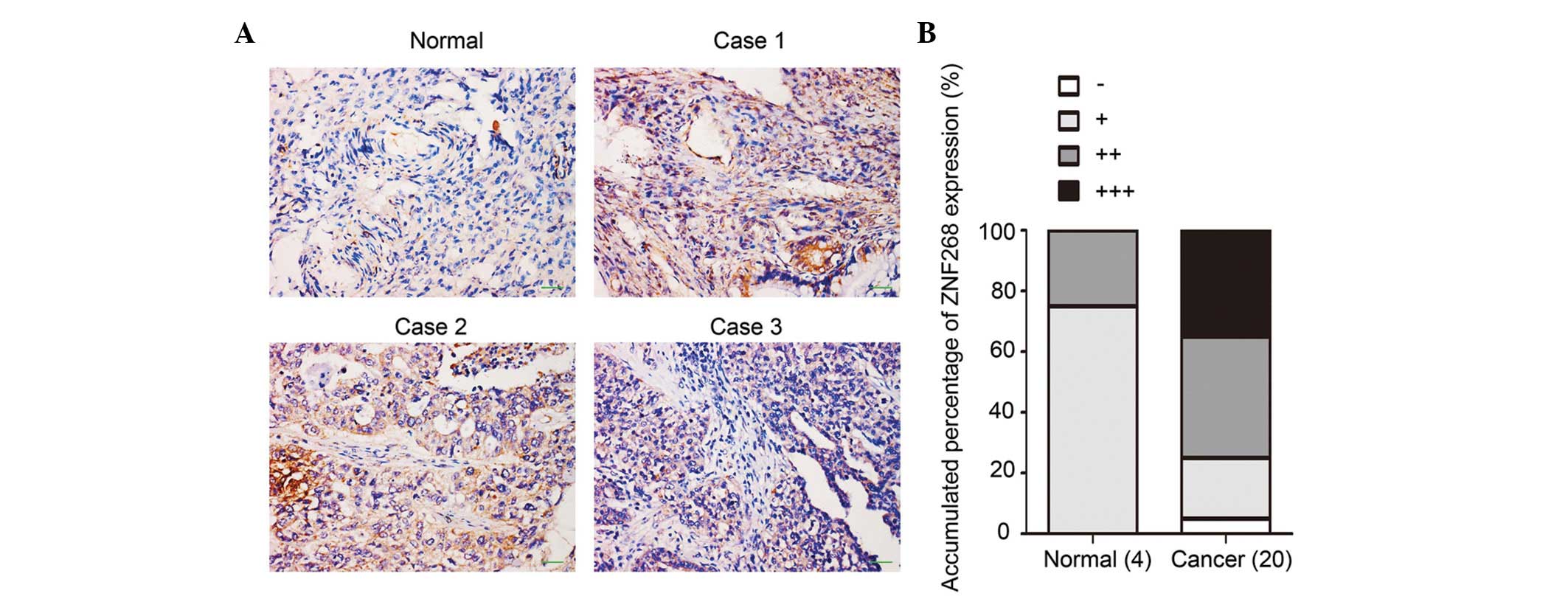
We previously observed different expression patterns
of ZNF268 in normal human ovarian tissues compared with ovarian
cancer tissues using the tissue microarray method. ZNF268 was
overexpressed in the majority of the ovarian cancer tissues (∼84%),
while ZNF268 expression was rarely detected in the normal tissues
(8). To confirm these results, more
ovarian tissues (four normal and 20 cancerous specimens) were
obtained and subjected to immunohistochemistry with an anti-SD
antibody to detect ZNF268 expression (6). The majority of the cancerous samples
exhibited high levels of ZNF268 expression (75% with ++/+++
staining) compared with the expression levels observed in the
normal tissues (75% with −/+ staining; Fig. 1A and B). These results suggested
that ZNF268 was overexpressed in human ovarian cancer tissues.
