Introduction
Gliomas of the brainstem account for ∼10–20% of all
central nervous system tumors (1).
The present study retrospectively analyzed clinical data from the
past 20 years and identified that brainstem gliomas (BSG) may be
divided into four types, diffuse, intrinsic focal, exophytic focal
and cervicomedullary, according to the tumor site. They may also be
separated into diffuse infiltrative BSG, known for their relentless
growth and poor outcome, and focal BSG, which are associated with a
favorable prognosis. BSG occur most often in children aged 7–9
years and in the fourth or fifth decade of adult life (2,3). All
BSG were previously regarded as malignant, since their location
rendered them inoperable. However, as modern technologies in
neuroimaging and microsurgery have developed, it has become
possible to remove certain types of BSG by surgery. However,
surgical intervention has only been beneficial in focal BSG
(4). There are currently no
effective therapeutic treatments for diffuse BSG (5–7). The
development of a satisfactory experimental model for these gliomas
is critical for understanding their biological behavior (8,9).
Numerous clinical studies have indicated that adult
BSG vary from those of children (3,10–12).
The majority of BSG in children are diffuse tumors, accounting for
up to 80% of brainstem tumors in children. The tumors cause diffuse
infiltration and swelling of the brainstem, with a clinical
presentation that includes involvement of the sixth nerve, ataxia,
cerebellar signs and long-tract signs in the form of a short
prodrome of symptoms (<6 months), which indicate a worse
prognosis. Surgery is not beneficial for these patients. Radiation
has been shown to provide temporary stabilization or a transient
improvement of clinical symptoms. The median survival time of
children with diffuse BSG is <1 year following diagnosis. In
adults, focal gliomas represent the majority of tumors, and these
displace the long tract of the brainstem. The tumors tend to
displace rather than infiltrate neural cells and have
well-demarcated borders. Surgery may be performed to remove the
tumor in this type of glioma. The median survival time is >4
years. Overall, BSG in adults are less aggressive and have a better
prognosis than those in children (1).
Genetic abnormalities associated with BSG in
children are different from those in adults, which may contribute
to the differences in the tumor growth patterns. The differing
anatomical characteristics may also contribute to the varied growth
patterns. Whether tumor cells in children are more invasive than
those of adults or if stronger nerve fibers in the adult brainstem
are able to block the invasion of tumors has not been determined.
The present study aimed to establish brainstem glioma models using
juvenile and adult rats. The study also investigated the difference
between BSG in juvenile and adult rats with regard to tumor growth,
survival, pathology and magnetic resonance imaging (MRI).
Materials and methods
Animals
Female juvenile Wistar rats (3 weeks old; body
weight, 40–50 g) and adult female Wistar rats (8 weeks old; body
weight, 200–220 g) were purchased from the Institute of Laboratory
Animals, Chinese Academy of Medical Sciences (Beijing, China). A C6
glioma cell line, originally cloned in N-nitrosomethylurea and in
an F-12 medium containing 2.5% fetal bovine serum (FBS) and 15%
horse serum (HoS), was purchased from the Institute of Basic
Medical Sciences, Chinese Academy of Medical Sciences (Beijing,
China). The main equipment used in the present study included a 3.0
Tesla MR machine and 5-inch surface coil (GE Healthcare, Stamford,
CT, USA; and Sigma, St. Louis, MO, USA), an inverted phase contrast
microscope (Nikon, Gotenba, Japan), a carbon dioxide
(CO2) incubator (Heraeus, Hong Kong), a microtome
(Leitz, Stuttgart, Germany), rat stereotaxic apparatus (Xibei
Optical Instrument Factory, Shanxi, China), a microinjection pump
(New Era Pump System, Inc., Farmingdale, NY, USA) and 10-μl
microsyringes (Feige, Shanghai, China). This study was carried out
in strict accordance with recommendations of the Guide for the Care
and Use of Laboratory Animals of the National Institutes of Health.
The animal use protocol was reviewed and approved by the
Institutional Animal Care and Use Committee (IACUC) of Beijing
Neurosurgical Institute (permit number, 20060828001).
