Introduction
Teratomas are ectopic tumors containing multiple
tissues from more than one mesoderm. Encephalic teratomas are rare
and account for 2 to 5% of infant teratomas and 0.3 to 0.6% of all
ventricular tumors. Teratomas may occur at any age, but with a
higher incidence rate in younger patients (1). In addition, the incidence rate in
males is marginally higher than that in females. Encephalic
teratomas always occur in the midline, where they are typically
detected in the pineal and suprasellar regions and rarely in the
posterior cranial fossa. Obstructive hydrocephalus is a common
complication of teratomas (2). This
disease is difficult to diagnose due to its rarity. Furthermore,
encephalic teratomas are occasionally misdiagnosed as highly
malgnant tumors. For this reason, the family members of patients
reject treatment and infant patients may lose the chance to be
cured of obstructive hydrocephalus. An accurate pre-operative
diagnosis is important and patients are usually in an extremely
dangerous condition when they are admitted to hospital. As such,
effective treatment strategies create conditions that promote the
post-operative recovery of patients and lay the foundation of the
desired long-term prognosis. In the present study, an infant
patient with a large immature teratoma in the posterior cranial
fossa, accompanied by obstructive hydrocephalus, was admitted to
the Department of Neurosurgery at Fuzhou General Hospital of
Nanjing Military Command (Fuzhou, China) in January 2011. The
treatment exhibited a positive effect on the patient. Strategies
identified in the associated literature were used to conduct the
analyses. Written informed consent was obtained from the patient’s
family.
Case report
A male infant aged 4 months and 9 days was admitted
to Fuzhou General Hospital of Nanjing Military Command with disease
symptoms, including binocular convergence, a downward gaze for
