Introduction
Pancreatic cancer (PC), which is highly malignant,
is a relatively common malignancy. Early surgical resection is the
only effective treatment. However, due to its deep location and
lack of pronounced early symptoms and specific clinical
manifestations, an early diagnosis is difficult to make. The rapid
development, fast growth and frequent lymph node metastasis all
contribute to the poor prognosis of PC. The majority of patients
with PC are diagnosed when the disease has reached an advanced
stage and only 1–3% of patients survive up to five years (1). Therefore, an early diagnosis and
future clinical developmental research is important. Tumor markers
are a considerable tool for early tumor detection and screening.
Tumor markers help improve the diagnosis of early PC and promote
the prognosis of PC. Carbohydrate antigen (CA) 19–9 is one of the
most common clinically used tumor markers for PC; however, it lacks
sufficient sensitivity and specificity in early diagnosis (2–4). Other
tumor markers, including carcinoembryonic antigen (CEA) and CA125,
are also being studied; however, not specifically for the early
diagnosis of PC. Therefore, it is crucial to identify more
sensitive and specific diagnostic tumor markers to screen high risk
patients and improve diagnosis (5,6).
Materials and methods
Ethical permission
The study protocol and consent forms conformed to
the Declaration of Helsinki and were approved by the Ethics Review
Board (ERB) Committee of the Affiliated Provincial Hospital of
Anhui Medical University (Hefei, Anhui, China). Informed consent
was obtained from all patients.
Patients and samples
Between May 2007 and May 2009, PC specimens from 38
cases that had complete medical records were collected from the
Department of Pathology of the Affiliated Provincial Hospital of
Anhui Medical University. These cases consisted of 18 males and 20
females, aged 16–81 years, with an average age of 60.5 years. The
specimens were divided according to their histological grade. A
total of 29 cases were highly-differentiated and nine cases were
poorly-differentiated, while according to the International Union
Against Cancer (UICC) staging of primary PC, there were seven stage
I cases and 31 stage II–IV cases. There were 23 cases with lymph
node metastasis and 15 without metastasis. These samples were used
for the immunohistochemical analysis. Between May 2007 and May
2009, a further 20 fresh specimens of PC and 20 adjacent normal
pancreatic tissues were collected, all of which were confirmed by
their pathology. Among these 20 fresh specimens, there were eight
males and 12 females, aged 16–76 years, with an average age of 60
years. These cases were also divided according to histological
grade. There were 13 highly-differentiated cases and seven
poorly-differentiated cases, while the UICC staging of the original
onset of PC showed four stage I cases and 16 stage II–IV cases.
There were 13 cases with lymph node metastasis and seven cases
without metastasis. These specimens were used for the RT-PCR
analysis. None of the patients received pre-operative adjuvant
treatment.
Immunohistochemistry
Immunohistochemical staining was performed on 4-mm
sections of formalin-fixed paraffin-embedded samples using a
standard avidin-biotin-peroxidase complex technique. Following
deparaffinization with xylene and rehydration with serial gradient
ethanol, the antigen was retrieved by heating the slides in 10 mM
citrate buffer (pH 6.0) for 6 min in a microwave. The endogenous
peroxidase was blocked with 0.3% hydrogen peroxide. The slides were
subsequently incubated with a blocking protein (normal goat serum)
for 10 min and primary antibody was added overnight at 4°C followed
by rinsing. Antibodies against C3, C4b1 and apolipoprotein E (ApoE;
Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) were used. The
secondary biotinylated antibodies, PV6000 (C3), PV9003 (C4b1) and
PV9003 (ApoE), were then applied for 30 min, followed by 30 min of
incubation with streptavidin peroxidase (Dako LSAB + HRP kit; DAKO,
Carpinteria, CA, USA). Subsequent to being rinsed, the slides were
visualized with diaminobenzidine (DAB) chromogen solution (Dako)
and counterstained with hematoxylin, followed by dehydration
through graded ethanol and slide mounting. The control group was
treated with phosphate-buffered saline (PBS) instead of the primary
antibody. To ensure the specificity of the primary antibodies,
consecutive tissue sections were incubated in the absence of the
primary antibody. No immunostaining was detected in these sections,
indicating the specificity of the primary antibodies used in this
study.
Reverse transcription (RT)-PCR
Total RNA (Invitrogen, Carlsbad, CA, USA) was
isolated from the fresh tissue samples using TRIzol. Cellular RNA
(1 μg) was used to perform RT using a Promega (Madison, WI,
USA) RT kit and Oligo (dT) primers (A3800). All primers (Table I) were purchased from Takara
(Dalian, China). Samples were amplified with an Applied Biosystem
PCR System (Foster City, CA, USA) for 35 cycles with the following
conditions: denaturation at 95°C for 30 sec, annealing at 51°C for
30 sec and extension at 72°C for 30 sec. β-actin was used as a
control.
 | Table I.Primers of C3, C4, ApoE and
β-actin. |
Table I.