Animal model
Wistar rats (25 adults and 25 juveniles) were
divided into groups A (experimental group, 15 juvenile rats), B
(control group, 10 juvenile rats), C (experimental group, 15 adult
rats) and D (control group, 10 adult rats). The rats were injected
with 1×105 C6 glioma cells (groups A and C) or
physiological saline solution (groups B and D). The treatment
solutions were stereotactically injected into the pons of each rat.
The C6 cells were cultured in an F-12 medium with 2.5% FBS and 15%
HoS at 37°C in a humidified incubator containing 5% CO2
(13). Cell passaging was performed
prior to injection and the culture fluid was replaced 24 h later.
In the logarithmic phase with 80% cell confluence, the cells were
digested with 2.5% trypsin. The action of trypsin was terminated by
the addition of the medium when the majority of cells were round,
the cell processes had retracted and the cell gaps were widening.
The collected cell suspension was centrifuged at 500 × g for 10
min. Viable cells were identified by Trypan blue exclusion and
counted with a hemocytometer. The cells were diluted to a
concentration of 1.0×105 cells/10 μl.
Surgical technique
The animals were anesthetized with 10% chloral
hydrate administered intraperitoneally. The head of each rat was
fixed on a stereotactic frame. The parieto-occipital skin was
shaved and prepared in sterile conditions. A midline incision of ∼1
cm in length was made. The lambdoid suture was identified and a
1-mm burr was drilled 1.5 mm posterior to this and 1.5 mm to the
left of the sagittal suture for the rats in groups A and B. A 1-mm
burr was drilled 2.0 mm posterior to the lambdoid suture and 2.0 mm
to the left of the sagittal suture in the rats of groups C and D. A
10-μl microsyringe containing 1.0×105 cells was
fixed onto the microinjection pump. The needle was inserted into
the burr hole to a depth of 8.5 mm from the bone surface in the
adult rats and 7.5 mm from the bone surface in the juvenile rats.
The suspended cells were injected at a speed of 1 μl/min.
Subsequent to the injection, the needle remained in place for 10
min to avoid a backflow of cells. The needle was then slowly
withdrawn from the burr hole, which was closed with bone wax, then
the skin was sutured (14,15).
Observation of behavior and survival
All rats were weighed every day. Neurological
deficits were observed and evaluated, including evaluation of the
corneal reflect using a cotton swab on the eyes, as well as
observation of movement to determine if the rats had weakness or
hemiparesis of the legs. The survival time of each rat was
recorded.
MR scan
At two weeks post-implantation, the animals were
anesthetized with 10% chloral hydrate administered
intraperitoneally. The animals were then scanned in the axial,
sagittal and coronal planes with a slice thickness of 1.5 mm. A
T1-weighted image (T1WI) was scanned following an intravenous
injection of gadolinium diethylenetriamine pentaacetic acid
(Gd-DTPA; 0.4 ml/kg).
Hematoxylin and eosin (HE) staining
One day after the MR scan, all animals were
sacrificed using an intraperitoneal injection of an excessive dose
of chloral hydrate and then perfused with 0.9% saline followed by
4% paraformaldehyde. Subsequent to the removal of the brainstem,
fixation, paraffin embedding and slicing (5 μm in thickness)
were performed and the slides were stained with HE.
Immunohistochemical analysis
The slides were deparaffinized, rehydrated and
boiled in 10 mM citrate buffer (pH 6.0) for 10 min in an oven to
expose the antigen. Non-specific binding sites were blocked by
incubating the slices with 10% normal goat serum for 30 min at room
temperature. The slides were incubated with primary antibodies
(MMP-2, MMP-9 and β-catenin; 1:100 dilution; Millipore, Billerica,
MA, USA) overnight at 4°C. The sections were then washed and
incubated with a secondary antibody at room temperature for 30 min.
Visualization was performed using the streptavidin-peroxidase
method combined with 3,3′-diaminobenzidine. The integrated optical
density (IOD) was calculated using medical image analysis software
(ImagePro Plus, Media Cybernetics Inc., Bethesda, MD, USA).