ZNF268-knockdown promotes SKOV-3 cell
growth
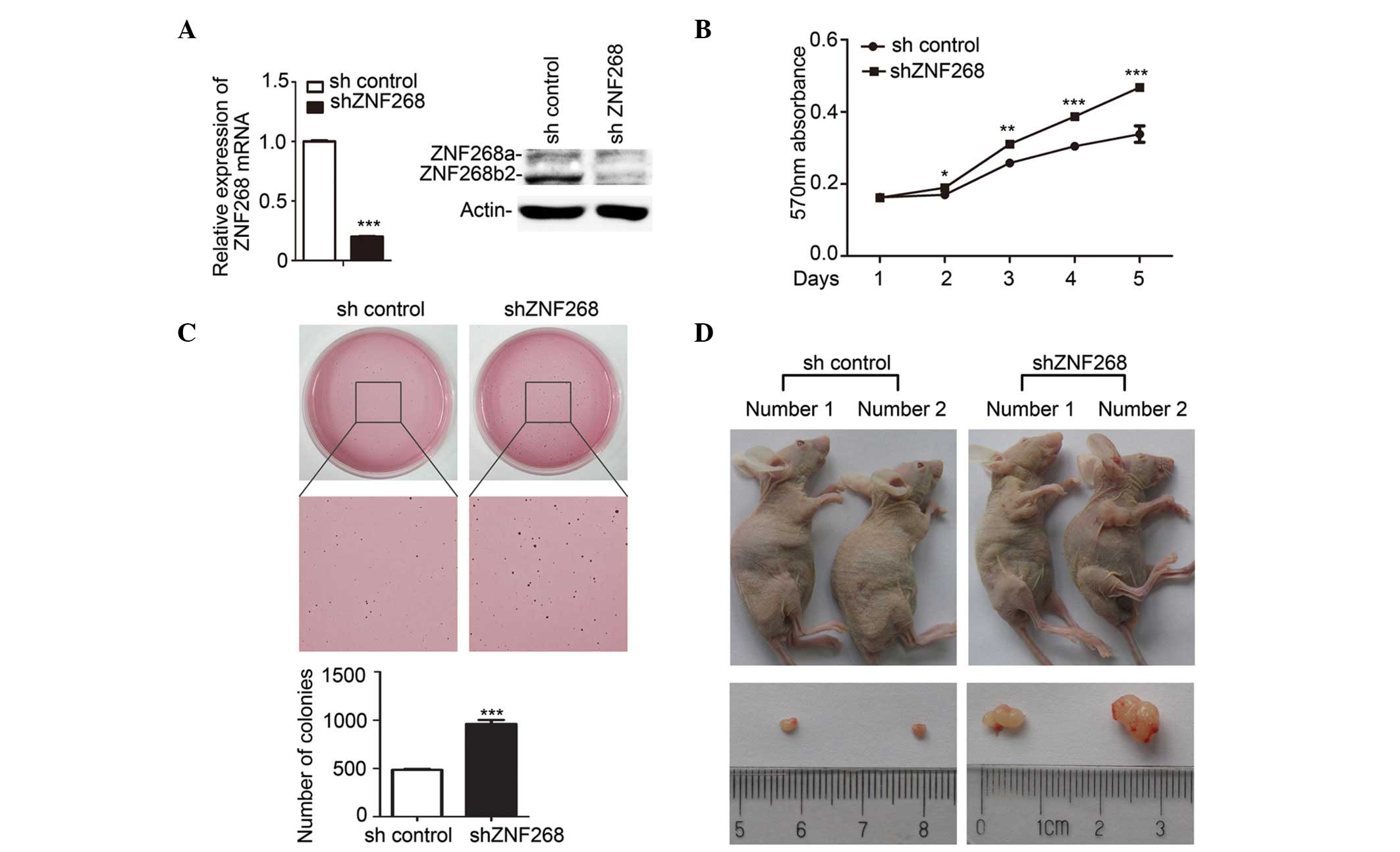
To investigate the biological function of ZNF268
overexpression in human ovarian cancer tissues, a ZNF268-knockdown
was established in the ovarian cancer SKOV-3 cells (shZNF268) using
the siRNA method (Fig. 2A). The
expression of the ZNF268 mRNA and the levels of the proteins
(ZNF268a and ZNF268b2) encoded by the ZNF268 gene were decreased in
the shZNF268 cells (Fig. 2A). Next,
the effect of ZNF268-knockdown on SKOV-3 cell growth was analysed
using the MTT assay. The results showed that the growth rate of the
shZNF268 cells was increased two days subsequent to the cells being
plated, and the greatest increase in growth rate occurred
subsequent to five days (Fig. 2B).
