Introduction
Colorectal cancer (CRC) is the third most frequently
diagnosed type of cancer worldwide, accounting for >1 million
cases and 639,000 mortalities every year (1). The incidence of CRC is currently
markedly increasing in developing countries, including China
(2). In a recent study, CRC was
ranked in the top five causes of mortality in urban and rural areas
(9.78 and 5.96 fatalities per 100,000, respectively) of China
(3). CRC is a heterogeneous disease
affected by genetic and environmental factors (4). It is widely accepted that the mutation
of the adenomatous polyposis coli (Apc) gene is important in
the early stages of the transformation of the colonic epithelium.
The Apc gene encodes a tumor suppressor protein that is
linked to 80% of cases of sporadic CRC, and which is involved in
the inherited condition, familial adenomatous polyposis syndrome
(5). Besides the loss of the APC
protein and exposure to environmental factors, including cigarette
smoke and alcohol (6), a poor diet
and high body mass index (BMI) (7)
also contribute to the risk of CRC. The mitogen-activated protein
kinase (MAPK) pathway is known to transduce these signals, leading
to various biological outcomes, including apoptosis, inflammation
and tumorigenesis (8). The MAPK
family of proteins comprises extracellular signal-regulated kinase
1/2 (ERK1/2), c-Jun N-terminal kinase (JNK) and p38. The p38
subfamily consists of four isoforms (p38α, -β, -γ, and -δ) that are
defined by the threonine-glycine-tyrosine (Thr-Gly-Tyr; TGY) dual
phosphorylation motif, and exhibit significant homology at the
amino acid level. p38α and -β are ubiquitously expressed and are
thought to have overlapping functions, while p38γ and -δ are
differentially expressed, depending on the tissue type. p38 is
activated through phosphorylation at the
Thr180-Gly-Tyr182 motif by MAP kinase kinase
3 (MKK3), MKK4 and MKK6 (9). The
activation of p38 results in various cellular changes, including
the regulation of transcription, protein synthesis, cell surface
receptor expression, cell cycle progression and apoptosis. The
oncogenic function of p38 is context-dependent. In CRC, p38α is
required for the proliferation and survival of CRC cells, and its
inhibition leads to cell cycle arrest and autophagic cell death
(10). p38 has been demonstrated to
be involved in CRC cell migration and metastasis in animal models
(11,12). Furthermore, it has been revealed
that the inhibition of p38 results in a loss of CRC cell resistance
to chemotherapy drugs (13).
Therefore, p38 activity is important in CRC tumorigenesis and
resistance to chemotherapy.
Regardless of its importance, few studies have
examined the contribution of p38 genetic polymorphisms to the risk
of CRC. The present study investigated the functional importance of
an SNP located in the promoter region of p38β, and correlated its
presence with the risk of sporadic CRC. To the best of our
knowledge, the study demonstrates for the first time that the p38β
promoter region SNP (rs2235356, -1628A>G) is correlated with an
increased risk of sporadic CRC, in a study cohort.
Materials and methods
Study subjects and sample collection
The study was approved by the Institutional Review
Board of Sun Yat-Sen University (Guangzhou, China). The study
cohort consisted of 855 patients with histologically confirmed
sporadic CRC and 871 cancer-free control subjects, who were
genetically unrelated Han Chinese individuals from Guangzhou and
the surrounding regions of Southern China (14). The response rate was 95%. The
exclusion criteria removed individuals with familial adenomatous
polyposis and those fulfilling the Amsterdam criteria for
hereditary nonpolyposis CRC from the study. The 871 cancer-free
control subjects were randomly selected from a pool of >10,000
individuals who had participated in the health check-up programs in
the community health stations in Guangzhou during the same period
of time as when the cases were recruited. The response rate was
85%. The controls were frequency-matched to the cases by gender and
age (±5 years). Following the receipt of written informed consent,
each participant was scheduled for an interview that used a
structured questionnaire to collect information on various factors,
including smoking status, alcohol use and the family history of
cancer. The definitions of these factors corresponded with those
used in a previous study by our group (14). The present study used the BMI cutoff
points suggested by the Cooperative Meta-Analysis Group of the
Working Group on Obesity in China (15). Subjects whose BMI was <18.0
kg/m2 were categorized as being underweight; whereas
18.0–25.0 kg/m2 constituted normal body weight and
>25.0 kg/m2 was classed as overweight. Each subject
was requested to donate 5 ml blood.