Statistical analysis
The statistical analysis was performed using SPSS
13.0 (SPSS, Inc., Chicago, IL, USA). The results are presented as
the mean ± SD. The survival, tumor size and IOD of groups A and C
were compared, and Kaplan-Meier survival curves and weight change
curves were generated for the two groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
Behavioral observation
The rats in groups A and C presented with secretions
from the left eye at 1 week post-implantation. The left corneal
reflex was blunt compared with the right reflex, which indicated an
extraocular muscle problem. Hemiparesis was also observed. The two
groups of rats exhibited weakness, apastia and a gradual reduction
in movement as the tumor grew. The rats then died. None of the rats
in groups B or D exhibited abnormal behavior.
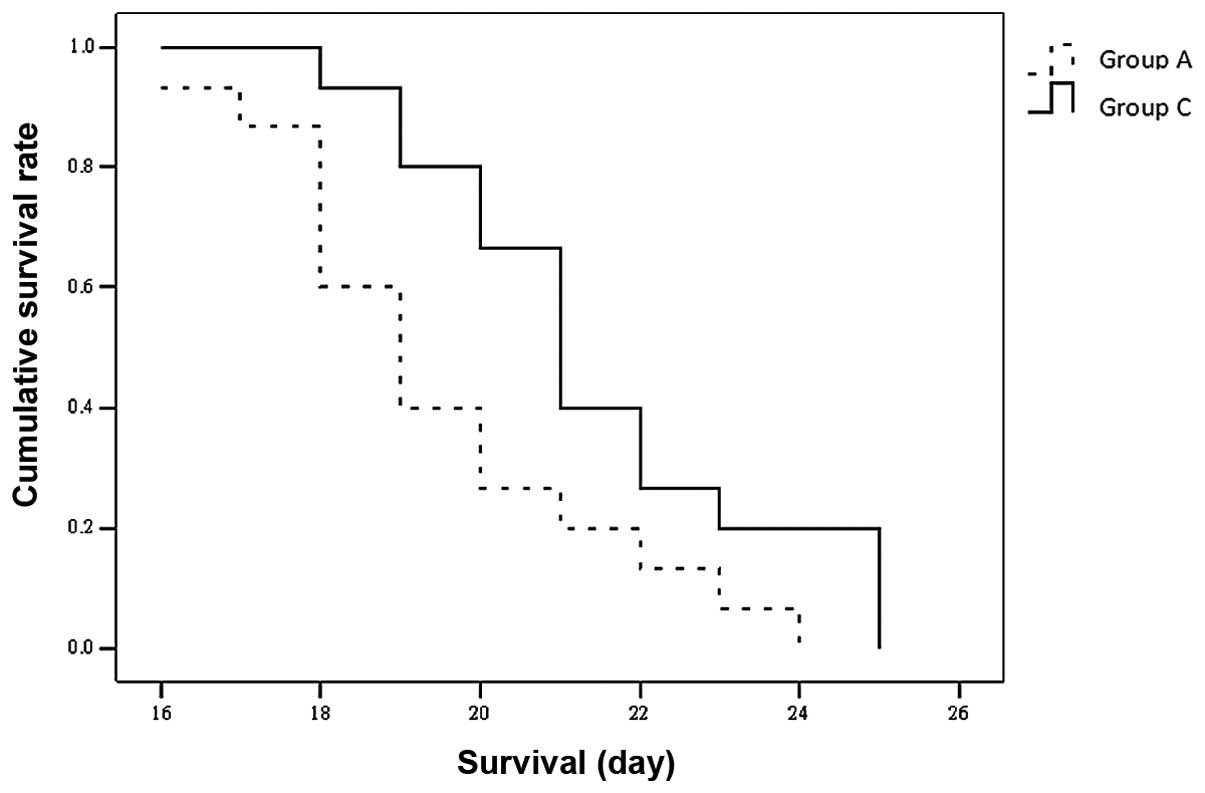
Survival rate
A total of 2, 3, 3 and 2 rats in groups A, B, C and
D, respectively, died on the day of the implantation or 1 day
post-operatively, due to epidural or subdural hemorrhage induced by
the drilling process. The survival time for the remaining rats was
recorded as 16–24 days (mean, 19.47±2.232 days) in group A and
18–25 days (mean, 21.47±2.232 days) in group C. The difference in
the survival time of groups A and C was statistically significant
(P<0.05; Fig. 1). All the rats
in group A and C were alive at the time when all the rats in groups
B and D had died.
