Introduction
Chaenomeles speciosa Nakai (C.
speciosa Nakai) has been used in traditional Chinese medicine
for thousands of years to treat a variety of diseases, including
sunstroke, edema and arthralgia. During the past decades, C.
speciosa Nakai has been employed to treat diarrhea (1) and hepatitis (2). More recently, C. speciosa Nakai
has also been used to treat arthritis (3–5).
Studies have revealed that C. speciosa Nakai has antioxidant
and immunomodulatory properties (6,7).
In addition, Chinese herbalists have used C.
speciosa Nakai to treat cancer, particularly hepatocellular
carcinoma. Curative treatments, including surgery, local
destruction techniques and liver transplantation, for
hepatocellular carcinoma (HCC) are only achievable in certain
patients. The majority of patients must undergo chemotherapy.
Although chemotherapy has been shown to improve the survival of
patients with HCC, there are several serious side-effects and drug
resistance may occur. These two obstacles to more successful
therapeutic outcomes have been major challenges for oncologists.
The challenge of identifying new therapeutic approaches that
alleviate the adverse effects of chemotherapy, while also improving
their efficiency, is clear and urgent. Consequently, the present
study investigated whether C. speciosa Nakai extracts may be
used for the treatment of HCC. The present study investigated the
effects of the ethanol extract of C. speciosa Nakai (EEC) in
inhibiting tumor growth in vivo and in vitro.
Materials and methods
Materials
RPMI-1640 medium and fetal bovine serum were
obtained from Invitrogen (Carlsbad, CA, USA). Unless stated
otherwise, all other chemicals were obtained from Sigma-Aldrich
(Zwijndrecht, the Netherlands).
Preparation of EEC
A total of 2 kg field-raised, air-dried C.
speciosa Nakai was ground to a fine powder and added to 4,000
ml ethanol [final ethanol concentration 60% (w/v)] for extraction
for 1 h at 75°C. The extract was incubated in a rotary evaporator
until all of the ethanol had evaporated. The extract was then
solubilized in saline and used for the present experiments.
Overall, 1 ml solution contained extract that was equal to 0.2 g
raw C. speciosa Nakai.
In vivo tumor model
Kunming mice were obtained from the Hubei Laboratory
Animal Center (Wuhan, China). The mice had free access to pellet
food and water. The animals were acclimated to laboratory
conditions at a temperature of 18–25°C, with a relative humidity of
55 to 65% and a 12-h light/dark cycle, for one week prior to the
experiments. All animal experiments were approved by the
Institutional Animal Control and Utilization Committee of the
Central Hospital of Handan City.
The murine H22 cell line was obtained
from the School of Basic Medical Science, Peking University
(Beijing, China). Cells were cultured in Dulbecco’s modified
Eagle’s medium (Gibco Laboratories, Grand Island, NY, USA)
supplemented with 10% fetal bovine serum, penicillin and
streptomycin (10 U/l, Gibco Laboratories) in a humidified
atmosphere with 5% CO2 at 37°C. The hepatoma model was
established by the subcutaneous inoculation of H22 cells
(1×106 cells per mouse) into the right flank of the mice
(8). At 48 h post-inoculation, the
mice were randomly divided into three groups: i) the
vehicle-treated control group, which received 0.5 ml saline by
gavage; ii) the EEC group, which received 0.5 ml EEC; and iii) the
cisplatin group, which received 5 g/kg cisplatin intraperitoneally.
Another eight mice, which were not inoculated with H22
cells, served as normal controls.
Red blood cells (RBCs; 5% 0.2 ml per mouse) were
injected into the peritoneal cavity on day 14. All the mice were
sacrificed at day 18 and the tumors were excised and weighed. The
tumor inhibition rate was calculated according to the formula:
inhibition rate (%) = (1 − tumor weight in test group / tumor
weight in control) × 100.
Hemolysis assay
Sheep RBCs (20%) were prepared with natural saline.
The RBCs (0.2 ml 20%) were injected intraperitoneally into the
mice. After four days, the specific IgM of the serum was detected
as previously described (9).
Briefly, 100 μl 50X diluted mouse serum, 50 μl 10X
diluted complement and 50 μl 5% sheep RBCs were added to the
ELISA plate and incubated for 30 min at 37°C. Following
centrifugation, 150 μl supernatant was transferred to a
96-well plate to detect the absorbance at OD415. The 50%
hemolysin value (HC50) was calculated as follows:
HC50 = sample dilution × ODsample /
(ODRBC / 2). ODRBC was the OD value of the
complete lysis of the RBCs.