Genotyping
The p38β (rs2235356, -1628A>G) polymorphism was
detected by the polymerase chain reaction-restriction fragment
length polymorphism (PCR-RFLP) method. In brief, the genomic DNA
from the peripheral blood of the participants was extracted by the
DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany) and subjected
to PCR using the forward primer, 5′-CCGAGGTTTTGTGCAGAGTT-3′, and
the reverse primer, 5′-CAGGTCAGCTCAGTGATGAGA-3′. The PCR conditions
used were as follows: 94°C for 5 min, 35 cycles of 94°C for 30 sec,
60°C for 45 sec and 72°C for 60 sec, and a final extension step of
72°C for 10 min. The PCR amplification was further digested with
FokI (New England Biolabs, Inc., Ipswich, MA, USA) overnight at
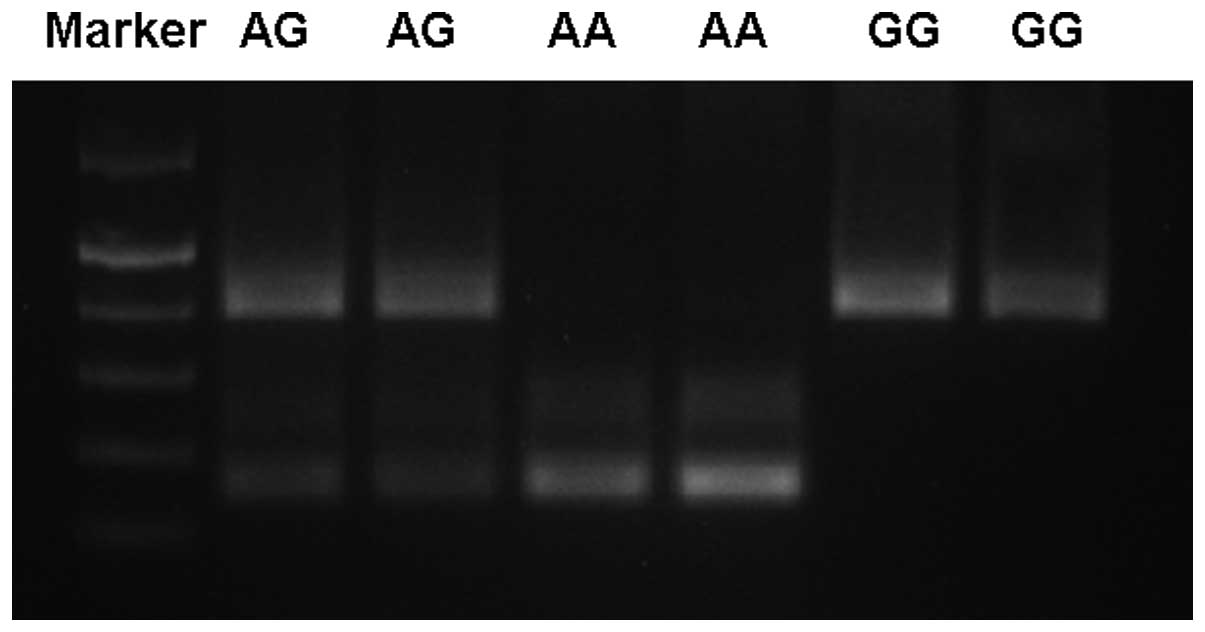