The ability to form colonies in soft agar is considered to be an
important characteristic of tumour growth in vitro.
Therefore, the ability of the shZNF268 SKOV-3 cells to form
colonies in soft agar was examined. Consistent with the results of
the MTT assay, the number of colonies was significantly increased
in the shZNF268 SKOV-3 cells compared with the sh control cells
(Fig. 2C).
To further study the function of ZNF268, an in
vivo xenograft model was used. Following the subcutaneous
injection of the SKOV-3 cells into six Balb/c-nu mice per group,
the mice in the shZNF268 group developed tumours earlier than the
mice of the sh control group (day 12 in the shZNF268 group vs. day
18 in the sh control group). In each group, two mice had developed
clear tumours at the time they were sacrificed (Fig. 2D). Consistent with the in
vitro results, the subcutaneous injection of the shZNF268
SKOV-3 cells into the nude mice resulted in increased tumour growth
(Fig. 2D). Together, these results
demonstrated that ZNF268-knockdown increases SKOV-3 cell growth in
in vitro models and in vivo xenografts.
ZNF268-knockdown alters SKOV-3 cell cycle
progression
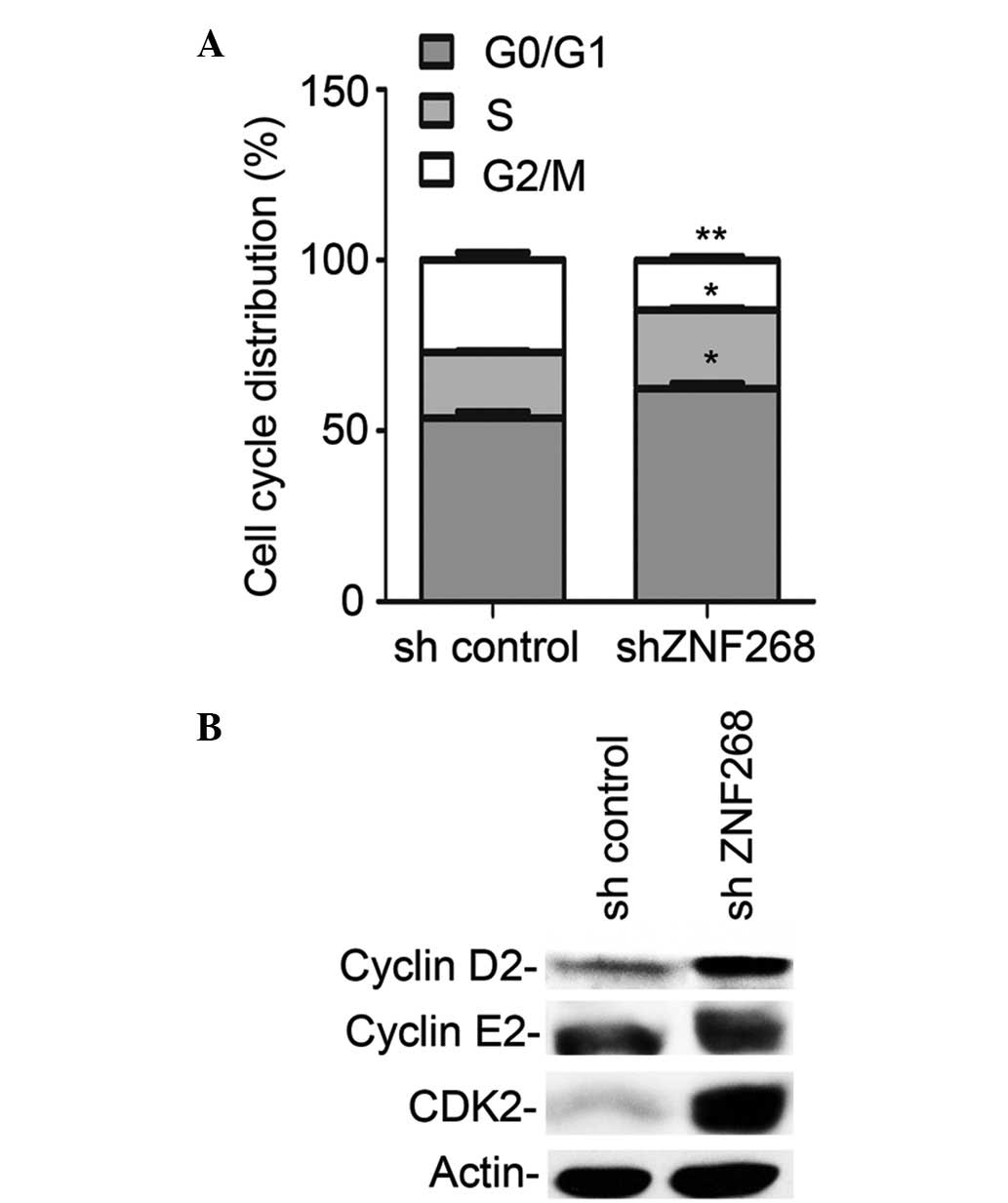
To further determine the potential mechanism by
which ZNF268-knockdown increased cell growth, the effects of
ZNF268-knockdown on cell cycle progression were analysed. The cells
were stained with PI and analysed using flow cytometry. Among the
shZNF268 cells, the proportion of cells in the
G0/G1 and S phases of the cell cycle
increased as the proportion of the cells in the G2/M
phase decreased (Fig. 3A).