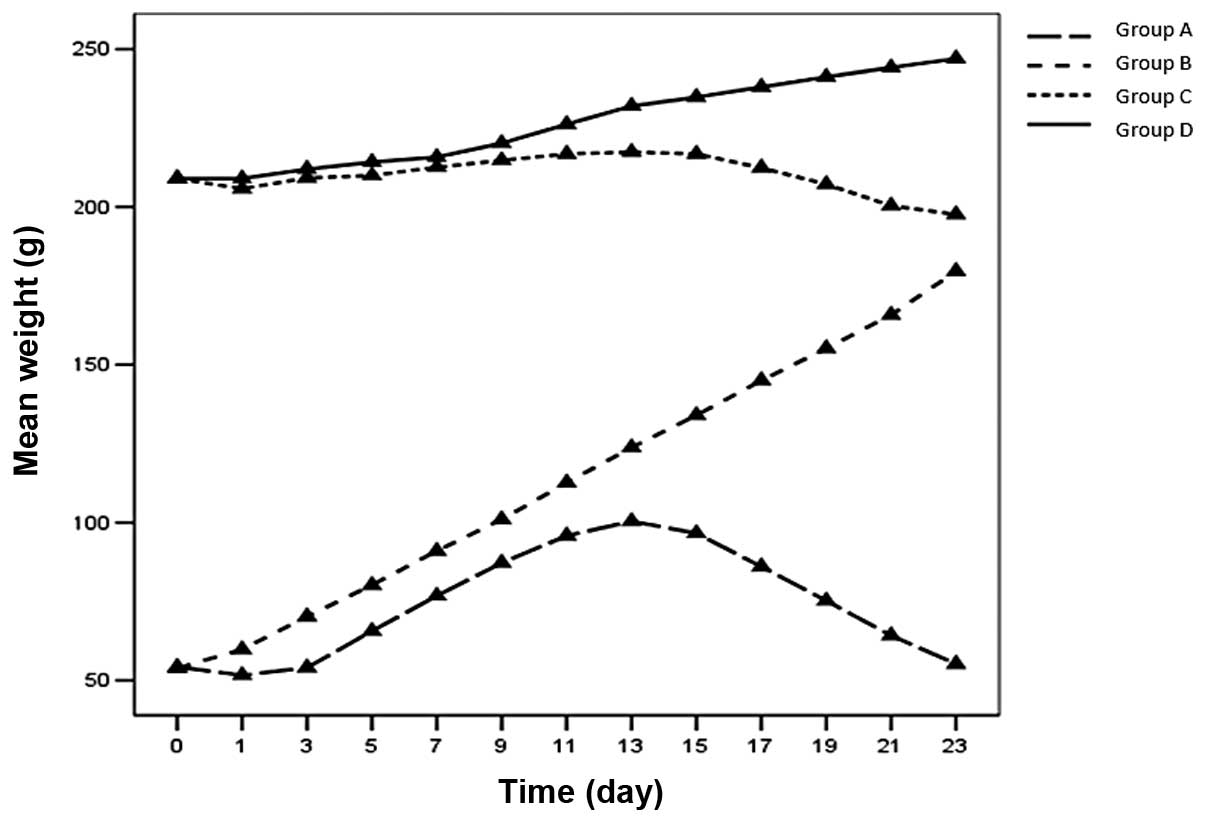
Weight observation
The weight of the rats in groups B and D continued
to increase, although the increase was not as rapid as predicted by
the age-matched control data on Wistar rats supplied by the
Institute of Laboratory Animals. The rats in groups A and C
exhibited a ‘decrease-increase-decrease’ mode of weight gain
(Fig. 2).