37°C, followed by agarose gel electrophoresis to reveal the
genotype of either -1628AA (two bands of 157 and 228 bp), -1628GG
(one band of 385 bp) or the heterozygous -1628AG (three bands of
157, 228 and 385 bp) (Fig. 1). A
GeneRuler Low Ranger DNA Ladder (100 bp; Thermo Scientific,
Waltham, MA, USA) was used. Two researchers who were blinded to the
CRC status of the participants independently evaluated the gel
electrophoresis results. The PCR-RFLP assay was repeated in 10% of
the samples, which yielded identical results (data not shown). For
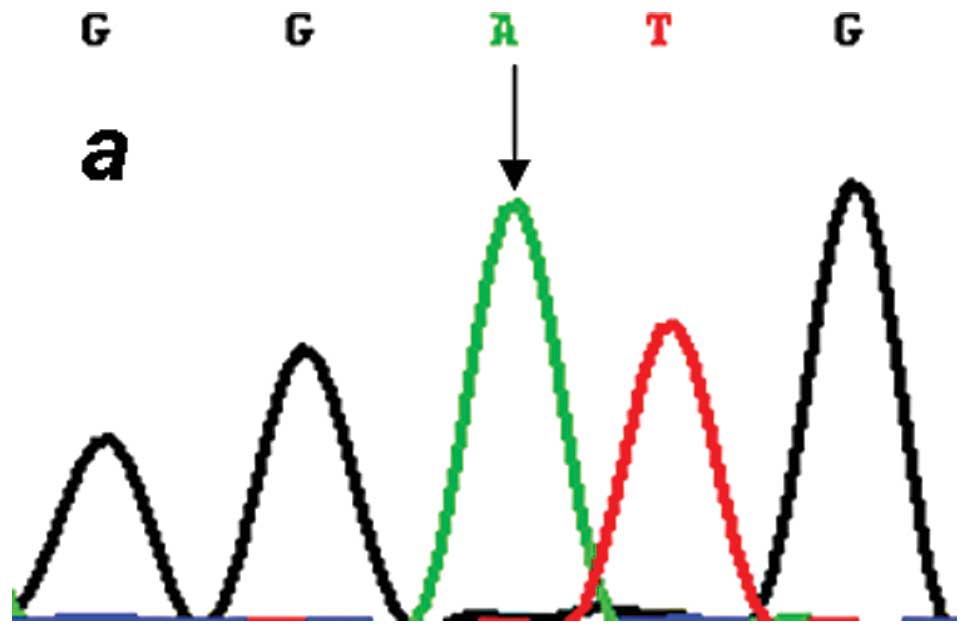
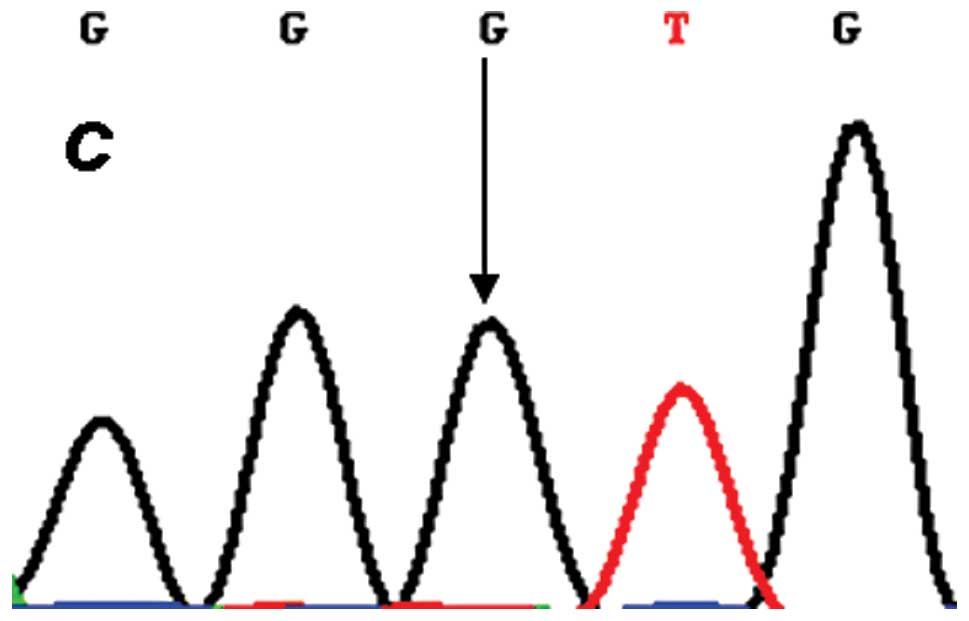
each target genotype the PCR products were purified and confirmed
by direct sequencing (Figs.