Consistent with these observations, increases in the expression of
positive regulators of cell cycle progression, including cyclin D2
and CDK2 (which were significantly increased) and cyclin E2 (which
was marginally increased) were also observed (21–23) in
the shZNF268 cells (Fig. 3B). These
results indicated that ZNF268-knockdown may increase SKOV-3 cell
growth by promoting cell cycle progression.
ZNF268-knockdown suppresses SKOV-3 cell
migration in vitro
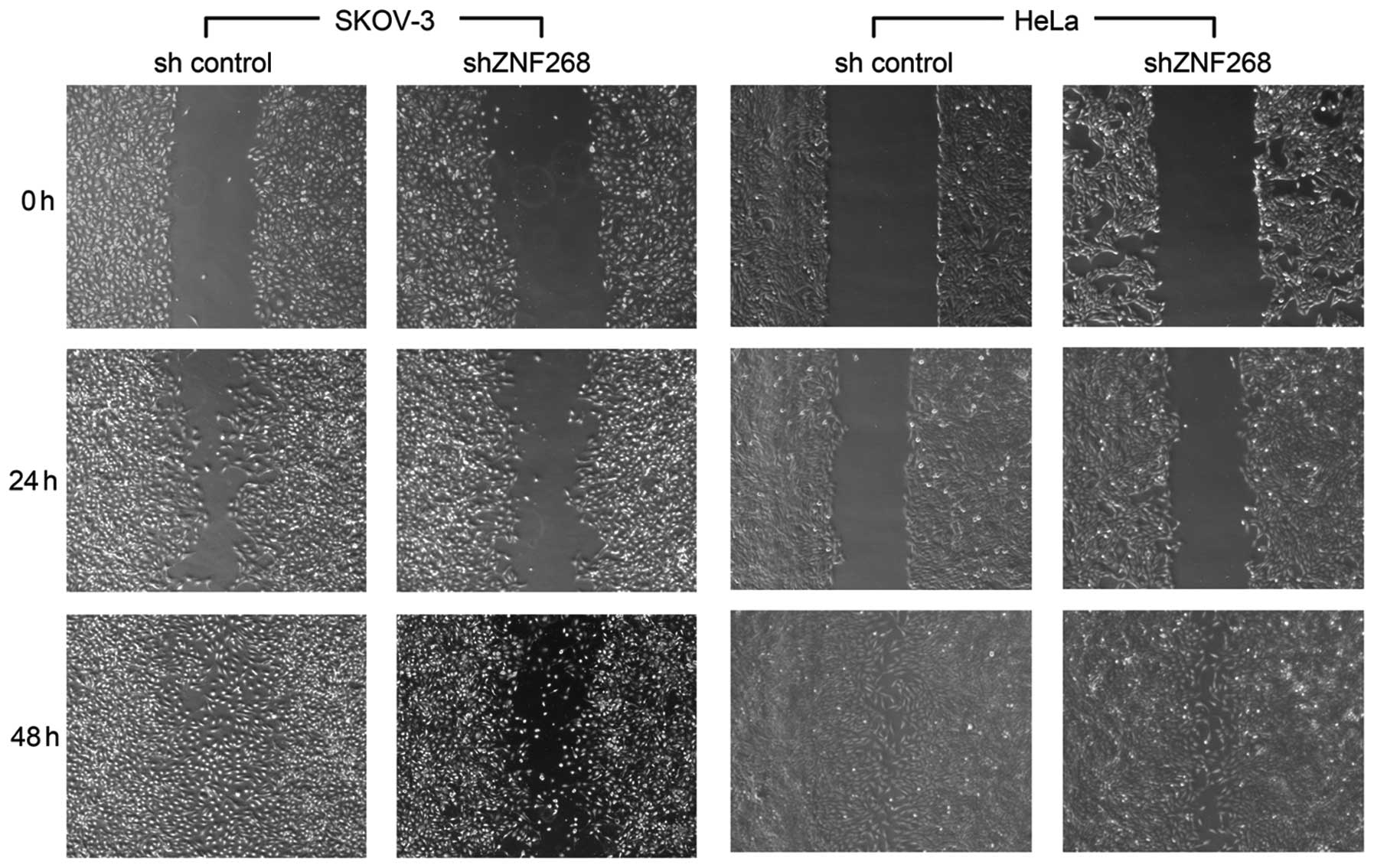
The scratch wound assay is a simple and reproducible
method for measuring cell migration (24). The function of ZNF268 was
investigated in SKOV-3 cell migration using the scratch wound
assay. As shown in Fig. 4,
migration was decreased in the shZNF268 SKOV-3 cells at 24 h and 48
h post-scratch, suggesting that ZNF268-knockdown suppressed SKOV-3
cell migration in vitro. ZNF268-knockdown in the HeLa cells
was also established in our previous study (8). However, no clear changes in cell
migration were observed in the shZNF268 HeLa cells (Fig. 4).
Discussion
Although considerable progress has been made in
cancer research, the mortality rate of ovarian cancer has not
improved over the past several decades (15), demonstrating the urgent requirement
for an improved understanding of the mechanisms underlying the
development of ovarian cancer. The present study revealed several
significant roles for ZNF268 in human ovarian cancer development
and progression. First, it was observed that ZNF268 is
overexpressed in ovarian carcinomas. Second, ZNF268-knockdown was
demonstrated to affect biological functions, including the
proliferation and migration of ovarian cancer SKOV-3 cells. The
results indicate that ZNF268-knockdown may increase the growth rate