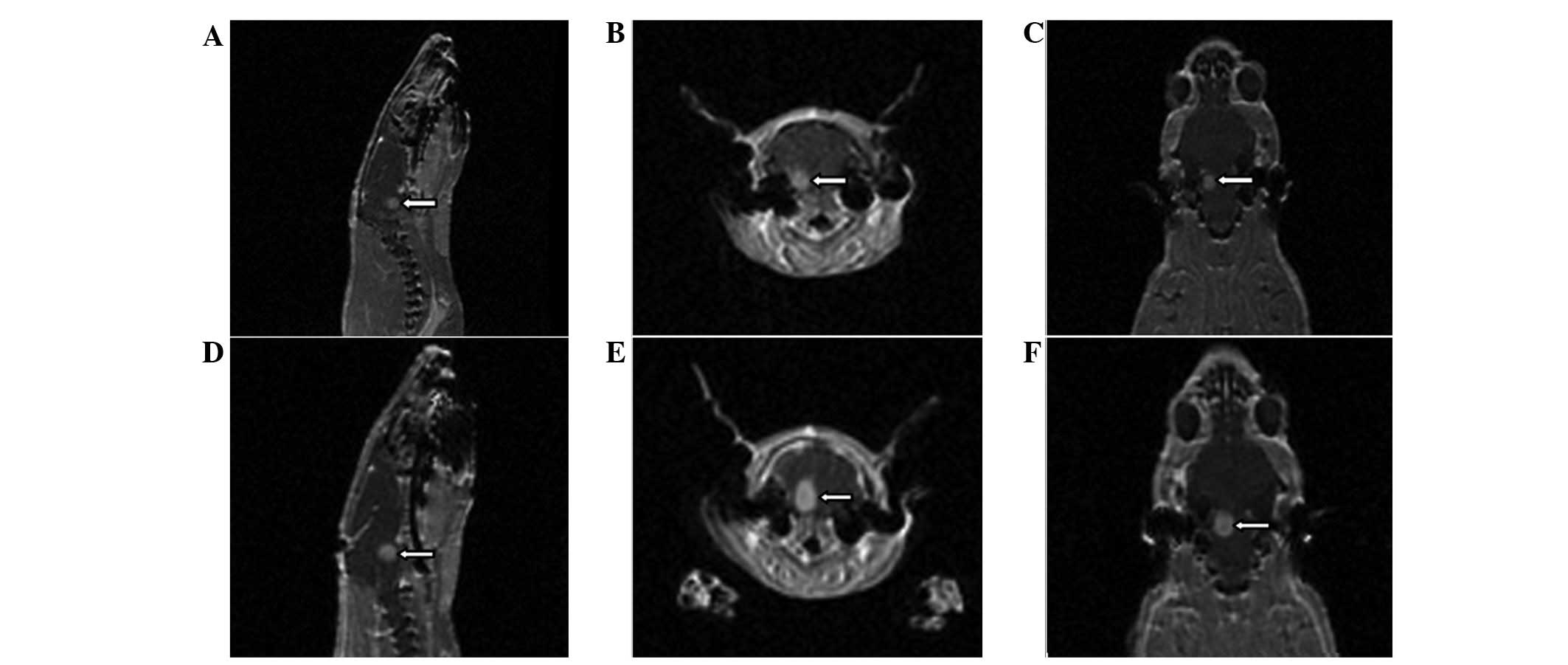
MRI scans
MRI scans were performed at 2 weeks
post-implantation and tumor growth was confirmed. The tumors were
round and confined within the pons, with a clear boundary. The
tumor formation rate was 84.6% (11/13) in group A and 83.3% (10/12)
in group C. The signals of the tumors were low in the T1WIs and
high in the T2WIs. Following the injection of Gd-DTPA, the signal
in the T1WIs was markedly enhanced and revealed necrosis inside the
tumor. The tumors exhibited marked mass effects, which resulted in
ventricular dilation since the tumors obstructed the pathway of the
cerebrospinal fluid (Fig. 3). The
tumor volume was calculated using the formula, V = 4/3 ×
πr3. The mean maximal diameter of the tumor was 4.55 mm
in group A and 4.62 mm in group C (P>0.05).