2–4). An in silico
analysis was performed using Genomatix (Genomatix Software GmbH,
Munich, Germany) to identify transcription factors that either
acquired or lost the ability to bind to -1628AA in the p38β
promoter region due to the SNP.
Statistical analysis
A two-sided χ2 test was used to evaluate
the differences in the distributions of age, gender, smoking
status, alcohol use, BMI and family history of cancer between the
CRC cases and the cancer-free controls. The Hardy-Weinberg
equilibrium (HWE), determined by a χ2 goodness of fit
test, was used to compare the expected and the observed genotype
frequencies in the cancer-free controls. Unconditional logistic
regression was used to calculate the odds ratios (ORs) and 95%
confident intervals (CIs) to estimate the correlation between the
presence of the SNP and the CRC risk, with and without adjustments
for age, gender, smoking status, alcohol drinking status, BMI and
family history of cancer. A logistic regression model was also used
for the trend test. In the stratification analysis, the main
effects of the p38β polymorphisms were assessed in each subgroup,
along with the effect of potential interactions between the p38β
polymorphisms and selected variables on cancer risk. All two-sided
statistical tests were performed using SAS software version 9.1
(SAS Institute, Inc., Cary, NC, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Characteristics of patients with CRC and
the control subjects
The demographic characteristics of the 855 CRC cases
and 871 cancer-free controls are presented in Table I. There was no significant
difference in the distributions of age (P=0.5773) and gender
(P=0.9319) between the two groups. However, the patients with CRC
were more likely to consume alcohol (46.8% in CRC cases, compared
with 23.4% in the controls; P<0.0001) and, in female
participants, be menopausal (80.5% in CRC cases, compared with
57.9% in controls; P<0.0001). Furthermore, there were a greater
number of CRC cases with an abnormal BMI or a family history of
cancer compared with the controls (P=0.0245 and P=0.0379,
respectively). These variables were therefore adjusted in the
multivariate logistic regression analysis, as well as in the
stratification and gene-environment interaction analysis, to reveal
the effect of the studied SNP on the risk of CRC.
 | Table I.Frequency distributions of selected
variables in patients with CRC and cancer-free control
subjects. |
Table I.
Frequency distributions of selected
variables in patients with CRC and cancer-free control
subjects.