of SKOV-3 cells by promoting cell cycle progression, suggesting
that ZNF268 may inhibit ovarian cancer cell growth in humans. It is
well known that oncogenes are usually mutated or expressed at high
levels in tumour cells, while suppressor genes are usually inactive
(25). Therefore, the inhibitory
function of ZNF268 on cell proliferation may appear controversial
due to its high expression levels in ovarian carcinomas. However,
exceptions to this rule exist in which there is no direct
correlation between function and expression. For example, the
tumour suppressor gene maspin is not detected in normal human
pancreatic cells, but is highly expressed in pancreatic cancers
(26). The tumour suppressor PTEN
also shows positive expression in ∼80% of squamous cell cervical
carcinomas (27,28) and is inactivated by the negative
regulator SIPL1 during cervical tumourigenesis (29). Other negative regulators may
interact with and inhibit the activity of ZNF268 in the same way
that SILP1 interacts with and inhibits PTEN. Using an
immunohistochemistry assay with anti-SD that recognizes the ZNF268a
and ZNF268b2 isoforms (6) the
present results demonstrated that total ZNF268 protein was
overexpressed in ovarian cancer and that ZNF268
(ZNF268a/ZNF268b2)-knockdown increases the growth of SKOV-3 cells.
Our previous study showed that the two isoforms (ZNF268a/ZNF268b2)
have varying expression patterns and function differently in human
cervical cancer development (8).
Further experiments should be performed to elucidate the functions
of these two isoforms in ovarian carcinogenesis, which are likely
to aid in the understanding of the mechanism of ZNF268 in SKOV-3
cell proliferation and ovarian carcinogenesis.
