Introduction
Hepatocellular carcinoma (HCC) is a major form of
liver cancer and has become the fifth most common malignancy and
the third leading cause of cancer-related mortality worldwide
(1,2). Hepatitis B virus (HBV)- or hepatitis C
virus (HCV)-induced liver cirrhosis is a significant risk factor
for HCC as >80% of HCCs feature a history of cirrhosis (3). Due to the particularly high prevalence
of HBV infection in the Chinese population, HBV-related liver
disease (e.g. chronic hepatitis, cirrhosis and HCC) is one of the
major healthcare burdens in China. There are an estimated 30
million individuals chronically infected with HBV. During a 5-year
period, 10% of these patients developed cirrhosis and 6.5% of the
cirrhotic patients suffered from HCC (4). Although surgical treatment (hepatic
resection and transplantation) is the most effective therapy, it is
associated with high tumor recurrence rates and consequently poor
prognoses (5). Furthermore,
numerous cases are not suitable for surgery as the diseases are at
the end-stage. Therefore, it is important to identify biomarkers
for HCC to enable the early diagnosis and monitoring of tumor
recurrence.
Annexin A2 (ANXA2), a member of the annexin family,
is involved in various biological functions including cell-cell
adhesion (6), cell proliferation
(7,8), cell surface fibrinolysis (9,10),
cell growth regulation and apoptosis (11–13)
and the cocarcinogenic effects of progastrin (14). It has been reported that ANXA2 is
frequently upregulated in HCC patients compared with controls
(15,16), suggesting that ANXA2 is associated
with HCC. In addition, ANXA2 has been demonstrated to be a
potential biomarker of immune liver fibrosis in a rat liver
fibrosis model (16), indicating
that ANXA2 may also be associated with liver fibrosis or even
cirrhosis.
The primary objective of the present study was to
clarify whether ANXA2 is a useful biomarker for distinguishing HCC
from cirrhosis; the secondary objective was to evaluate the role of
ANXA2 in predicting HCC recurrence following liver
transplantation.
Materials and methods
Patients and samples
A total of 130 adult patients with HBV-related HCC
(with cirrhosis; n=88) and HBV-related cirrhosis (n=42) were
prospectively enrolled between October 2006 and January 2008 at the
First Affiliated Hospital, Zhejiang University School of Medicine,
Hangzhou, Zhejiang, China. All patients received a primary liver
transplantation and anti-HBV treatment according to the standard
protocol (17). Patients with HCC
fell within the Hangzhou criteria (18). A total of four patients were
excluded as three cases suffered early mortality due to
hemorrhaging following transplantation and one was lost to
follow-up. The healthy controls (n=27) were donors for living donor
liver transplantation. The patient characteristics are shown in
Table I. The median follow-up time
was 52 months (range, 14–74 months).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | HCC (n=87) | Cirrhosis (n=39) | Healthy controls
(n=27) |
|---|
| Age (years) | 48.1±8.6 | 45.5±5.1 | 31.5±4.7 |
| Male/female, n | 66/21 | 29/10 | 18/9 |
| HBV DNA (+), n | 48 | 21 | - |
| HBV relapse, n | 18 | 9 | - |
| MELD score | 12.1±7.4 | 19.1±5.2 | - |
| Tumor size (cm),
n | | | |
| ≤5 | 51 | - | - |
| 5< n ≤8 | 22 | - | - |
| >8 | 14 | - | - |
| Multiple tumors,
n | 40 | - | - |
| Tumor
differentiation, n | | | |
| Good | 11 | - | - |
| Moderate | 76 | - | - |
| Poor | 0 | - | - |
| AFP (≥20 ng/ml),
n | 66 | - | - |
Peripheral venous blood samples were obtained prior
to surgery. Serum samples were allowed to clot and stored at −80°C.