| Variables | No. cases (n=855)
(%) | No. controls (n=871)
(%) | P-value |
|---|
| Age (years) | | | 0.5773 |
| ≤49 | 198 (23.1) | 198 (22.7) | |
| 50-60 | 234 (27.4) | 258 (29.6) | |
| >60 | 423 (49.5) | 415 (47.7) | |
| Gender | | | 0.9319 |
| Male | 521 (60.9) | 529 (60.7) | |
| Female | 334 (39.1) | 342 (39.3) | |
| Smoking status | | | 0.4520 |
| Smoker | 414 (48.4) | 406 (46.6) | |
| Non-smoker | 441 (51.6) | 465 (53.4) | |
| Alcohol status | | | <0.0001 |
| Not teetotal | 400 (46.8) | 204 (23.4) | |
| Teetotal | 455 (53.2) | 667 (76.6) | |
| Family history of
cancer | | | 0.0379 |
| Yes | 96 (11.2) | 72 (8.3) | |
| No | 759 (88.8) | 799 (91.7) | |
| BMI
(kg/m2) | | | 0.0245 |
| <18 | 56 (6.5) | 35 (4.0) | |
| 18–25 | 557 (65.2) | 608 (69.8) | |
| >25 | 242 (28.3) | 228 (26.2) | |
| Menstrual
history | | | <0.0001 |
| Premenopause | 65 (19.5) | 144 (42.1) | |
| Menopause | 269 (80.5) | 198 (57.9) | |
Distribution of the p38β promoter region
SNP and the risk of sporadic CRC
The genotypic and allelic distributions of the p38β
-1628A>G polymorphism among the cases and controls are
summarized in Table II. The
observed genotype frequencies of this SNP were in agreement with
the Hardy-Weinberg equilibrium in the control subjects (P=0.796).
As demonstrated in Table II, the
logistic regression analysis indicated that the subjects carrying
the -1628G variant allele (-1628AG and -1628GG) had a 1.99-fold
increased risk of sporadic CRC compared with those who were
homozygous for the -1628A allele (95% CI, 1.6–2.47; P<0.0001).
The increase in CRC risk was particularly evident in the subjects
who were heterozygous for the -1628G allele, with a 2.22-fold
increase in risk (95% CI, 1.78-2.79; P<0.0001). There was a
significant trend for the allele dose effect on the risk of CRC
(Ptrend<0.0001). The correlation between the -1628G
variant allele and the risk of CRC was also statistically
significant at a 5% type I error level, having adjusted for
multiple tests using a Bonferroni correction (P<0.0001).
 | Table II.p38β promoter region polymorphism
(rs2235356, -1628A>G) is correlated with an increased risk of
CRC. |
Table II.
p38β promoter region polymorphism
(rs2235356, -1628A>G) is correlated with an increased risk of
CRC.
| No. cases (%) | No. controlsa (%) | P-valueb | Crude OR (95%
CI) | Adjusted ORc (95% CI) |
|---|
| Total no.
subjects | 855 | 871 | | | |
| Total no.
alleles | 1710 | 1742 | | | |
| Rs2235356A>G
genotype | | | <.0001 | | |
| AA | 214 (25.0) | 343 (39.4) | | 1.00 (ref.) | 1.00 (ref.) |
| AG | 551 (64.5) | 410 (47.1) | | 2.15 (1.74–2.76) | 2.22 (1.78–2.79) |
| GG | 90 (10.5) | 118 (13.5) | | 1.22 (0.89–1.69) | 1.21 (0.86–1.70) |
| AG+GG | 641 (75.0) | 528 (60.6) | | 1.95 (1.58–2.39) | 1.99 (1.60–2.47) |
| Trend test
P-value | | | | 0.0002 | 0.0004 |
| G allele | 0.427 | 0.371 | <.0001 | | |
Stratification analyses of the p38β
promoter region SNP and the risk of CRC
A stratification analysis of the correlations
between the p38β variant genotypes and the risk of sporadic CRC was
performed by subdividing the subjects by age, gender, smoking and
alcohol consumption statuses, family history of cancer and BMI
(Table III). With the exception of
the subjects who had a family history of cancer, the subjects
carrying the -1628G variant allele (-1628AG and -1628GG) were
correlated with an increased risk of CRC in all remaining subgroups
(Table III).
 | Table III.Stratification analysis of the p38β
promoter region polymorphism (rs2235356, -1628A>G) genotypes by
selected variables in patients with CRC and cancer-free control
subjects. |
Table III.