>10 days and sudden seizures for one day. The infant was
vaccinated according to the recommended immunization schedule and
no clear abnormalities were observed. The size of the infant’s head
was normal for his age. The infant was born mature to a 34-year-old
G3P3 mother, without any history of exposure to X-rays and toxic
substances. No abnormalities were observed during the routine
antenatal care. The infant weighed 3.5 kg at birth and did not have
a history of asphyxia rescue. The patient’s Apgar score was 9.
Breast-feeding was conducted after birth. A special examination of
the infant revealed the following: a 5×5 cm bregma that was plump
and bulging to a height of ∼3 mm, and a head size of 49 cm.
Macewen’s sign was observed upon skull percussion. Additionally,
the infant was in a moderate coma. The setting-sun sign was
observed in the patient’s eyeball, while the bilateral pupil of the
infant faced downwards, had a diameter of 1.5 mm and lagged in
response to direct and indirect light. Muscular tension in the four
limbs of the infant patient was reduced and limbs bent upon pain
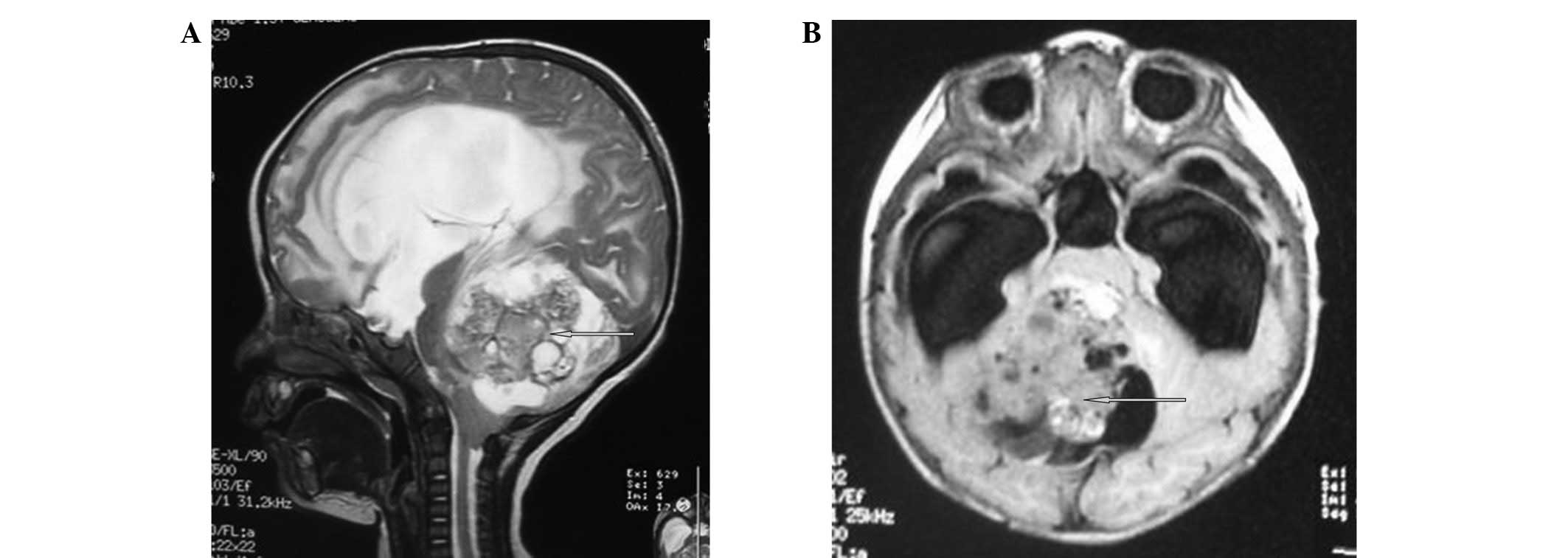
stimulation. Head magnetic resonance imaging (MRI) scans of the
infant showed a quasi-circular long signal in the cerebellar
vermis, which exhibited a marginally high signal in the
water-suppression image. The lesion was ∼5.8×5.7×6.0 cm in size and
had a clear boundary. The signal of the lesional substance was
uneven. Small, circular long T1 and T2 signals and short, irregular
T1 and T2 signals were also observed. The brain stem was compressed
and deviated forward. The fourth cerebral ventricle had become
narrowed due to compression. The third cerebral and two lateral
ventricles were expanded. A small patch of long T1 and T2 signals
was observed around the lateral ventricles, which showed a high
signal in the water-suppression image. The fissures and sulci on