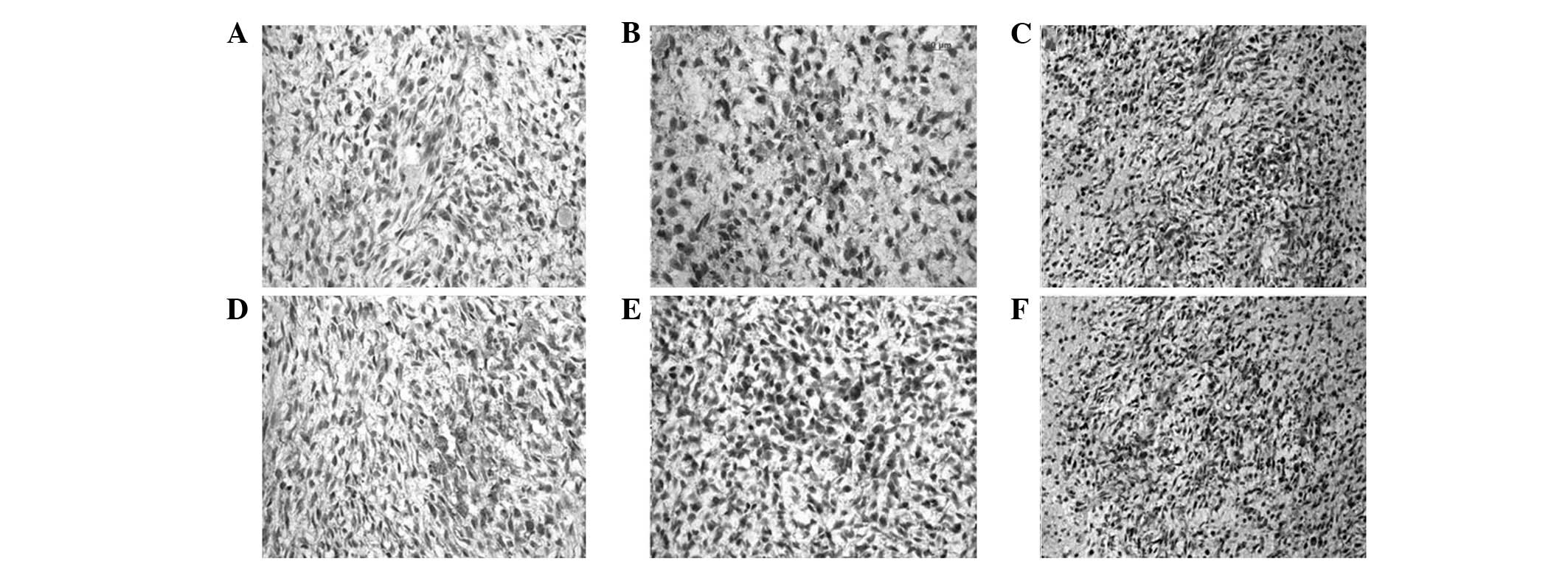
HE staining
The tumors were mass-like with relatively clear
boundaries upon gross examination. Using HE staining, the boundary
was evident without a membrane envelope, and intratumoral necrosis
was also clearly observed. Tumor growth was characterized as
invasive. The new vessels were abundant and palisade-like necrosis
was evident. The tumor cells were active, exhibiting several shapes
(including diamond, triangle and oval shapes) and showing an
increased nucleus-cytoplasm ratio. HE staining revealed no
significant differences between groups (Fig. 4).
Immunohistochemical staining
The MMP-2, MMP-9 and β-catenin staining in juvenile
and adult rats was positive in the cytoplasm and nucleus (Fig. 5). The number of positive cells and
expression levels (IOD value) were not significantly different
between groups (Table I).
 | Table I.IOD values of MMP-2, MMP-9 and
β-catenin in juvenile and adult rats. |
Table I.
IOD values of MMP-2, MMP-9 and
β-catenin in juvenile and adult rats.
| Group | MMP-2 | MMP-9 | β-catenin |
|---|
| A | 46.99±8.23 | 71.18±12.53 | 100.48±9.11 |
| C | 48.13±9.02 | 66.28±14.42 | 103.69±7.50 |
Discussion
In clinical studies, gliomas of the brainstem
exhibit marked differences in children and adults with regard to
incidence rate, form of onset, progression speed and prognosis
(16,17). The prognosis of diffuse tumors in
children is usually poor, whereas the prognosis for the focal type
typically observed in adults is better (18). Studies have attempted to investigate
whether this difference is caused by the biological behavior of the
tumor cells or by intensive nerve fibers and nuclei in the adult
brainstem, which block invasion of the tumor. However, the answer
this conundrum remains unknown. In the present study, the
proliferation and invasion of BSG were compared in juvenile and
adult rats using morphology and imaging anayses. The main factors
that determine the different features of BSG in the two age groups
were also explored.