Primers of C3, C4, ApoE and
β-actin.
| Gene | Forward primer | Reverse primer |
|---|
| C3 |
5′-TATCATCACCCCCAACATCT-3′ |
5′-CCCTCCACTTTCTTCCCGTA-3′ |
| C4 |
5′-GGAAGCAAACGAGGACTAT-3′ |
5′-TTCAGCAGAACACAAGGTG-3′ |
| ApoE |
5′-CTGCGTTGCTGGTCA-3′ |
5′-GCTCCTCGGTGCTCT-3′ |
| β-actin |
5′-CAACTTCATCCACGTTCACC-3′ | 5′-GAA
GAGCCAAGGACAGGTAC-3′ |
Western blot analysis
The tissues were washed twice with cold PBS, then
incubated in cold lysis buffer (1% Nonidet P40, 0.1% SDS, 150 mM
Tris, 50 U/ml aprotinin and 1 mmol/l PMSF; pH 7.4) for 20 min at
4°C. The cellular lysate was centrifuged for 2 min at 12,000 × g at
4°C. The supernatants were collected as total cellular proteins.
Quantitative analysis of the content was performed by the Lowry
method and an equal amount of total protein from each sample was
loaded onto SDS-PAGE gels. Following electrophoresis, the separated
proteins were transferred to polyvinylidene fluo-ride membranes
(Bio Trace PVDF; Pall Corporation, Port Washington, NY, USA) by
electroblotting. Subsequently, the membrane was blocked with
Tris-buffered saline (TBS) containing 5% skimmed milk for 1 h at
room temperature, followed by incubation with primary antibodies
against human C3, C4b1 and ApoE/TBS solution at 4°C overnight.
Subsequent to being washed, the membrane was incubated in
HRP-linked secondary antibody/TBS solution for 1 h at room
temperature. The western blotting reaction products with
chemiluminescence reagents (SuperSignal-West Femto Trial kit;
Pierce, Woburn, MA, USA) were visualized by radiography.
Data analysis
All experiments were repeated at least three times