each side were not broadened and the middle structures of the brain
remained at the midline (Fig. 1).
The clinical diagnosis was as follows: i)
cerebellar-space-occupying lesion, local cystic lesion and
bleeding; and ii) obstructive hydrocephalus.
Ventricular drainage was performed through a
puncture in the anterior fontanelle as an emergency treatment once
the patient had been admitted to the hospital. The patient’s
consciousness gradually returned following the surgery. Tension in
the anterior fontanelle was reduced. Thus, a fluid infusion was
performed to stabilize the patient’s condition. After full
preparations had been made, including fluid infusion, blood
preparation and the use of intraoperative pathology, the cerebellar
vermis tumor was resected from the posterior midline of the patient
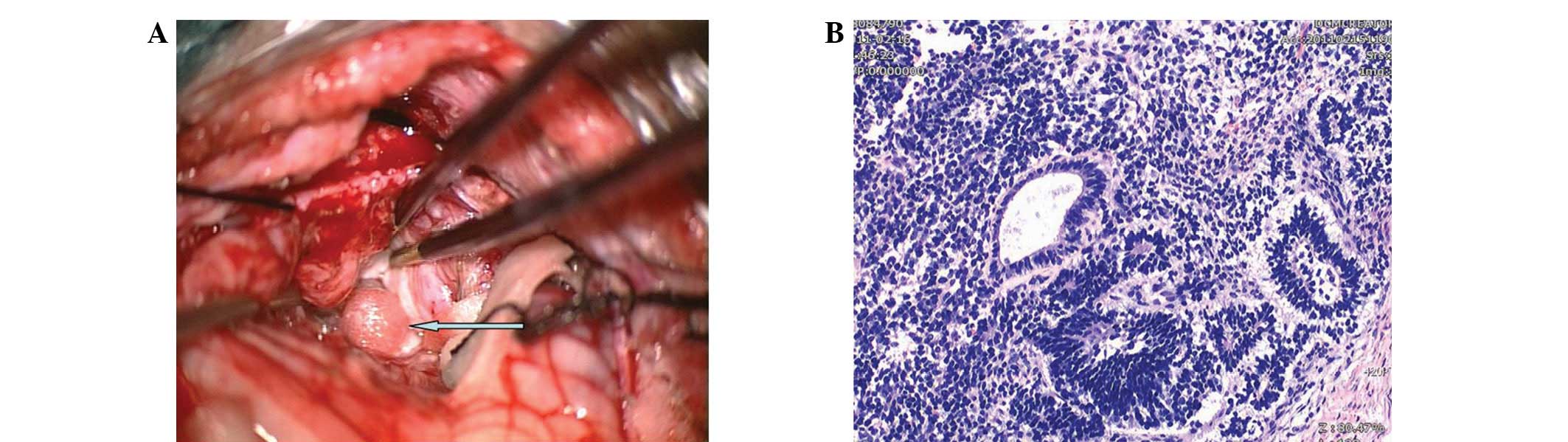
under general anesthesia on the third day. The tumor was located in
front of the cerebellar vermis during the surgery and was lobulated
with an irregular shape and uneven texture. Certain sections of the
tumor were soft, whereas others were tough. Cystic changes were
observed in several places. A large amount of the cyst fluid was
yellow, whereas the remainder was white and dirty. In addition,
several light-red small circular nodes, with a diameter of ∼3 mm
and rough surfaces, were observed (Fig.
2A). Calcified points and sporadic white hair were also
observed. The tumor had a full membranous envelope with an average
blood supply and a clear dividing line. The tumor pressed on the
cerebellum and the bottom of the four ventricles, with unclear
peripheral adhesions. The tumor was fully resected under the
guidance of a microscope. The pathological result was a Grade II
immature teratoma (Fig. 2B). The
post-operative recovery was good, with the patient showing an
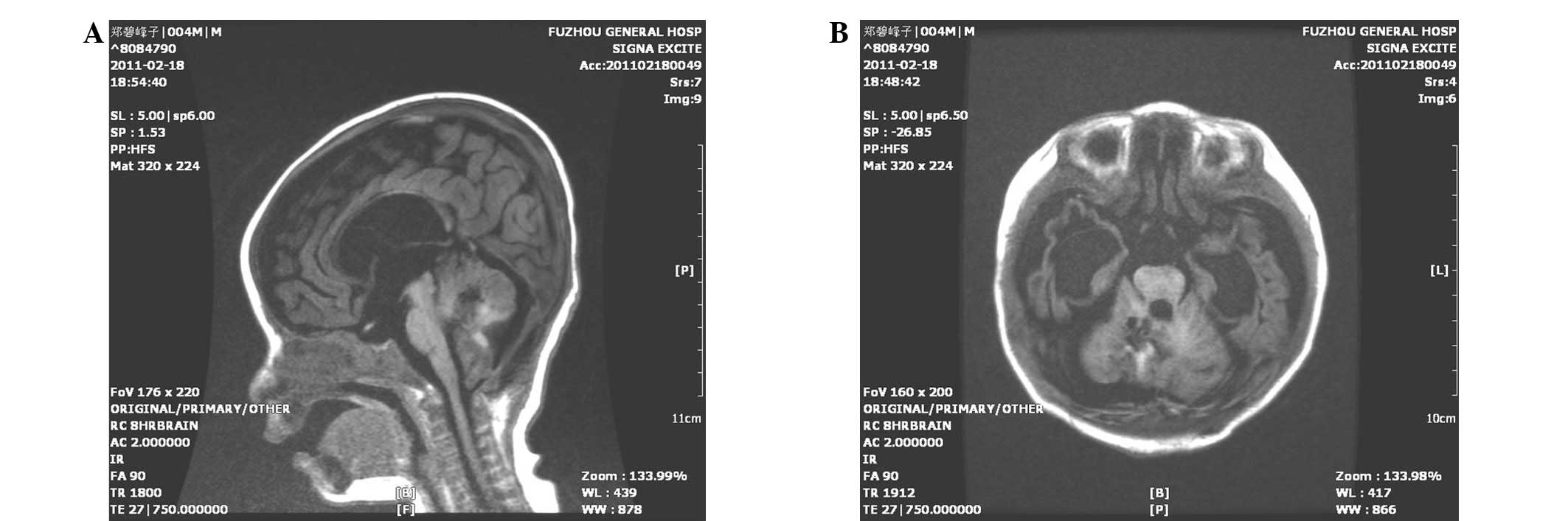
improved mental response. MRI scans revealed that the tumor had
been completely removed (Fig. 3).