With the growth of the tumors, the nerve nuclei and
cranial nerves (V, VI, VII and VIII) in, or derived from the
brainstem were compressed. Rats in groups A and C exhibited a blunt
corneal reflex in the left eye, which suggested an impaired
function of the extraocular muscles. Hemiparesis resulting from
motor fiber damage was also observed. The rats exhibited gradually
increasing weakness, apastia and eventually died.
Several rats bled when the needle pierced the dura
mater. They died the same day or the day after implantation,
possibly due to epidural or subdural hemorrhage. Rats in groups A
and C received the same quantity of cells; however, the survival of
the adult rats was longer than that of juvenile rats (P<0.05).
This difference in survival may be due to the larger size of the
adult rats. In juvenile rats, the space within the skull is smaller
than that of adult rats. Therefore, the compensatory space in the
skulls of juvenile rats is likely to be limited compared with that
in adult rats, as the tumor volumes increase at a similar speed and
size in all rats. This causes the tumor to compress the brainstem
more severely in juvenile rats. Additionally, in the present study,
hydrocephalus appeared earlier in the juvenile rats, which resulted
in shorter survival times. This is consistent with the results from
a study by Liu et al (19).
Rats in groups A and C exhibited a
‘decrease-increase-decrease’ pattern of weight gain, which is in
contrast with the data reported by Liu et al (19). Subsequent to implantation, the
stress response following surgery and anesthesia caused a
short-term reduction in eating and drinking, which resulted in
weight loss. As rats recovered from the stress response, they began
to eat and drink normally again, thus increasing their weight.
However, the rate of increase was lower than that of the control
group. With the growth of the tumors, the rats exhibited apastia
and their weight began to decrease. The weight of the juvenile rats
at the time of death was heavier than at the beginning of the
study, whereas the adult rats weighed less at the time of death
compared with their baseline weight.
The majority of studies on BSG in animal models
focus on pathology and survival (20–22).
In the present study, MRI was used to evaluate tumor growth for the
first time. This approach permitted observation in vivo,
therefore, the continuous observation of growth was possible. The
tumors of rats in groups A and C were ball-like and had a low
signal in the T1WIs and a high signal in the T2WIs and the T1WIs
enhanced by Gd-DTPA. These imaging results are similar to those
reported for glioblastomas in humans (23).
HE staining showed that the tumor cells grew
actively and were densely arrayed with evident mitosis. The growth
was invasive with palisade-like necrosis. These characteristics are
similar to those exhibited in human glioblastoma (24). However, no differences were observed
between the juvenile and adult rats. The tumors in all rats were
focal rather than diffuse. Therefore, the present study was unable
to obtain conclusive results on the morphological differences
between the two age groups. MMP-2, MMP-9 and β-catenin were also
used to test for differences between adult and juvenile rats for
the first time. Positive staining for all three antibodies was
observed in the cytoplasm and nuclei in all sections; however, the
difference between the two age groups was not statistically
significant. In summary, MRI and HE and immunohistochemical
staining were performed in the present study, however, no
statistically significant morphological or image-based differences
were identified between the BSG in the juvenile and adult rats.
This may be due to the use of the same tumor cell type in the two
groups of rats.
Clinical studies on BSG have demonstrated
differences between children and adults. From the results obtained
in the present study, we conclude that the differences are likely
to be caused by the heterogeneity of the tumor cells themselves,
rather than by the nerve fibers and nuclei in the adult brainstem
blocking tumor invasion. Different types of cells should be used in
future experiments. Tumor cells from the BSG of children and adults
should be acquired, cultured and purified as two different cell
lines to establish an immunodeficient animal model.
References
|
1.
|
Recinos PF, Sciubba DM and Jallo GI:
Brainstem tumors: where are we today? Pediatr Neurosurg.
43:192–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Hargrave D, Bartels U and Bouffet E:
Diffuse brainstem glioma in children: critical review of clinical
trials. Lancet Oncol. 7:241–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Landolfi JC, Thaler HT and DeAngelis LM:
Adult brainstem gliomas. Neurology. 51:1136–1139. 1998. View Article : Google Scholar
|
|
4.
|
Geoerger B, Hargrave D, Thomas F, et al:
Innovative Therapies for Children with Cancer pediatric phase I
study of erlotinib in brainstem glioma and relapsing/refractory
brain tumors. Neuro Oncol. 13:109–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Yang B, Wu X, Mao Y, et al: Dual-targeted
antitumor effects against brainstem glioma by intravenous delivery
of tumor necrosis factor-related, apoptosis-inducing,
ligand-engineered human mesenchymal stem cells. Neurosurgery.