and representative results are presented. Data are expressed as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Immunohistochemical analysis of C3, C4b1
and ApoE
The expression levels of C3, C4b1 and ApoE were
significantly increased in the human PC samples
(immunohistochemistry, magnification, ×200; Figs. 1–3).
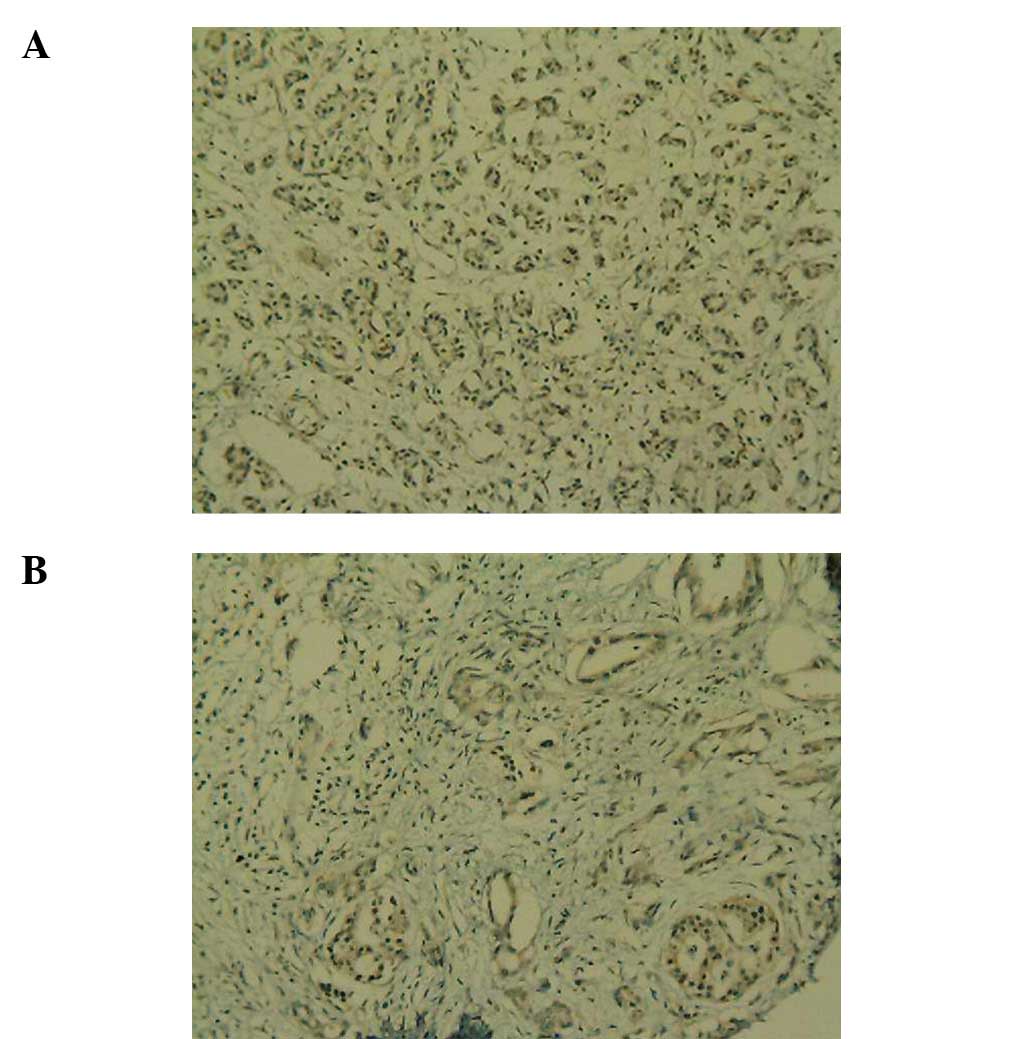
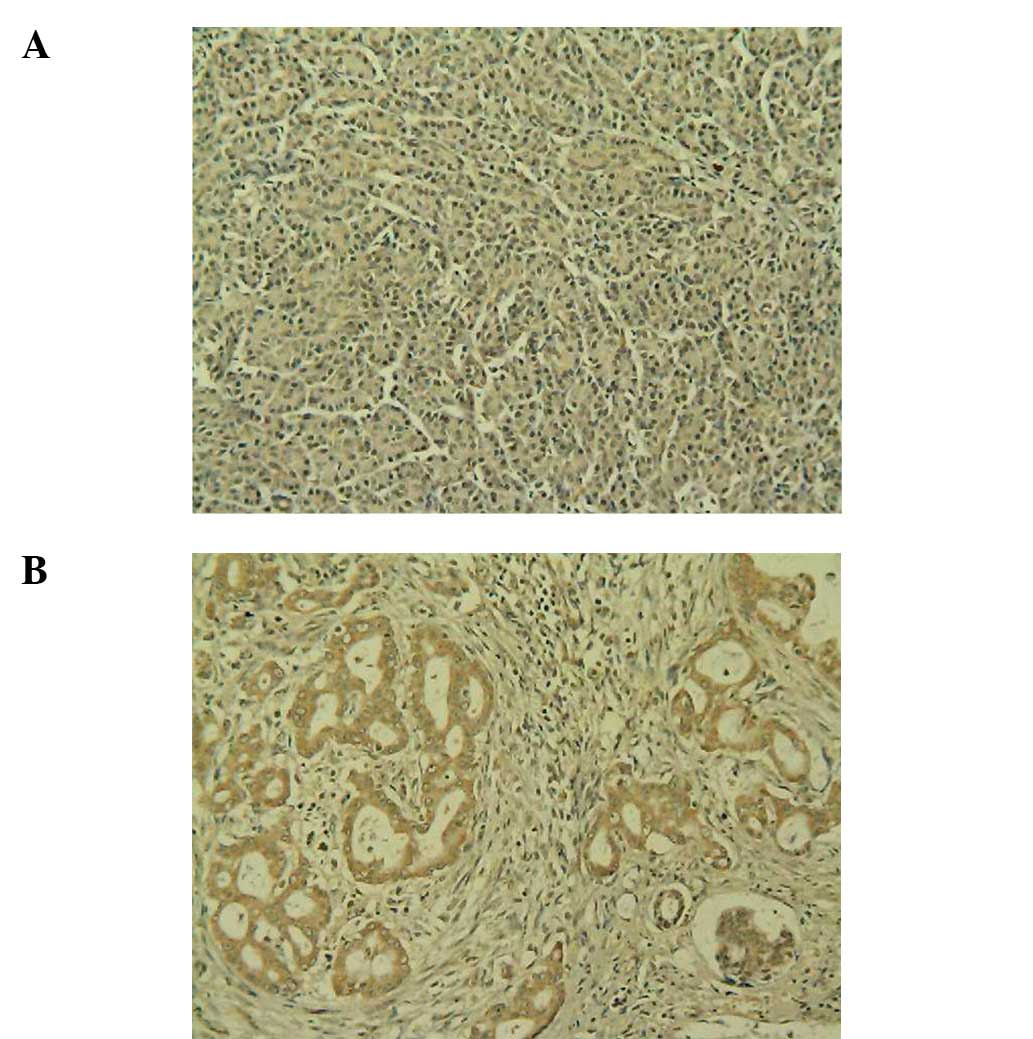
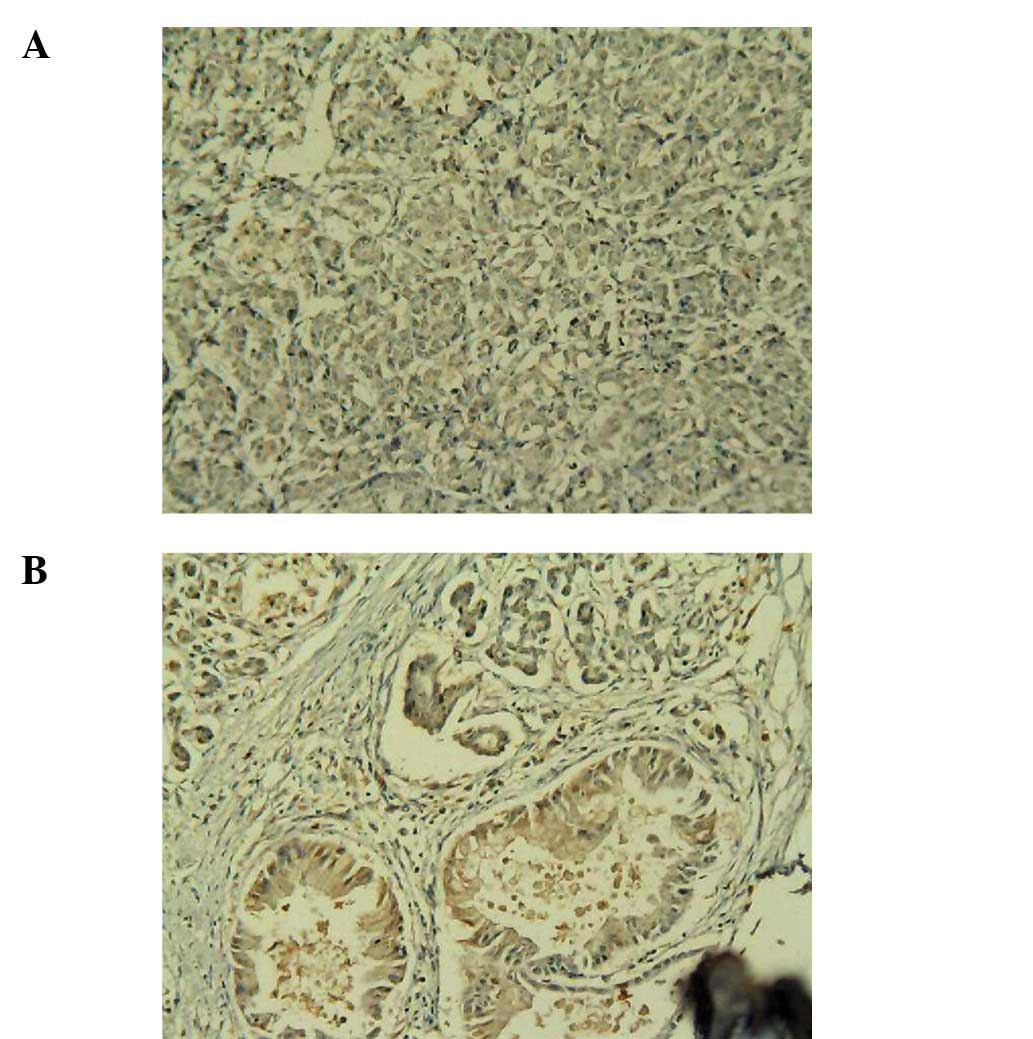
Weak positive C3 expression was observed in the normal human
pancreas specimens (Fig. 1A), while
positive C3 expression was observed in the PC specimens (Fig. 1B). Weak positive C4b1 expression was
observed in the normal human pancreas tissues (Fig. 2A), while positive C4b1 expression
was observed in the PC specimens (Fig.
2B). Weak positive ApoE expression was observed in the normal
human pancreas tissues (Fig. 3A),
while positive ApoE expression was observed in the PC specimens
(Fig. 3B).
The immunohistochemical staining showed that the
expression of C3, C4b1 and ApoE occurred in the cytoplasm. The C3,
C4b1 and ApoE expression levels in PC and normal human pancreatic
tissues were compared and the staining results showed that the
human PC tissue had higher C3, C4b1 and ApoE expression levels
(Table II).
 | Table II.Correlations of the expression levels
of C3, C4b1 and ApoE with pancreatic cancer and pathological
factors. |
Table II.
Correlations of the expression levels
of C3, C4b1 and ApoE with pancreatic cancer and pathological
factors.
| Factor | Category | No. | C3 | C4b1 | ApoE |
|---|
| Group | Pancreatic
cancer | 38 | 73.68% (28/38) | 76.31% (29/38) | 86.84% (33/38) |