Stratification analysis of the p38β
promoter region polymorphism (rs2235356, -1628A>G) genotypes by
selected variables in patients with CRC and cancer-free control
subjects.
| Variable | No. cases (n=855)
(%)
| No. controls
(n=871) (%)
| Crude OR (95% CI)
AG+GG vs. AA | Adjusted ORa (95% CI) AG+GG vs. AA | P-valueb |
|---|
| AA | AG+GG | AA | AG+GG |
|---|
| Age (years) | | | | | | | 0.2772 |
| ≤60 | 103 (23.8) | 329 (76.2) | 182 (39.9) | 274 (60.1) | 2.12
(1.59–2.84) | 2.11
(1.56–2.87) | |
| > 60 | 111 (26.2) | 312 (73.8) | 161 (38.8) | 254 (61.2) | 1.78
(1.33–2.39) | 1.97
(1.43–2.72) | |
| Gender | | | | | | | 0.2622 |
| Male | 135 (25.9) | 386 (74.1) | 204 (38.6) | 325 (61.4) | 1.80
(1.38–2.33) | 1.82
(1.36–2.44) | |
| Female | 79 (23.7) | 255 (76.3) | 139 (40.6) | 203 (59.4) | 2.21
(1.59–3.08) | 2.10
(1.50–2.94) | |
| Smoking status | | | | | | | 0.0770 |
| Smoker | 112 (27.1) | 302 (72.9) | 154 (37.9) | 252 (62.1) | 1.65
(1.23–2.21) | 1.68
(1.20–2.35) | |
| Non-smoker | 102 (23.1) | 339 (76.9) | 189 (40.6) | 276 (59.4) | 2.28
(1.71–3.04) | 2.22
(1.66–2.97) | |
| Drinking
status | | | | | | | 0.0501 |
| Not teetotal | 114 (28.5) | 286 (71.5) | 76 (37.3) | 128 (62.7) | 1.49
(1.04–2.13) | 1.47
(1.02–2.12) | |
| Teetotal | 100 (22.0) | 355 (78.0) | 267 (40.0) | 400 (60.0) | 2.37
(1.81–3.11) | 2.37
(1.79–3.14) | |
| BMI
(kg/m2) | | | | | | | 0.3030 |
| <18 | 9 (16.1) | 47 (83.9) | 12 (34.3) | 23 (65.7) | 2.72
(1.01–7.39) | 3.63
(1.01–13.0) | |
| 18–25 | 129 (23.2) | 428 (76.8) | 231 (38.0) | 377 (62.0) | 2.03
(1.57–2.63) | 2.01
(1.53–2.62) | |
| >25 | 76 (31.4) | 166 (68.6) | 100 (43.9) | 128 (56.1) | 1.71
(1.17–2.49) | 1.94
(1.29–2.92) | |
| Family history of
cancer | | | | | | | 0.0578 |
| Yes | 23 (24.0) | 73 (76.0) | 19 (26.4) | 53 (73.6) | 1.14
(0.56–2.30) | 1.04
(0.49–2.21) | |
| No | 191 (25.2) | 568 (74.8) | 324 (40.5) | 475 (59.5) | 2.03
(1.63–2.52) | 2.12
(1.69–2.66) | |
In silico analyses of the affect of the
p38β promoter region SNP on p38β gene expression
The presence of the SNP in the p38β promoter region
may affect the gene expression of p38β. An in silico
analysis was performed using Genomatix (Genomatix Software GmbH,
Munich, Germany), to identify the transcription factors that had
either acquired or lost the ability to bind to this locus due to
the SNP. It was determined that the -1628G variant allele was
correlated with a loss of binding ability for chorion-specific
transcription factor (GCMa), but also with an acquired binding
ability for basic Krüppel-like factor (KLF3), erythoid Krüppel-like
factor (EKLF), the rat C2H2 zinc finger protein (involved in
olfactory neuronal differentiation), Wilms' tumor suppressor, the
zinc finger with Krüppel-associated box (KRAB) and SCAN (named
after SRE-ZBP, CTfin51, AW-1 and Number 18 cDNA) domains 3 (data
not shown).