65:610–624. 2009. View Article : Google Scholar
|
|
6.
|
Lee DH, Ahn Y, Kim SU, et al: Targeting
rat brainstem glioma using human neural stem cells and human
mesenchymal stem cells. Clin Cancer Res. 15:4925–4934. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Sharp JR, Bouffet E, Stempak D, et al: A
multi-centre Canadian pilot study of metronomic temozolomide
combined with radiotherapy for newly diagnosed paediatric brainstem
glioma. Eur J Cancer. 46:3271–3279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Choi SA, Hwang SK, Wang KC, et al:
Therapeutic efficacy and safety of TRAIL-producing human adipose
tissue-derived mesenchymal stem cells against experimental
brainstem glioma. Neuro Oncol. 13:61–69. 2011. View Article : Google Scholar
|
|
9.
|
Jing C, Yuan L, Xingguo P, et al:
Promising fusion protein design to target the U87 MG glioma cell
line. Asian Pac J Cancer Prev. 12:935–937. 2011.PubMed/NCBI
|
|
10.
|
Selvapandian S, Rajshekhar V and Chandy
MJ: Brainstem glioma: comparative study of clinico-radiological
presentation, pathology and outcome in children and adults. Acta
Neurochir (Wien). 141:721–727. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Freeman CR and Farmer JP: Pediatric brain
stem gliomas: a review. Int J Radiat Oncol Biol Phys. 40:265–271.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Walker DA, Punt JA and Sokal M: Clinical
management of brain stem glioma. Arch Dis Child. 80:558–564. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Wang DC, Zhang Y, Chen HY, et al:
Hyperthermia promotes apoptosis and suppresses invasion in C6 rat
glioma cells. Asian Pac J Cancer Prev. 13:3239–3245. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Jallo GI, Volkov A, Wong C, Carson BS and
Penno MB: A novel brainstem tumor model: functional and
histopathological characterization. Childs Nerv Syst. 22:1519–1525.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Sho A, Kondo S, Kamitani H, Otake M and
Watanabe T: Establishment of experimental glioma models at the
intrinsic brainstem region of the rats. Neurol Res. 29:36–42. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Guillamo JS, Monjour A, Taillandier L, et
al: Brainstem gliomas in adults: prognostic factors and
classification. Brain. 124:2528–2539. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Mauffrey C: Paediatric brainstem gliomas:
prognostic factors and management. J Clin Neurosci. 13:431–437.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Hargrave D: Paediatric high and low grade
glioma: the impact of tumour biology on current and future therapy.
Br J Neurosurg. 23:351–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Liu Q, Liu R, Kashyap MV, et al: Brainstem
glioma progression in juvenile and adult rats. J Neurosurg.
109:849–855. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Hashizume R, Ozawa T, Dinca EB, et al: A
human brainstem glioma xenograft model enabled for bioluminescence
imaging. J Neurooncol. 96:151–159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Thomale UW, Tyler B, Renard V, et al:
Neurological grading, survival, MR imaging and histological
evaluation in the rat brainstem glioma model. Childs Nerv Syst.
25:433–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Becher OJ, Hambardzumyan D, Walker TR, et
al: Preclinical evaluation of radiation and perifosine in a
genetically and histo-logically accurate model of brainstem glioma.
Cancer Res. 70:2548–2557. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Poussaint TY, Kocak M, Vajapeyam S, et al:
MRI as a central component of clinical trials analysis in brainstem
glioma: a report from the Pediatric Brain Tumor Consortium (PBTC).
Neuro Oncol. 13:417–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Yamasaki F, Kurisu K, Kajiwara Y, et al:
Magnetic resonance spectroscopic detection of lactate is predictive
of a poor prognosis in patients with diffuse intrinsic pontine
glioma. Neuro Oncol. 7:791–801. 2011. View Article : Google Scholar : PubMed/NCBI
|