| Normal pancreas | 38 | 42.11% (16/38) | 26.32% (10/38) | 42.11% (16/38) |
| P-value | | <0.01 | <0.01 | <0.01 |
| UICC | Stage I | 7 | 57.14% (4/7) | 28.57% (2/7) | 57.14% (4/7) |
| Stage II-IV | 31 | 77.42% (24/31) | 87.10% (27/31) | 93.55% (29/31) |
| P-value | | >0.05 | <0.05 | <0.05 |
| Lymph nodes
metastasis | No | 15 | 46.67% (7/15) | 40.00% (6/15) | 33.33% (5/15) |
| Yes | 23 | 56.52% (13/23) | 73.91% (17/23) | 78.26% (18/23) |
| P-value | | >0.05 | <0.05 | <0.05 |
In the human PC specimens, an average of 73.68
(28/38), 76.32 (29/38) and 86.84% (33/38) expressed C3, C4b1 and
ApoE, respectively, whereas only 42.11 (16/38), 26.32 (10/38) and
42.11% (16/38) of normal human pancreatic specimens expressed C3,
C4b1 and ApoE, respectively (χ2=7.77, 19.01 and 16.6,
respectively; P<0.01).
Of the stage I PC specimens, 57.14% (4/7) expressed
C3, while the rate of positive expression was 77.42% (24/31) in
stage II–IV PC (P=0.19). In the PC specimens with lymphatic
metastasis, 56.52% (13/23) expressed C3. The positive expression
rate was 46.67% (7/15) in the PC tissues without lymphatic
metastasis (P=0.74).
In the stage I PC specimens, 28.57 (2/7) and 57.14%
(4/7) expressed C4b1 and ApoE, respectively, while the rates of
positive expression were 87.10 (27/31) and 93.55% (29/31) in the
stage II–IV PC specimens (P<0.05). In the PC tissues with
lymphatic metastasis, 73.91 (17/23) and 78.26% (18/23) expressed
C4b1 and ApoE, respectively, while the positive expression rates
were 40.00 (6/15) and 33.33% (5/15) in the PC tissues without
lymphatic metastasis (P<0.05).