Moreover, the hydrocephalus was also significantly improved. The
infant was discharged from the hospital and follow-ups were
performed for 18 months. No recurrence of the neoplasm was noted.
At present, the patient is experiencing normal growth and
development.
Discussion
Intracranial teratomas exhibit different clinical
features according to the growth zones. In the majority of infant
patients, the main clinical feature of these tumors is intracranial
hypertension. Intracranial teratomas are commonly accompanied by
symptoms that include obstructive hydrocephalus, which results in
headaches, vomiting, papilledema, outreach paralysis and
progressive increases in head size. A small number of infant
patients also experience epileptic attacks, even when they are in a
coma, which may be associated with the intracranial hypertension
and brainstem compression caused by the tumor. Infant patients with
posterior cranial fossa teratomas do not exhibit dystaxia since
their motor function is not yet fully developed. The reason that a
medical consultation is required for these infant patients is
typically the presentation of hydrocephalus or epilepsy (3). MRI has advantages in characterizing
the shape, texture, outline, composition and original position of
the tumor, as well as its association with surrounding structures,
particularly in enhanced scanning. MRI is able to clearly identify
the original position of the tumor and its invasiveness and
subsequently provide guidance in surgery (4).
In the present study, the patient was admitted to
the Fuzhou General Hospital of Nanjing Military Command due to
binocular cohesion and a downward gaze for >10 days, and sudden
hyperspasmia for one day. These symptoms are features of
hydrocephalus and epilepsy. The patient did not undergo immediate
tumor resection since he was weak and the hydrocephalus was
serious. Therefore, the hydrocephalus was resolved first. Tumor
resection was then performed from the posterior midline under
general anesthesia on the third day after full pre-operative
preparations had been made. A good therapeutic effect was
subsequently achieved. A lateral ventricle puncture through the
bregma was a suitable choice since the infant bregma was not yet
closed. This surgery is simple and easy and is able to rapidly
relieve severe intracranial hypertension caused by hydrocephalus,
thereby stabilizing the vital signs of infant patients. This allows
doctors more time to perfect the pre-operative preparations and
improve the safety of the tumor resection. However, a ventricle
puncture may cause transtentorial herniation when posterior cranial
fossa occupation is accompanied by obstructive hydrocephalus. Such
a case would require more attention during treatment. Resection is
the main method for treating posterior cranial fossa teratomas. In
solving the hydrocephalus, doctors should attempt to remove as much
of the tumor as possible. The key aspect of treating a teratoma is
opening the circulation passage of the cerebrospinal fluid. This
type of tumor has a complete membranous envelope with a clear
boundary and a low blood supply. Therefore, as much of the tumor as
possible should be removed. Since tumors are often located within
important brain structures, the surgery should be performed
carefully to avoid damaging other organs. Teratoma surgeries should
also be conducted carefully for sections of teratomas that exhibit
cystic changes, in order to prevent cyst fluid from flowing into
the resorption cavity of the arachnoid space, thereby preventing
post-operative pyrexia.
Platinum-based chemotherapy is the main
post-operative chemotherapy for immature teratomas (5). The most common chemotherapy regimen
consists of cis-platinum, bleocin and etoposide or
cyclophosphamide, combined with taxol. The difference in the
sensitivity to chemotherapy among teratomas is high due to the
various compositions of the tumor tissue (6). Overall, teratomas are not highly
sensitive to post-operative chemotherapy. In addition,
chemotherapeutics may have certain toxic effects, such as digestive
tract symptoms, bone marrow arrest, renal toxicity, auditory nerve
damage, pneumoedema and pulmonary fibrosis. Infant patients are
weak and have difficulty enduring these complications. Thus,
post-operative chemotherapy is not recommended as a routine
treatment for infant patients (7).