The tissues were paraffin-embedded following fixation in 10%
formalin for 24–48 h. The non-cancer tissues were the cirrhotic
tissues surrounding the cancer tissue. All surgeries were performed
by the same group led by Professor Shusen Zheng. HCC and cirrhosis
were diagnosed by imaging examinations and biopsy and verified by
histological examination following surgery.
Informed consent was obtained from each patient. The
present study was approved by the Ethics Committee of the First
Affiliated Hospital, Zhejiang University, and performed strictly
according its guidelines, the regulations of the Organ Transplant
Committee of Zhejiang province and the Declaration of Helsinki.
Enzyme immunoassay
Serum ANXA2 levels were determined using a sensitive
enzyme immunoassay (ANXA2 kit; Uscn Life Science Inc., Wuhan,
China) according to the manufacturer’s instructions. Briefly, 100
μl of standard, blank or samples were added into the
appropriate wells, which were precoated with a monoclonal antibody
specific for ANXA2, and incubated at 37°C for 2 h. Detection
reagent A working solution was added to each well for 1 h at 37°C,
then 100 μl of detection reagent B working solution was
added to each well for 30 min at 37°C. At 10 min, following color
development, the intensity was read at 450 nm. The results were
calculated from a standard curve (recombinant human ANXA2; range,
0.625–40 ng/ml) generated from a four-parameter logistic curve fit.
Measurements were performed in duplicate and the mean values were
taken.
Immunohistochemical staining
Immunohistochemical staining was performed as
described previously (19). The
cases were semi-quantitatively evaluated with a four-tiered system
by two independent pathologists and assessed using the
immunoreactive score (IRS) (20).
Individual cases were considered immunoreactive (IR) for antigens
when >10% of the cells were stained. The percentage of positive
cells was assessed semi-quantitatively: 0, absent; 1, <10% of
positive cells; 2, 10–49%; 3, 50–80%; and 4>80%. To achieve the
final score, the quantity of immunoreactive cells was multiplied by
the staining intensity: 0, absent; 1, weak; 2, moderate; and 3,
marked. The final score was considered as follows: 0, negative;
1–4, +; 5–8, ++; and 9–12, +++. The staining assessment was
performed by two independent pathologists. The concordance on
agreed scores was achieved with a high k coefficient value
(>0.80).
Statistical analysis
Categorical data are presented as a number and
percentage, while continuous data are presented as the mean and
standard deviation or median (25th to 75th percentile of the
inter-quartile range). Categorical data were compared using the
Chi-squared test and continuous data were compared with the
Student’s t-test or Mann-Whitney test. A Spearman’s rank
correlation was performed to analyze the correlation. The cutoff
value was selected according to receiver-operating characteristic
curves. P<0.05 was considered to indicate a statistically
significant difference. Interobserver variability was assessed with
the κ index. All statistical analyses were performed using SPSS
13.0 software (SPSS, Chicago, IL, USA).