Flow cytometry
Splenocytes were prepared and cultured in 24-well
plates for 48 h, then the cells were washed and fixed in 70%
alcohol overnight at 4°C. The cells were washed with PBS and 500
μl RNase (30 μl/ug) was added into Eppendorf tubes
for 30 min. Finally, propidium iodide dye was added in a dark room
(10,11) and the cell cycle was analyzed using
flow cytometry (FCM). The lymphocyte proliferation rate (%) was
calculated as follows: proliferation rate (%) = [(S +
G2M) / (G0/1 + S + G2M)] ×
100.
Reverse transcription-polymerase chain
reaction (RT-PCR)
The H22 tumor tissues were collected and
the total RNA was isolated using the RNase mini kit (Invitrogen).
First-strand cDNA was synthesized from the total RNA with Super
Script II reverse transcriptase (Invitrogen) using oligodT as the
reverse primer.
The cDNA was amplified by PCR in a programmable DNA
thermal cycler for 30 cycles (95°C for 3 min, 95°C for 30 sec, 55°C
for 30 sec and 72°C for 30 sec). The primer sequences used for the
PCR are shown in Table I.
 | Table I.Oligonucleotide primers. |
Table I.
Oligonucleotide primers.
| Gene | Primer sequences
(5′-3′) |
|---|
| PD-L1 | AGG CAA GCT TAT GTG
GGT CCG GCA GGT AC |
| AGG CGA ATT CTC AAA
GAG GCC AAG AAC AAT |
| Foxp3 | CCC TTT CAC CTA TGC
CAC CCT |
| GCT CCC TTC TCG CTC
TCCAC |
| TGF-β | ACG GCA TGG ATC TCA
AAG AC |
| GTG GGT GAG GAG CAC
GTA GT |
| β-actin | TCA CCC ACA CTG TGC
CCC ATC TAC GA |
| CAG CGG AAC CGC TCA
TTG CCA ATG G |
Proliferation assay
The percentage of growth inhibition was determined
with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay (12).
Hepatocellular carcinoma H22 cells (4,000 cells/well)
were seeded into 96-well plates and treated with EEC. The cells
were incubated overnight at 37°C with 5% CO2.
Subsequently, 100 μl MTT, at a concentration of 2.5 mg/ml,
was added to each well and the cells were incubated for an
additional 4 h. The supernatant was then aspirated and dimethyl
sulfoxide was added to the wells to dissolve the precipitate. The
absorbance was determined at 570 nm using a micro-plate reader
(Multiskan Spectrum; Thermo Electron Corporation, Vantaa, Finland).
The cell survival rate (%) = 100 × ODEEC group /
ODcontrol group.
DNA fragmentation assay
The H22 cells were cultured in the
presence or absence of EEC for 48 h, then gently scraped and
harvested by centrifugation. The cells were incubated for 20 min in
DNA fragmentation lysis buffer [50 mmol/l Tris-HCl (pH 8.0), 20
mmol/l ethylenediaminetetraacetic acid and 0.5% Triton X-100] on
ice. The cells were then centrifuged at 12,000 × g for 30 min.
DNA was extracted with a mixture of phenol and
chloroform (1:1) and precipitated with twice the volume of cold
ethanol and sodium acetate. The precipitates were resuspended in 10
μl 10 mM Tris (pH 7.8) and 1 mM EDTA buffer and incubated
for 30 min at 37°C with 1 μg/ml RNase (Roche Molecular
Biochemicals, Indianapolis, IN, USA) to remove the RNA. The DNA
pellets were electrophoresed for 90 min at 100 V on 2% agarose
gels. The gel was stained with ethidium bromide and the DNA
fragments were visualized under ultraviolet light.
Measurement of mitochondrial membrane
potential (Δψm)
The H22 cells (2×104
cells/well) were seeded into a 96-well plate and incubated
overnight at 37°C, with 5% CO2. Subsequently, the cells
were treated with various concentrations of EEC and incubated for
an additional 10 h (13). To
measure Δψm, the cells were incubated with DiOC6 (100 nM; Molecular
Probes, Eugene, OR, USA) during the last 30 min of treatment. DiOC6
is a fluorescent dye that is incorporated into the mitochondria in
a Δψm-dependent manner. Once the cells had been sufficiently
washed, the fluorescence intensity was determined using a
multifunctional micro-plate reader (14).
Statistical analysis
All values are expressed as the mean results of the
triplicate experiments ± standard deviation. The unpaired Student’s
t-test was used to identify differences between the groups.
P<0.05 was considered to indicate a statistically significant
difference. SPSS 10.0 (SPSS, Inc., Chicago, IL, USA) was used for
the statistical analyses.