The infant in the present study was only four months old and was
weak, therefore, chemotherapy was not administered.
The prognosis for an immature teratoma is associated
with tumor differentiation. Highly-differentiated tumors have a
better prognosis, while those that are mainly composed of
undifferentiated embryonal tissue have a worse prognosis. Ogawa
et al (8) reported that the
10-year survival rate of immature teratoma was up to 70%. Full
tumor resection may suspend the development of the disease to a
certain extent, although infants who survive surgery may have
varying levels of developmental retardation (9). Teratomas may be detected in various
locations and generate germ cell tumors with different histological
types. Therefore, periodic checkups and a long-term follow-up
should be performed, even if the computed tomography (CT) and MRI
results indicate a full resection. The early recurrence of a
teratoma may be identified by monitoring the cerebrospinal fluid
and serum tumor markers. Therefore, periodic head MRI scans are
essential between 6 months and 3 years post-surgery (10). Long-term recurring tumors should not
be blindly diagnosed as the recurrence of a teratoma and the
possibility of the patient having other malignant germ cell tumors
should also be considered (11). In
the present study, follow-ups were conducted for 18 months. No
recurrence or abnormal development were noted. However, attention
continues to be given to the prognosis of this infant patient due
to the short follow-up duration.
In conclusion, infant patients with immature
teratomas in the posterior cranial fossa are brought to see doctors
mainly due to the symptoms of hydrocephalus and epilepsy.
Ventricular drainage through an anterior fontanelle puncture is an
effective measure for treating patients with severe hydrocephalus.
Immature teratomas have the potential to change into other
malignant tumors, thus, a long-term follow-up should be conducted
for patients even if a full resection has been performed.
References
|
1.
|
Desai K, Nadkarni T, Muzumdar D and Goel
A: Midline posterior fossa teratoma - case report. Neurol Med Chir
(Tokyo). 41:94–96. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Burger PC, Yu IT, Tihan T, et al: Atypical
teratoid/rhabdoid tumor of the central nervous system: a highly
malignant tumor of infancy and childhood frequently mistaken for
medulloblastoma: a Pediatric Oncology Group study. Am J Surg
Pathol. 22:1083–1092. 1998. View Article : Google Scholar
|
|
3.
|
Nouri A, Khuja M, Wilczynski J, et al:
Massive fetal intracranial teratoma with hydrocephalus detected at
33 weeks of gestation. Neuro Endocrinol Lett. 31:174–177.
2010.PubMed/NCBI
|
|
4.
|
Liang L, Korogi Y, Sugahara T, et al: MRI
of intracranial germ-cell tumours. Neuroradiology. 44:382–388.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Furtado SV, Ghosal N, Rokade VB and Hegde
AS: Fourth-ventricular immature teratoma. J Clin Neurosci.
18:296–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Lee YH, Park EK, Park YS, Shim KW, Choi JU
and Kim DS: Treatment and outcomes of primary intracranial
teratoma. Childs Nerv Syst. 25:1581–1587. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Huang X, Zhang R and Zhou LF: Diagnosis
and treatment of intracranial immature teratoma. Pediatr Neurosurg.
45:354–360. 2009. View Article : Google Scholar
|
|
8.
|
Ogawa K, Toita T, Nakamura K, et al:
Treatment and prognosis of patients with intracranial
nongerminomatous malignant germ cell tumors: a multiinstitutional
retrospective analysis of 41 patients. Cancer. 98:369–376. 2003.
View Article : Google Scholar
|
|
9.
|
Arslan E, Usul H, Baykal S, Acar E,
Eyüboğlu EE and Reis A: Massive congenital intracranial immature
teratoma of the lateral ventricle with retro-orbital extension: a
case report and review of the literature. Pediatr Neurosurg.
43:338–342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Shim KW, Kim DS and Choi JU: Mixed or
metachronous germ-cell tumor? Childs Nerv Syst. 23:713–718. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Wang ZD, Jia G, Ma ZY, Zhang YQ and Yao
HX: Long-term recurrence after total resection of intracranial
mature teratoma: 2 case report and literature review. Chinese J
Minim Invas Neurosurg. 14:18–19. 2009.(In Chinese).
|