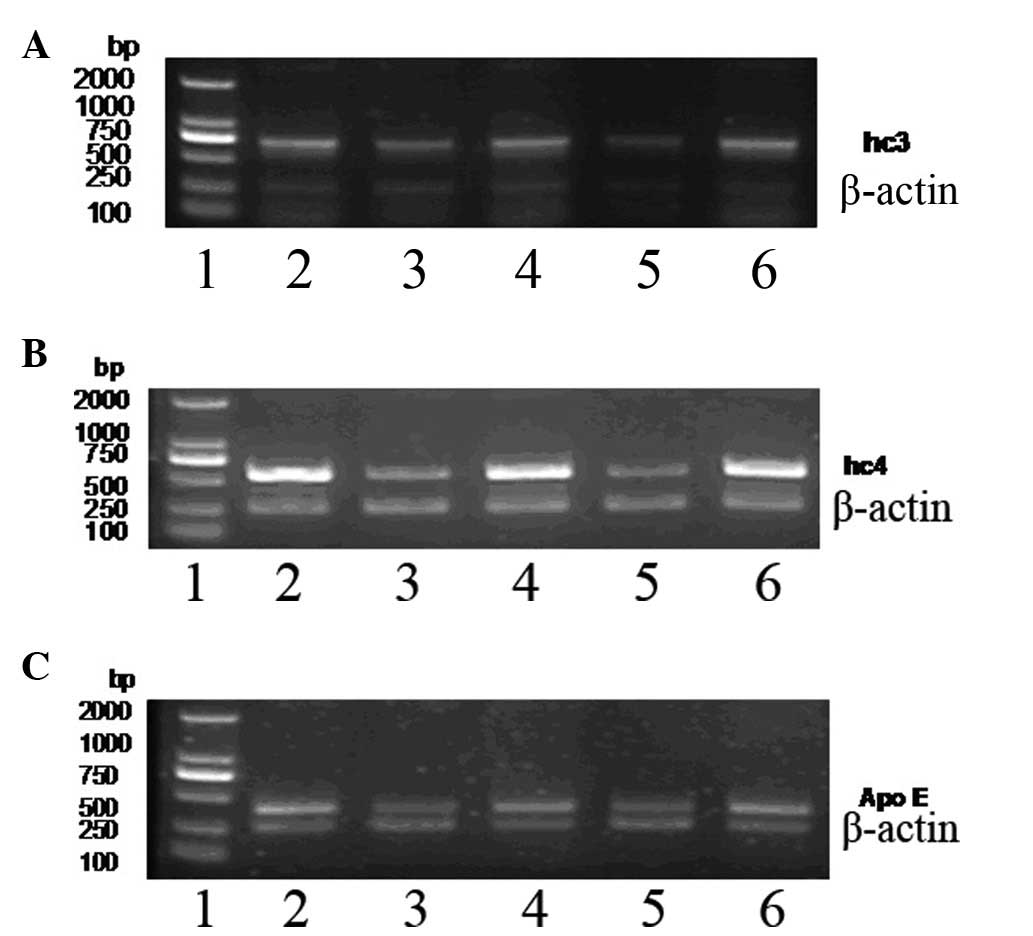
C3, C4b1 and ApoE mRNA expression in
PC
The expression levels of C3, C4b1 and ApoE in the PC
specimens were determined by RT-PCR (Fig. 4).
The expression levels of C3 (5.93±0.82), C4b1
(7.94±0.95) and ApoE (4.83±0.65) mRNA in PC were significantly
higher compared with the normal pancreas tissue levels of C3
(4.05±1.12; t=6.03, P<0.01), C4b1 (1.22±0.57; t=27.21,
P<0.01) and ApoE (1.78±0.74; t=13.77, P<0.01; Table III).
 | Table III.Expression levels of C3, C4b1 and ApoE
mRNA in pancreatic cancer. |
Table III.
Expression levels of C3, C4b1 and ApoE
mRNA in pancreatic cancer.
| Factor | Category | No. | C3 | C4b1 | ApoE |
|---|
| Group | Pancreatic
cancer | 20 | 5.93±0.82 | 7.94±0.95 | 4.83±0.65 |
| Normal pancreas | 20 | 4.05±1.12 | 1.22±0.57 | 1.78±0.74 |
| t-value | | 6.03 | 27.21 | 13.77 |
| P-value | | <0.01 | <0.01 | <0.01 |
| UICC | Stage I | 4 | 6.51±0.28 | 7.21±0.12 | 4.28±0.12 |
| Stage II–IV | 16 | 5.78±0.85 | 9.14±1.02 | 5.28±0.81 |
| t-value | | 1.66 | 7.33 | 4.74 |
| P-value | | >0.05 | <0.01 | <0.01 |
| Lymph nodes
metastasis | No | 7 | 6.06±0.55 | 7.39±0.15 | 4.42±0.25 |
| Yes | 13 | 5.86±0.95 | 8.24±1.07 | 5.05±0.71 |
| t-value | | 0.52 | 2.81 | 2.25 |
| P-value | | >0.05 | <0.05 | <0.05 |
In the stage I PC tissues, the expression level of
C3 was 1.85±0.10, while in stage II–IV tissues, the expression
level was 1.57±0.29 (t=1.85, P=0.08). In the PC specimens with
lymphatic metastasis, the expression level of C3 was 1.56±0.31,
while the expression level was 1.76±0.14 in the PC specimens
without lymphatic metastasis (t=2.05, P=0.06; Table IV).
 | Table IV.The GRAVY levels of C3, C4b1 and
ApoE. |
Table IV.