Results
Serum ANXA2 for the diagnosis of HCC
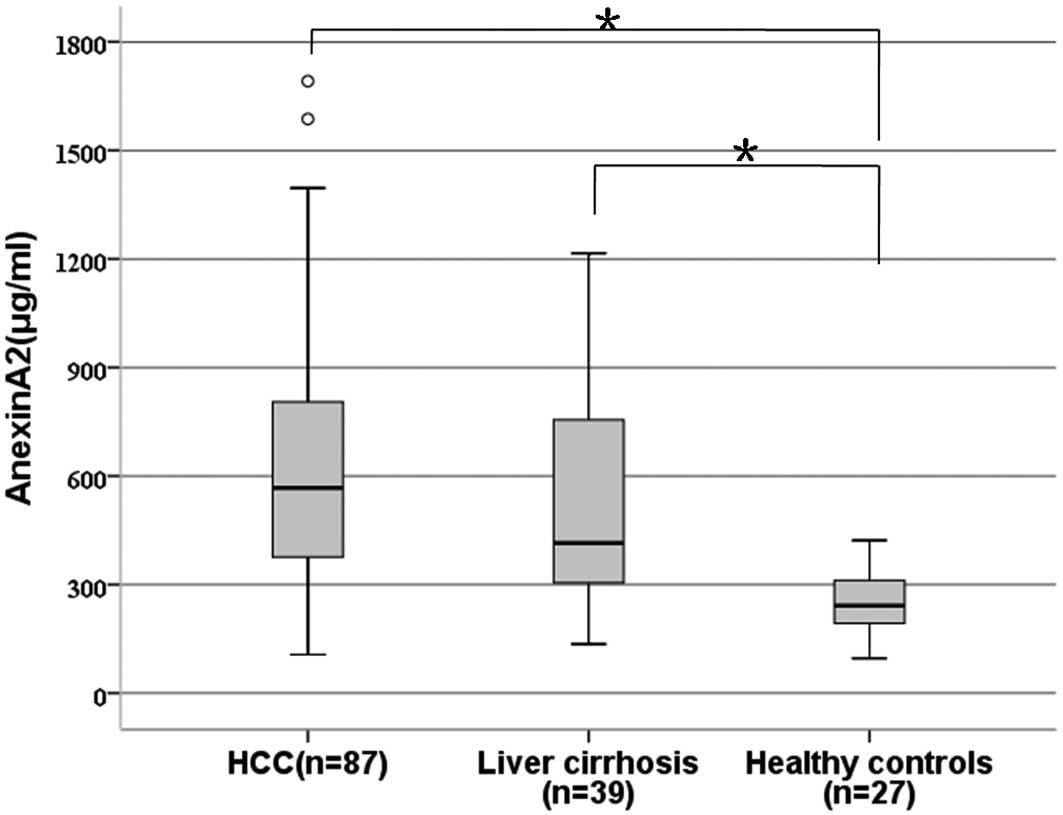
The serum levels of ANXA2 were significantly
increased in the patients with HCC (median, 567.2 vs. 241.9
μg/ml; P=0.003) and cirrhosis (median, 414.8 μg/ml
vs. 241.9 μg/ml, P=0.011) compared with the healthy controls
(Fig. 1). However, there was no
significant difference in the serum ANXA2 levels between the
patients with HCC and those with cirrhosis (P=0.342).
There was no statistical association between serum
ANXA2 and age, gender, tumor differentiation or tumor size (data
not shown).
Tissue ANXA2 for the diagnosis of
HCC
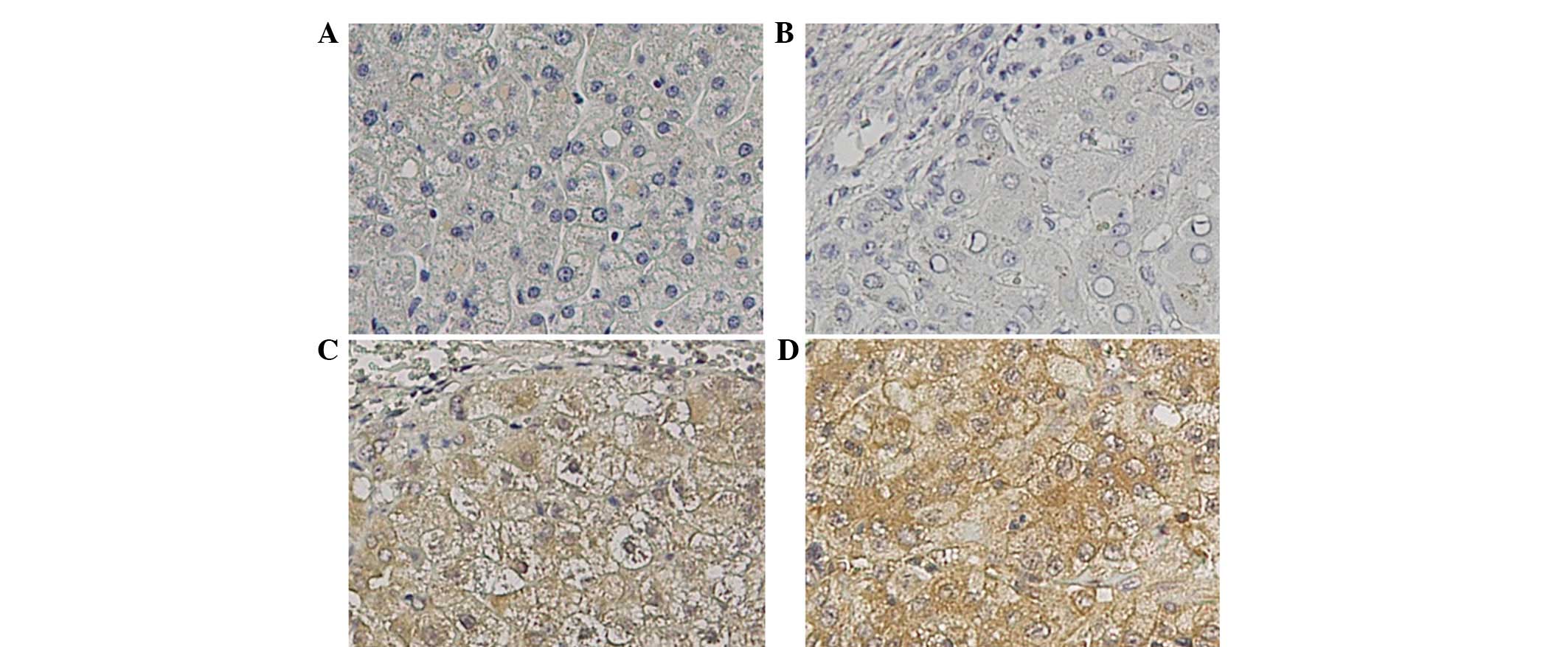
ANXA2 protein in the tissue was expressed either at
the cell membrane or in the cytoplasm of cirrhotic and tumor cells
(Fig. 2). Healthy controls were not
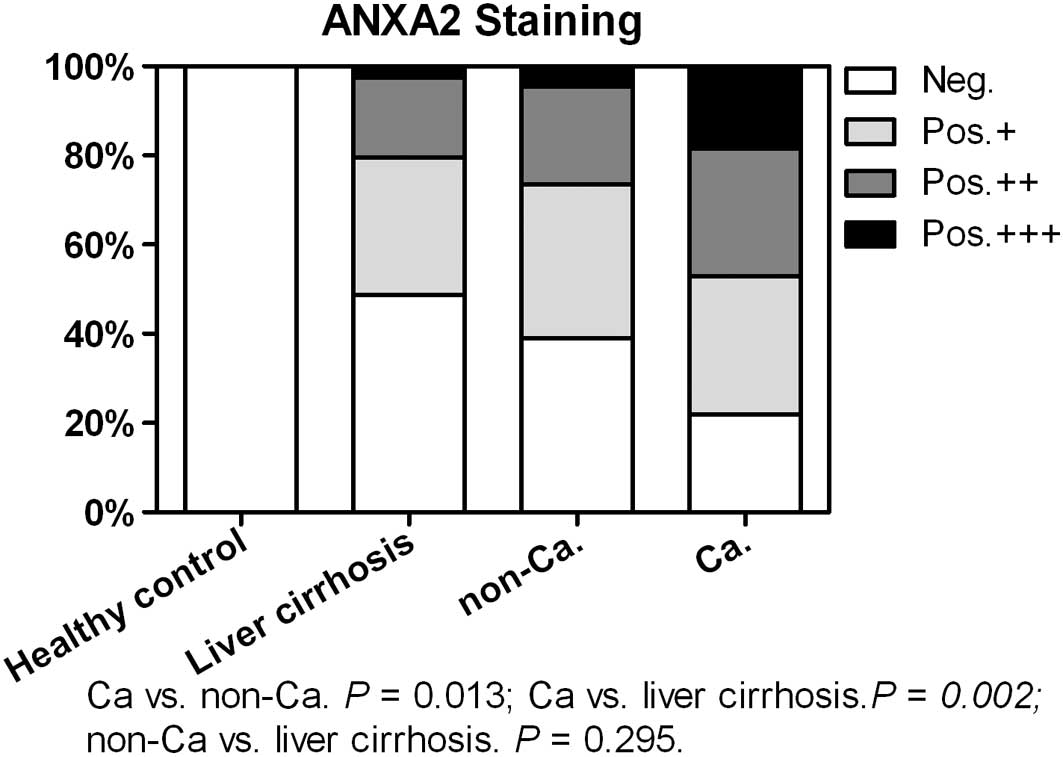
immunostained while the number of cases immunoreactive for ANXA2
steadily increased from the liver cirrhosis tissues (20/39, 51.3%)
to the non-cancer (53/87, 60.9%) and cancer (68/87, 78.2%; Fig. 3) tissues. The cancer tissues
exhibited a significantly higher ANXA2-positive rate than the
non-cancer (P=0.013) and liver cirrhosis tissues (P=0.002).
Furthermore, the cancer tissues (16/87, 18.4%) showed a higher