The high expression levels of ZNF268 in human
ovarian carcinomas may be associated with the ability of ZNF268 to
promote cell migration. Due to the asymptomatic nature of the early
stages of ovarian cancer and the lack of a reliable method for
early detection, the majority of ovarian cancer patients are
diagnosed with metastatic disease, the cure rate of which is
significantly less than that of non-metastatic disease (17,18).
From this perspective, ZNF268-knockdown suppresses SKOV-3
migration, indicating that ZNF268 may serve as a potential
therapeutic target for ovarian cancer. However, the function of
ZNF268 requires further validation in vivo and the molecular
mechanism underlying the suppression of migration requires
investigation.
Several lines of evidence, based on gene structure,
suggest that ZNF268 may be important in human development. First,
ZNF268 contains a typical KRAB domain, which is only present in
tetrapod vertebrate genomes (2).
Second, ZNF268 consists of up to 24 zinc fingers, and the number of
zinc finger repeats tends to increase during the evolutionary
process (30). Third, no ZNF268
ortholog has been detected in the mouse genome. In the present
study, the knockdown of ZNF268 increased SKOV-3 cell growth. This
is similar to the effects of ZNF268-knockdown on growth promotion
observed in erythroleukemia K562 cells (14), but contrary to the effects of
ZNF268-knockdown on the growth of cervical HeLa cells (8). The underlying reason for these
differences may be that the functional mechanism varies in these
cells; ZNF268-knockdown increases the proportion of cells in the S
phase in SKOV-3 and K562 cells (14), but inhibits HeLa cell cycle
progression by causing G0/G1 phase arrest
(8). Notably, the proportions of
the SKOV-3 cells in the G0/G1 and G2/M phases
were also increased and decreased, respectively, by
ZNF268-knockdown, suggesting that ZNF268-knockdown also affects the
other phases, and their coordinative effect on cell cycle
progression may explain the increased SKOV-3 cell growth. In
addition, the role of ZNF268 in cell migration has also been
demonstrated to be different in the SKOV-3 and HeLa cells. These
results suggest that ZNF268 may act as a multifunctional molecule
in humans by utilising various mechanisms in different cell
types.
Acknowledgements
The present study was supported by
grants from the National High Technology Research and Development
Program of China (863 Program; 2006AA02A306), the National Natural
Science Foundation of China (30871245 and 31271511) and from the
Specialized Research Fund for the Doctoral Program of Higher
Education of China (200804861004).
References
|
1.
|
Huntley S, Baggott DM, Hamilton AT,
Tran-Gyamfi M, et al: A comprehensive catalog of human
KRAB-associated zinc finger genes: insights into the evolutionary
history of a large family of transcriptional repressors. Genome
Res. 16:669–677. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Urrutia R: KRAB-containing zinc-finger
repressor proteins. Genome Biol. 4:2312003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Margolin JF, Friedman JR, Meyer WK,
Vissing H, et al: Krüppel-associated boxes are potent
transcriptional repression domains. Proc Natl Acad Sci USA.
91:4509–4513. 1994.
|
|
4.
|
Mark C, Abrink M and Hellman L:
Comparative analysis of KRAB zinc finger proteins in rodents and
man: evidence for several evolutionarily distinct subfamilies of
KRAB zinc finger genes. DNA Cell Biol. 18:381–396. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Gou DM, Sun Y, Gao L, Chow LM, et al:
Cloning and characterization of a novel Krüppel-like zinc finger
gene, ZNF268, expressed in early human embryo. Biochim Biophys
Acta. 1518:306–310. 2001.
|
|
6.
|
Shao H, Zhu C, Zhao Z, Guo M, et al:
KRAB-containing zinc finger gene ZNF268 encodes multiple
alternatively spliced isoforms that contain transcription
regulatory domains. Int J Mol Med. 18:457–463. 2006.PubMed/NCBI
|
|
7.
|
Sun Y, Gou DM, Liu H, Peng X and Li WX:
The KRAB domain of zinc finger gene ZNF268: a potential
transcriptional repressor. IUBMB Life. 55:127–131. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Wang W, Guo M, Hu L, Cai J, et al: The
zinc finger protein ZNF268 is overexpressed in human cervical
cancer and contributes to tumorigenesis via enhancing NF-κB
signaling. J Biol Chem. 287:42856–42866. 2012.PubMed/NCBI
|
|
9.