The GRAVY levels of C3, C4b1 and
ApoE.
| Factor | Category | No. | C3 | C4b1 | ApoE |
|---|
| Group | Pancreatic
cancer | 20 | 1.63±0.28 | 1.25±0.18 | 2.57±0.22 |
| Normal
pancreas | 20 | 0.88±0.19 | 0.65±0.13 | 1.28±0.24 |
| t-value | | 9.93 | 11.81 | 17.71 |
| P-value | | <0.01 | <0.01 | <0.01 |
| UICC | Stage I | 4 | 1.85±0.10 | 1.11±0.08 | 2.32±0.03 |
| Stage II–IV | 16 | 1.57±0.29 | 1.28±0.19 | 2.63±0.20 |
| t-value | | 1.85 | 2.87 | <0.05 |
| P-value | | >0.05 | <0.05 | 2.99 |
| Lymph nodes
metastasis | No | 7 | 1.76±0.14 | 1.10±0.07 | 2.37±0.07 |
| Yes | 13 | 1.56±0.31 | 1.34±0.17 | 2.67±0.20 |
| t-value | | 2.05 | 4.26 | 3.70 |
| P-value | | >0.05 | <0.01 | <0.01 |
In the stage I PC tissues, the expression levels of
C4b1 and ApoE were 1.11±0.08 and 2.32±0.03, respectively, while the
levels were 1.28±0.19 and 2.63±0.20, respectively, in the stage
II–IV PC samples (t=2.87 and 2.99, respectively; P<0.05). In the
PC tissues with lymphatic metastasis, the expression levels of C4b1
and ApoE were 1.34±0.17 and 2.67±0.20, respectively, while the
levels were 1.10±0.07 and 2.37±0.07, respectively, in the PC
tissues without lymphatic metastasis (t=4.26 and 3.70,
respectively; P<0.01; Table
IV).
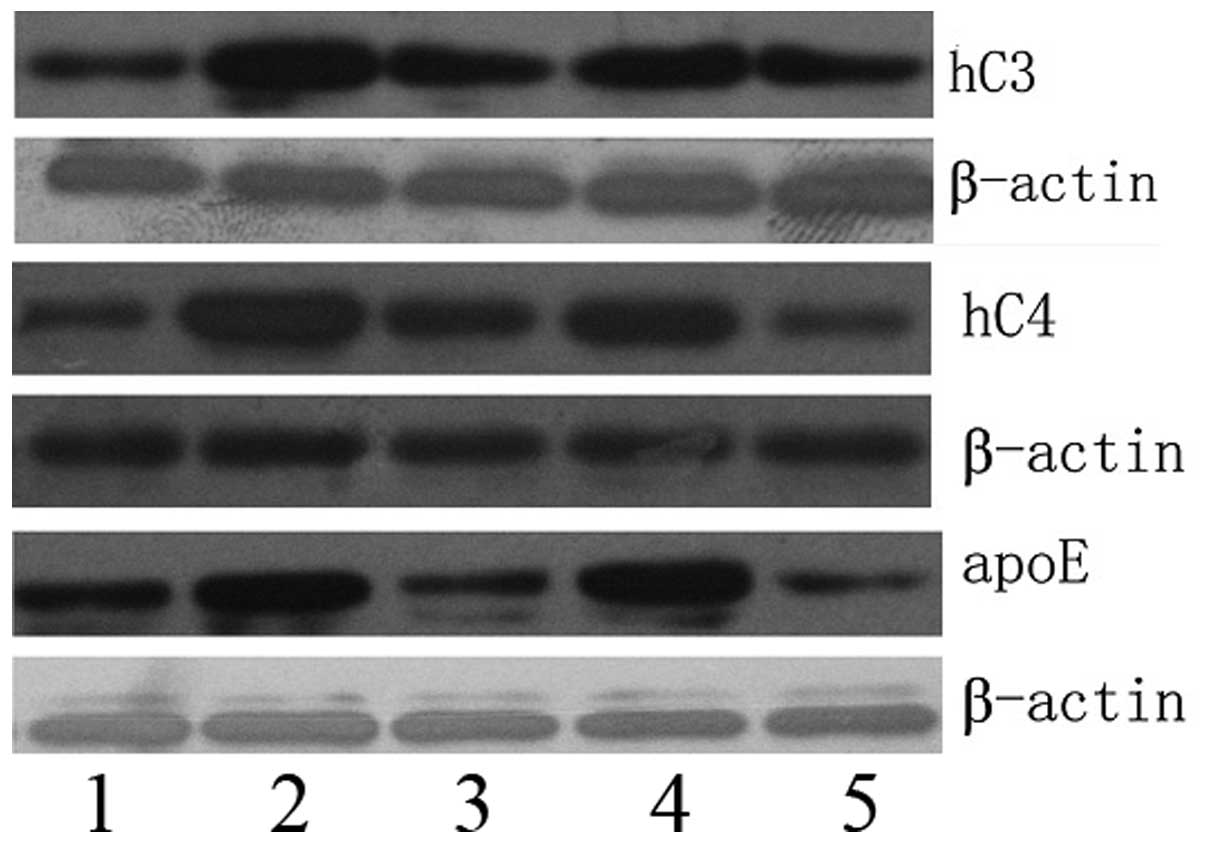
Western blotting of C3, C4b1 and ApoE
protein expression
Western blotting was performed to determine the
relative C3, C4b1 and ApoE protein expression levels in five
pancreatic group types (Fig. 5). As
expected, C3, C4b1 and ApoE were each detected as single bands.
β-actin was used as a control. The C3, C4b1 and ApoE protein
expression levels were higher in the PC samples and lower in the
normal pancreas samples. Furthermore, the expression levels of C3,
C4b1 and ApoE were observed to be significantly different in the
various phases of PC (Table
IV).