marked ANXA2 staining rate (+++) compared with the non-cancer
(4/87, 4.6%, P=0.004) and cirrhosis (1/39, 2.6%, P=0.016) tissues.
There was no significant difference between the non-cancer and
liver cirrhosis tissues (P=0.295).
Considering the healthy controls and the cirrhosis
and non-cancer tissues as non-malignant, the sensitivity,
specificity and accuracy of ANXA2 for HCC detection were 78.2, 52.3
and 61.7%, respectively. Considering only the cirrhosis and
non-cancer tissues as non-malignant, the sensitivity, specificity
and diagnostic accuracy of ANXA2 were 78.2, 42.1 and 56.8%,
respectively.
There was no statistical association between the
positive expression of tissue ANXA2 and age, gender, tumor
differentiation, tumor size or HCC recurrence.
Association between serum and tissue
ANXA2 levels
A significant correlation was identified between the
serum and cancer tissue ANXA2 levels (r=0.364, P=0.017), but not
between the serum and the non-cancer tissues (r=0.243, P=0.160) in
the HCC patients. A significant correlation was also observed
between the serum and tissue ANXA2 levels in the cirrhotic patients
(r=0.312, P=0.023).
Tissue/serum ANXA2 and prognosis in HCC
patients
The serum ANXA2 levels did not differ significantly
between the patients with HCC recurrence and those without HCC
recurrence following liver transplantation (P>0.05). Patient
survival did not differ significantly between the patients with
high serum ANXA2 expression and those with low expression
(P>0.05). Additionally, no significant differences were observed
in patient survival or tumor-free survival between the positive
expression of tissue ANXA2 and the negative expression of tissue
ANXA2 (P>0.05).
Discussion
In the present study, a typical multistage
hepatocarcinogenesis model (healthy, cirrhosis and HCC) was used to
evaluate the roles of serum and tissue ANXA2 in the diagnosis of
HCC. In accordance with the previous study (15), the results demonstrated that the
analysis of serum ANXA2 was able to discriminate between the HCC
and healthy patients. However, the serum ANXA2 levels were also
clearly elevated in the patients with cirrhosis compared with the
healthy controls. Furthermore, no significant differences were
observed in the serum ANXA2 levels between the patients with HCC
and those with cirrhosis, indicating that ANXA2 was not a good
serological diagnostic marker for HCC, particularly in patients
with a history of cirrhosis. ANXA2 may serve as a biomarker for
liver cirrhosis.