Discussion
The present study investigated the correlation
between a putative functional SNP of the p38β promoter region
(rs2235356) and the risk of CRC in a Chinese population, with a
sample size of 855 patients with sporadic CRC and 871 cancer-free
control subjects. We developed a PCR-based method for detecting
this SNP, and demonstrated that the -1628G allele was correlated
with an increased risk of sporadic CRC in our study cohort (95% CI,
1.6–2.47; P<0.0001).
p38 promotes the survival of CRC cells, primarily
through its functions in the DNA repair pathway and in autophagy.
p38 is one of the effector kinases of the DNA damage sensor system,
following the activation of ataxia telangiectasia mutated (ATM)
kinase, ataxia telangiectasia and Rad3-related (ATR) kinase and
DNA-dependent protein kinase (DNA-PK) (16). Therefore, p38 may enhance the DNA
repair response following chemotherapy in colon cancer cells,
resulting in drug resistance. Furthermore, p38 has been
demonstrated to inhibit the autophagy and cell death of CRC cells
(17), indicating that p38 activity
is necessary for the survival of these cells. The present study
identified an SNP in the p38β promoter region that may be
biologically significant in the tumorigenesis of sporadic CRC,
which is consistent with the importance of p38 in mediating
CRC.
One of the primary downstream effectors of p38 is
activator protein-1 (AP-1), a heterodimeric transcription factor
composed of proteins belonging to the c-fos, c-Jun and activating
transcription factor (ATF) families. In CRC, AP-1 activity is
activated by ERK and JNK (18), or
by the hyper-activation of the Wnt pathway. p38 phosphorylates
ATF-2, which forms a heterodimer with the Jun family transcription
factors to form active AP-1 (19).
Aberrant c-Jun activity has been observed in CRC, which is
consistent with the function of p38 in the regulation of AP-1
(20). Abnormal p38 activity may
increase the risk of CRC by modulating AP-1. In addition to JNK and
ATF-2, AP-1 activity is also regulated by the level of c-fos. The
expression of c-fos is dependent on sterol regulatory element,
which interacts with ternary complex factors (TCFs). Sap-1a is a
TCF that is phosphorylated by p38, thus AP-1 activity may also be
regulated by p38 through Sap-1a. Therefore, aberrant levels of the
p38 pathway constituents may promote CRC through the activation of
the AP-1 transcription factor.
In conclusion, the present study has provided
evidence that the -1628A>G genetic variation in the p38β
promoter region may contribute to the susceptibility to CRC in
Chinese populations. It would be of interest to determine if this
SNP is correlated with the risk of sporadic CRC in other ethnic
groups. Furthermore, future efforts are required to determine the
functional importance of this SNP in controlling the level of
p38β.
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (grant nos.
81072042 and 81172040). The authors would like to thank the
participants for taking part in the study, and, in particular, Ms.
Peihuang Wu, Dr Zhenyu Xian and Dr Dechang Diao for their
laboratory assistance.
References
|
1.
|
World Health Organization: World Health
Organization Mortality Database. http://www-dep.iarc.fr/.
Accessed May 5, 2010.
|
|
2.
|
Yang L, Parkin DM, Ferlay J, Li L and Chen
Y: Estimates of cancer incidence in China for 2000 and projections
for 2005. Cancer Epidemiol Biomarkers Prev. 14:243–250.
2005.PubMed/NCBI
|
|
3.
|
Zhao P, Dai M, Chen W and Li N: Cancer
trends in China. Jpn J Clin Oncol. 40:281–285. 2010. View Article : Google Scholar
|
|
4.
|
Lichtenstein P, Holm NV, Verkasalo PK,
Iliadou A, Kaprio J, Koskenvuo M, et al: Environmental and
heritable factors in the causation of cancer - analyses of cohorts
of twins from Sweden, Denmark, and Finland. N Engl J Med.