Discussion
Complement is a sophisticated regulatory mechanism
of the protein response system that is activated by
antigen-antibody complexes or microorganisms and is involved in
mediating inflammation, opsonophagocytosis, cell lysis, immune
regulation and clearance of immune complexes. C3 is a mediatory
inflammatory factor that, when activated, releases a variety of
vasoactive substances (7,8), causing inflammation. In the present
study, immunohistochemistry and western blotting demonstrated that
the expression levels of C3 in the PC tissues were high, although
the expression of C3 did not differ between the various stages of
PC. The expression of C3 was not observed to be significantly
different between the PC tissues with and without lymphatic
metastasis. These findings indicated that C3 is involved in the
early stages of PC, but not advanced PC. Therefore, C3 may be used
as a serum biomarker in the early diagnosis of PC. The presence of
C3, an important cytokine precursor involved in early PC, indicates
that inflammation may be involved in the mechanism of PC. This is
consistent with the study by Wang et al (9), which showed that the inflammatory
regulator NF-κB was upregulated in PC tissues and cell lines.
Certain studies have also reported that the expression of C3 is
high in colon cancer serum (10),
which may indicate that C3 is expressed specifically in the
digestive tract.
C4b1 is not only closely associated with immune
diseases, particularly systemic lupus erythematosus (SLE) (11), but it also has a crucial role in the
induction of B cell self-tolerance (12). Theoretically, these are favorable
factors for organisms fighting tumors, since antibodies bind to
tumor antigens on tumor cells and are involved in directly killing
them. Furthermore, immunoglobulin in B cell surfaces may combine
with tumor antigens, inducing T cells to respond to tumors and
creating antibodies, in collaboration with K cells, macrophages and
complement, which are also able to kill the tumor cells
[antibody-dependent cell-mediated cytotoxicity (ADCC)] and control
tumor development and metastasis to a certain extent. Fujita et
al analyzed the serum C4 levels of 43 cases of gastrectomy in
gastric cancer patients (13). The
results showed that higher levels of serum C4 were associated with
higher recurrence rates. The present study also demonstrated that
the expression of C4b1 in PC is high and markedly associated with
the TNM stage of the tumor and with lymph node metastasis. The
expression of C4b1 in the stage II–IV tumors and those with lymph
node metastasis was significantly higher compared with the stage I
tumors and those without lymph node metastasis, demonstrating that
C4b1 is a marker of PC progression. We propose that the high
expression levels of C4b1 in advanced PC may be the body’s
protective immune response against the developmental process of PC.
Therefore, this may generate new ideas for researching immune
therapy for PC.
ApoE is a low-density lipoprotein (LDL) receptor
ligand and its main biological function is the delivery of fat.
ApoE is closely associated with lipoprotein metabolism, as well as
the promotion of macrophage cholesterol overflow and the inhibition
of platelet aggregation functions (14). The ApoE gene polymorphism produces
the major human ApoE, generated in the liver and brain and other
tissues, including mononuclear cells (such as macrophages). Between
60 and 80% of ApoE is synthesized in the liver. In addition, the
human kidney, adrenal gland, bones and macrophages also synthesize
ApoE. A previous study of ApoE was concerned with the association
between ApoE and hyperlipidemia or related cardiovascular and
cerebrovascular diseases (15),
although in recent years, with more in-depth study, an increasing
amount of research has been concerned with the role of ApoE in
tumors. The expression of ApoE has been reported to be increased in
a variety of tumors (16). Grant
(17) reported that the
upregulation of ApoE is a risk factor for prostate cancer.
Andreotti et al (18)
conducted a survey in Shanghai, China and showed that males with
ApoE expression had an increased risk of suffering from bile duct
cancer. The study by Moore et al (19) also revealed that ApoE and its gene
variants increase the risk of renal cell cancer. Moreover, in the
study of ApoE, the same close association was observed with
gastrointestinal cancer (20).
Mrkonjic et al (21)
reported that diet (saturated fatty acid content) and ApoE isoforms
synergistically increased the colorectal cancer risk. Grønborg
et al (22) observed that
the expression of ApoE in PC cells (Panc-1) was 20-fold higher
compared with the normal control group. Yu et al (23) analyzed the expression of serum
proteins in PC and normal controls by two-dimensional gel
electrophoresis combined with a comparative analysis using tandem
mass spectrometry and showed that the ApoE levels in the serum of
patients with PC were significantly higher compared with the normal
control group. Gillard et al (24) proposed that ApoE is distributed in
the cells and is secreted by hepatic carcinoma cells. Huvila et
al (25) demonstrated that ApoE
has a positive correlation with endometrial adenocarcinoma and the
degree of malignancy of the tumor. A higher expression of ApoE was
associated with higher degrees of malignancy, indicating that ApoE
reflects certain tumor biological characteristics. The present
results showed that ApoE is highly expressed in PC. Furthermore,
the ApoE levels were significantly higher in stage II–IV and lymph
node metastatic PC tissues compared with stage I PC tissues and
those without PC lymph node metastasis, thus showing that ApoE has
an important role in the development of PC. This also indicates
that ApoE reflects the biological characteristics of PC. The
present results were consistent with those of Huvila et al
(25). Martínez-Clemente et
al (26) observed that ApoE was
able to inhibit the expression of TNF-α, which is able to kill
tumor cells. This also suggests that ApoE may be involved in tumor
development. Additionally, the present results indicated that ApoE
promotes the development of PC. Whether the mechanism is due to the
inhibition of TNF-α remains to be clarified.
In conclusion, C3 may be used as a marker for the
early diagnosis of PC. C4b1 and ApoE are closely correlated with
tumor development, reflecting the biological behavior of PC, and
may be used as diagnostic markers of advanced PC.