|
Guo MX, Wang D, Shao HJ, Qiu HL, et al:
Transcription of human zinc finger ZNF268 gene requires an
intragenic promoter element. J Biol Chem. 281:24623–24636. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Sun Y, Shao H, Li Z, Liu J, et al: ZNF268,
a novel kruppel-like zinc finger protein, is implicated in early
human liver development. Int J Mol Med. 14:971–975. 2004.PubMed/NCBI
|
|
11.
|
Krackhardt AM, Witzens M, Harig S, Hodi
FS, et al: Identification of tumor-associated antigens in chronic
lymphocytic leukemia by SEREX. Blood. 100:2123–2131. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Wang D, Guo MX, Hu HM, Zhao ZZ, et al:
Human T-cell leukemia virus type 1 oncoprotein tax represses ZNF268
expression through the cAMP-responsive element-binding
protein/activating transcription factor pathway. J Biol Chem.
283:16299–16308. 2008. View Article : Google Scholar
|
|
13.
|
Zhao Z, Wang D, Zhu C, Shao H, et al:
Aberrant alternative splicing of human zinc finger gene ZNF268 in
human hematological malignancy. Oncol Rep. 20:1243–1248.
2008.PubMed/NCBI
|
|
14.
|
Zeng Y, Wang W, Ma J, Wang X, et al:
Knockdown of ZNF268, which is transcriptionally downregulated by
GATA-1, promotes proliferation of K562 cells. PLoS One.
7:e295182012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Jemal A, Bray F, Center MM, Ferlay J, et
al: Global cancer statistics. CA Cancer J Clin. 61:69–90. 2011.
View Article : Google Scholar
|
|
16.
|
Marugame T and Hirabayashi Y: Comparison
of time trends in ovary cancer incidence (1973–1997) in East Asia,
Europe, and the USA, from Cancer Incidence in Five Continents Vols
IV VIII. Jpn J Clin Oncol. 37:802–803. 2007.PubMed/NCBI
|
|
17.
|
Agarwal R and Kaye SB: Ovarian cancer:
strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Paley PJ: Ovarian cancer screening: are we
making any progress? Curr Opin Oncol. 13:399–402. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Bast RC Jr, Hennessy B and Mills GB: The
biology of ovarian cancer: new opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Ropponen KM, Eskelinen MJ, Lipponen PK,
Alhava EM and Kosma VM: Reduced expression of alpha catenin is
associated with poor prognosis in colorectal carcinoma. J Clin
Pathol. 52:10–16. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Lauper N, Beck AR, Cariou S, Richman L, et
al: Cyclin E2: a novel CDK2 partner in the late G1 and S phases of
the mammalian cell cycle. Oncogene. 17:2637–2643. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Lundberg AS and Weinberg RA: Functional
inactivation of the retinoblastoma protein requires sequential
modification by at least two distinct cyclin-cdk complexes. Mol
Cell Biol. 18:753–761. 1998.PubMed/NCBI
|
|
24.
|
Cory G: Scratch-wound assay. Methods Mol
Biol. 769:25–30. 2011. View Article : Google Scholar
|
|
25.
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Maass N, Hojo T, Ueding M, Lüttges J, et
al: Expression of the tumor suppressor gene Maspin in human
pancreatic cancers. Clin Cancer Res. 7:812–817. 2001.PubMed/NCBI
|
|
27.
|
El-Mansi MT and Williams AR: Evaluation of
PTEN expression in cervical adenocarcinoma by tissue microarray.
Int J Gynecol Cancer. 16:1254–1260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Lee JS, Choi YD, Lee JH, Nam JH, et al:
Expression of PTEN in the progression of cervical neoplasia and its
relation to tumor behavior and angiogenesis in invasive squamous
cell carcinoma. J Surg Oncol. 93:233–240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
He L, Ingram A, Rybak AP and Tang D:
Shank-interacting protein-like 1 promotes tumorigenesis via PTEN
inhibition in human tumor cells. J Clin Invest. 120:2094–2108.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Looman C, Abrink M, Mark C and Hellman L:
KRAB zinc finger proteins: an analysis of the molecular mechanisms
governing their increase in numbers and complexity during
evolution. Mol Biol Evol. 19:2118–2130. 2002. View Article : Google Scholar : PubMed/NCBI
|