343:78–85. 2000. View Article : Google Scholar
|
|
5.
|
Radtke F, Clevers H and Riccio O: From gut
homeostasis to cancer. Curr Mol Med. 6:275–289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Tsong WH, Koh WP, Yuan JM, Wang R, Sun CL
and Yu MC: Cigarettes and alcohol in relation to colorectal cancer:
the Singapore Chinese Health Study. Br J Cancer. 96:821–827. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Moghaddam AA, Woodward M and Huxley R:
Obesity and risk of colorectal cancer: a meta-analysis of 31
studies with 70,000 events. Cancer Epidemiol Biomarkers Prev.
16:2533–2547. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Fang JY and Richardson BC: The MAPK
signalling pathways and colorectal cancer. Lancet Oncol. 6:322–327.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Wilson KP, Fitzgibbon MJ, Caron PR,
Griffith JP, Chen W, McCaffrey PG, et al: Crystal structure of p38
mitogen-activated protein kinase. J Biol Chem. 271:27696–27700.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Chiacchiera F and Simone C:
Signal-dependent regulation of gene expression as a target for
cancer treatment: inhibiting p38alpha in colorectal tumors. Cancer
Lett. 265:16–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Gout S, Morin C, Houle F and Huot J: Death
receptor-3, a new E-Selectin counter-receptor that confers
migration and survival advantages to colon carcinoma cells by
triggering p38 and ERK MAPK activation. Cancer Res. 66:9117–9124.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Wolf MJ, Hoos A, Bauer J, Boettcher S,
Knust M, Weber A, et al: Endothelial CCR2 signaling induced by
colon carcinoma cells enables extravasation via the JAK2-Stat5 and
p38MAPK pathway. Cancer Cell. 22:91–105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Paillas S, Boissière F, Bibeau F, Denouel
A, Mollevi C, Causse A, et al: Targeting the p38 MAPK pathway
inhibits irinotecan resistance in colon adenocarcinoma. Cancer Res.
71:1041–1049. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Wei Y, Wang L, Lan P, Zhao H, Pan Z, Huang
J, et al: The association between -1304T>G polymorphism in the
promoter of MKK4 gene and the risk of sporadic colorectal cancer in
southern Chinese population. Int J Cancer. 125:1876–1883. 2009.
|
|
15.
|
Zhou BF; Cooperative Meta-Analysis Group
of the Working Group on Obesity in China: Predictive values of body
mass index and waist circumference for risk factors of certain
related diseases in Chinese adults - study on optimal cut-off
points of body mass index and waist circumference in Chinese
adults. Biomed Environ Sci. 15:83–96. 2002.PubMed/NCBI
|
|
16.
|
Reinhardt HC, Aslanian AS, Lees JA and
Yaffe MB: p53-deficient cells rely on ATM- and ATR-mediated
checkpoint signaling through the p38MAPK/MK2 pathway for survival
after DNA damage. Cancer Cell. 11:175–189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Comes F, Matrone A, Lastella P, Nico B,
Susca FC, Bagnulo R, et al: A novel cell type-specific role of
p38alpha in the control of autophagy and cell death in colorectal
cancer cells. Cell Death Differ. 14:693–702. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Mechta F, Lallemand D, Pfarr CM and Yaniv
M: Transformation by ras modifies AP1 composition and activity.
Oncogene. 14:837–847. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Zhao M, New L, Kravchenko VV, Kato Y, Gram
H, di Padova F, et al: Regulation of the MEF2 family of
transcription factors by p38. Mol Cell Biol. 19:21–30.
1999.PubMed/NCBI
|
|
20.
|
Chen D, Song S, Lu J, Luo Y, Yang Z, Huang
Q, et al: Functional variants of -1318T > G and -673C > T in
c-Jun promoter region associated with increased colorectal cancer
risk by elevating promoter activity. Carcinogenesis. 32:1043–1049.
2011.
|