References
|
1.
|
Guo X and Cui Z: Current diagnosis and
treatment of pancreatic cancer in China. Pancreas. 31:13–22. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Willett CG, Daly WJ and Warshaw AL: CA
19–9 is an index of response to neoadjunctive chemoradiation
therapy in pancreatic cancer. Am J Surg. 172:350–352. 1996.
|
|
3.
|
Goonetilleke KS and Siriwardena AK:
Systematic review of carbohydrate antigen (CA 19–9) as a
biochemical marker in the diagnosis of pancreatic cancer. Eur J
Surg Oncol. 33:266–270. 2007.PubMed/NCBI
|
|
4.
|
Marrelli D, Caruso S, Pedrazzani C, et al:
CA19-9 serum levels in obstructive jaundice: clinical value in
benign and malignant conditions. Am J Surg. 198:333–339. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Grønborg M, Bunkenborg J, Kristiansen TZ,
et al: Comprehensive proteomic analysis of human pancreatic juice.
J Proteome Res. 3:1042–1055. 2004.
|
|
6.
|
Cecconi D, Palmieri M and Donadelli M:
Proteomics in pancreatic cancer research. Proteomics. 11:816–828.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Hugli TE: Structure and function of the
anaphylatoxins. Springer Semin Immunopathol. 7:193–219. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Scholz W, McClurg MR, Cardenas GJ, et al:
C5a-mediated release of interleukin 6 by human monocytes. Clin
Immunol Immunopathol. 57:297–307. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Wang W, Abbruzzese JL, Evans DB, et al:
The nuclear factor-kappa B RelA transcription factor is
constitutively activated in human pancreatic adenocarcinoma cells.
Clin Cancer Res. 5:119–127. 1999.PubMed/NCBI
|
|
10.
|
Habermann JK, Roblick UJ, Luke BT, et al:
Increased serum levels of complement C3a anaphylatoxin indicate the
presence of colorectal tumors. Gastroenterology. 131:1020–1029.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Pickering MC, Botto M, Taylor PR, et al:
Systemic lupus erythematosus, complement deficiency, and apoptosis.
Adv Immunol. 76:227–324. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Einav S, Pozdnyakova OO, Ma M and Carroll
MC: Complement C4 is protective for lupus disease independent of
C3. J Immunol. 168:1036–1041. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Fujita T, Hara A and Yamazaki Y: The value
of acute-phase protein measurements after curative gastric cancer
surgery. Arch Surg. 134:73–75. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Greenow K, Pearce NJ and Ramji DP: The key
role of apolipoprotein E in atherosclerosis. J Mol Med (Berl).
83:329–342. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Trompet S, Jukema JW, Katan MB, et al:
Apolipoprotein e genotype, plasma cholesterol, and cancer: a
Mendelian randomization study. Am J Epidemiol. 170:1415–1421. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Trost Z, Marc J, Sok M and Cerne D:
Increased apolipoprotein E gene expression and protein
concentration in lung cancer tissue do not contribute to the
clinical assessment of non-small cell lung cancer patients. Arch
Med Res. 39:663–667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Grant WB: A multicountry ecological study
of risk-modifying factors for prostate cancer: apolipoprotein E
epsilon4 as a risk factor and cereals as a risk reduction factor.
Anticancer Res. 30:189–199. 2010.PubMed/NCBI
|
|
18.
|
Andreotti G, Chen J, Gao YT, et al:
Polymorphisms of genes in the lipid metabolism pathway and risk of
biliary tract cancers and stones: a population-based case-control
study in Shanghai, China. Cancer Epidemiol Biomarkers Prev.
17:525–534. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Moore LE, Brennan P, Karami S, et al:
Apolipoprotein E/C1 locus variants modify renal cell carcinoma
risk. Cancer Res. 69:8001–8008. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Beppu T, Gil-Bernabe P, Boveda-Ruiz D, et
al: High incidence of tumors in diabetic thrombin activatable
fibrinolysis inhibitor and apolipoprotein E double-deficient mice.
J Tromb Haemost. 8:2514–2522. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Mrkonjic M, Chappell E, Pethe VV, et al:
Association of apolipoprotein E polymorphisms and dietary factors
in colorectal cancer. Br J Cancer. 100:1966–1974. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Grønborg M, Kristiansen TZ, Iwahori A, et
al: Biomarker discovery from pancreatic cancer secretome using a
differential proteomic approach. Mol Cell Proteomics. 5:157–171.
2006.PubMed/NCBI
|
|
23.
|
Yu KH, Rustgi AK and Blair IA:
Characterization of proteins in human pancreatic cancer serum using
differential gel electrophoresis and tandem mass spectrometry. J
Proteome Res. 4:1742–1751. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Gillard BK, Lin HY, Massey JB and Pownall
HJ: Apolipoproteins A-I, A-II and E are independently distributed
among intracellular and newly secreted HDL of human hepatoma cells.
Biochim Biophys Acta. 1791:1125–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Huvila J, Brandt A, Rojas CR, et al: Gene
expression profiling of endometrial adenocarcinomas reveals
increased apolipoprotein E expression in poorly differentiated
tumors. Int J Gynecol Cancer. 19:1226–1231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Martínez-Clemente M, Ferré N,
González-Périz A, et al: 5-lipoxygenase deficiency reduces hepatic
inflammation and tumor necrosis factor alpha-induced hepatocyte
damage in hyperlipidemia-prone ApoE-null mice. Hepatology.
51:817–827. 2010.PubMed/NCBI
|