Ultrasound-guided fine-needle aspiration biopsy is a
much safer and less traumatic procedure than open surgery in the
diagnosis of cancer. The acquired histopathological results may
guide the therapeutic strategy and predict a patient prognosis
(21). In the present study, the
diagnostic and prognostic role of tissue ANXA2 in HCC was assessed.
The results revealed that the positive expression rate of ANXA2 was
significantly higher in the cancer tissues compared with the
cirrhotic and normal liver tissues, suggesting that the analysis of
tissue ANXA2 was more likely to distinguish HCC from non-malignant
cirrhotic tissues than the analysis of serum ANXA2. Although tissue
ANXA2 had a high sensitivity, the specificity was extremely low,
contributing to a low diagnostic accuracy. Therefore, neither serum
nor tissue ANXA2 would be a good biomarker for HCC patients with a
history of cirrhosis. Furthermore, it was observed that the tissue
ANXA2 levels were not associated with tumor-recurrence and
mortality following liver transplantation, indicating that the
early detection of ANXA2 in cancer tissues by biopsy does not aid
in the prediction of a patient prognosis.
In addition, there were no significant associations
between the expression levels of serum or tissue ANXA2 and the
tumor characteristics, including size, number and differentiation.
A marked correlation was observed between the serum and tissue
ANXA2 levels in the patients with HCC and cirrhosis. The present
results provided further evidence that ANXA2 may not be a unique
product of tumors but that it is also produced by cirrhotic tissue.
Several previous studies have demonstrated that ANXA2 is
differentially expressed between normal tissues and tissues of
liver fibrosis induced by various causes (e.g. alcohol, the immune
system or HBV) (16,22–24).
Certain researchers have even considered ANXA2 to be an early
effector molecule during the progression of fibrosis (22). Therefore, ANXA2 may not be produced
and released by cancer only and it is therefore not a valid
biomarker.
In conclusion, the expression of serum and tissue
ANXA2 was not only elevated in the patients with HBV-related HCC
but also in the patients with liver cirrhosis. ANXA2 expression is
not a good serological or immunological diagnostic marker for
differentiating HCC from cirrhosis. In the HCC patients, ANXA2 in
the serum or cancer tissues was not associated with prognosis
following liver transplantation.
Acknowledgements
The present study was supported by the
Chinese High Tech Research & Development (863) Program
(2012AA020204) and the Natural Science Foundation of Zhejiang
Province (Q12H030010).
References
|
1.
|
Gomaa AI, Khan SA, Toledano MB, Waked I
and Taylor-Robinson SD: Hepatocellular carcinoma: epidemiology,
risk factors and pathogenesis. World J Gastroenterol. 14:4300–4308.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Zhou L, Liu J and Luo F: Serum tumor
markers for detection of hepatocellular carcinoma. World J
Gastroenterol. 12:1175–1181. 2006.PubMed/NCBI
|
|
3.
|
Inagaki Y, Xu HL, Hasegawa K, et al:
Des-gamma-carboxyprothrombin in patients with hepatocellular
carcinoma and liver cirrhosis. J Dig Dis. 12:481–488. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Liu J and Fan D: Hepatitis B in China.
Lancet. 369:1582–1583. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Fattovich G, Stroffolini T, Zagni I and
Donato F: Hepatocellular carcinoma in cirrhosis: incidence and risk
factors. Gastroenterology. 127(5 Suppl 1): S35–S50. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Fritz K, Fritz G, Windschiegl B, Steinem C
and Nickel B: Arrangement of Annexin A2 tetramer and its impact on
the structure and diffusivity of supported lipid bilayers. Soft
Matter. 6:4084–4094. 2010. View Article : Google Scholar
|
|
7.
|
Mai J, Waisman DM and Sloane BF: Cell
surface complex of cathepsin B/annexin II tetramer in malignant
progression. Biochim Biophys Acta. 1477:215–230. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Chiang Y, Rizzino A, Sibenaller ZA, Wold
MS and Vishwanatha JK: Specific down-regulation of annexin II
expression in human cells interferes with cell proliferation. Mol
Cell Biochem. 199:139–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Jacovina AT, Deora AB, Ling Q, et al:
Homocysteine inhibits neoangiogenesis in mice through blockade of
annexin A2-dependent fibrinolysis. J Clin Invest. 119:3384–3394.
2009.PubMed/NCBI
|
|
10.
|
Hajjar KA and Acharya SS: Annexin II and
regulation of cell surface fibrinolysis. Ann NY Acad Sci.
902:265–271. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Takahashi S, Reddy SV, Chirgwin JM, et al:
Cloning and identification of annexin II as an autocrine/paracrine
factor that increases osteoclast formation and bone resorption. J
Biol Chem. 269:28696–28701. 1994.PubMed/NCBI
|
|
12.
|
Huang Y, Jin Y, Yan CH, et al: Involvement
of Annexin A2 in p53 induced apoptosis in lung cancer. Mol Cell
Biochem. 309:117–123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Bao H, Jiang M, Zhu M, Sheng F, Ruan J and
Ruan C: Overexpression of Annexin II affects the proliferation,
apoptosis, invasion and production of proangiogenic factors in
multiple myeloma. Int J Hematol. 90:177–185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Sarkar S, Swiercz R, Kantara C, Hajjar KA
and Singh P: Annexin A2 mediates up-regulation of NF-κB, β-catenin,
and stem cell in response to progastrin in mice and HEK-293 cells.
Gastroenterology. 140:583–595. e583–e595. 2011
|
|
15.
|
Ji NY, Park MY, Kang YH, et al: Evaluation
of annexin II as a potential serum marker for hepatocellular
carcinoma using a developed sandwich ELISA method. Int J Mol Med.
24:765–771. 2009.PubMed/NCBI
|
|
16.
|
Zhang L, Peng X, Zhang Z, et al:
Subcellular proteome analysis unraveled annexin A2 related to
immune liver fibrosis. J Cell Biochem. 110:219–228. 2010.PubMed/NCBI
|
|
17.
|
Lu AW, Zheng SS, Wu MP, Shen Y and Yang
RW: Reevaluation of the effect of lamivudine therapy preoperative
to prevent HBV recurrence after liver transplantation.
Hepatobiliary Pancreat Dis Int. 7:357–361. 2008.PubMed/NCBI
|
|
18.
|
Zheng SS, Xu X, Wu J, et al: Liver
transplantation for hepatocellular carcinoma: Hangzhou experiences.
Transplantation. 85:1726–1732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Sun YL, Yin SY, Xie HY, et al: Stem-like
cells in hepatitis B virus-associated cirrhotic livers and adjacent
tissue to hepatocellular carcinomas possess the capacity of
tumorigenicity. J Gastroenterol Hepatol. 23:1280–1286. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German).
|
|
21.
|
Ozkan G, Tutar M, Bayram M, et al: The
impact of ultra-sonography-guided fine needle aspiration of no
palpable supraclavicular lymph nodes on diagnosis and staging in
advanced lung cancer. Tuberk Toraks. 57:186–191. 2009.PubMed/NCBI
|
|
22.
|
Zhang L, Jia X, Feng Y, et al: Plasma
membrane proteome analysis of the early effect of alcohol on liver:
implications for alcoholic liver disease. Acta Biochim Biophys Sin
(Shanghai). 43:19–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Seth D, Hogg PJ, Gorrell MD, McCaughan GW
and Haber PS: Direct effects of alcohol on hepatic fibrinolytic
balance: implications for alcoholic liver disease. J Hepatol.
48:614–627. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Jia XF, Peng X, Feng YL, Yang H, Yuan ZH
and Zhang LJ: Subcellular proteome analysis of immune or alcohol
induced rat liver fibrosis. Zhonghua Gan Zang Bing Za Zhi.
18:826–830. 2010.(In Chinese).
|