Results
EEC has marked antiproliferative and
apoptotic effects in cancer cells
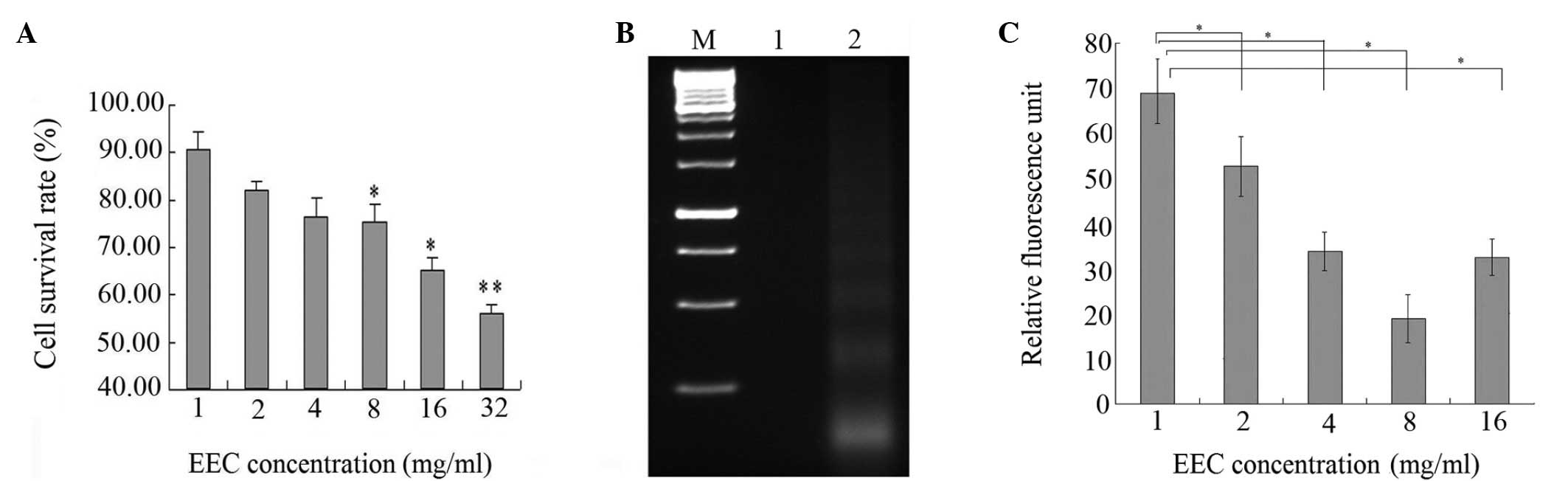
The effect of EEC on the growth of cancer cells is
expressed as the percentage of cell viability relative to the
control. As shown in Fig. 1A, EEC
inhibited the proliferation of the H22 cells in a
dose-dependent manner. Compared with the saline-treated controls,
EEC at a dose of 32 mg/ml caused 57.5% inhibition of H22
cell proliferation (P<0.001). The cells treated with EEC showed
typical apoptotic morphologies, including cell shrinkage and
rounding and cell membrane blebbing, as well as nuclear
fragmentation and condensation (data not shown). Apoptosis was
characterized by the activation of endogenous endonucleases with
the subsequent cleavage of chromatin DNA into internucleosomal
fragments of 180 to 200 bp. The extracted cell DNA was detected by
agarose gel electrophoresis. Fragmented DNA was clearly observable
in the H22 cells following treatment with EEC for 48 h,
whereas the cells did not produce ladders without treatment. Thus,
the apoptotic effect of EEC on the tumor cells was further
demonstrated by DNA fragmentation (Fig.
1B).
Mitochondrial damage is significant in cell
apoptosis. Therefore, to determine whether Δψm is involved in the
regulation of apoptosis induced by EEC, the fluorescent lipophilic
cation DiOC6 was used as an indicator of the energy state of the
mitochondria. As shown in Fig. 1C,
EEC treatment led to a rapid drop in mitochondrial energy, as
demonstrated by a decrease in fluorescence from the baseline
following 12 h of treatment (M1, 47.55% in the control cells vs.
77.43% in the EEC-treated H22 cells; the Δψm peak
shifted to the left, indicating that fewer cells retained DiOC6 in
their mitochondria). These results suggested that the inhibitory
effect of EEC on tumor cell growth may be through the induction of
apoptosis.
EEC inhibits tumor growth in vivo
The antitumor activity of EEC was investigated in
the H22 murine hepatoma model. The results showed that
EEC and cisplatin significantly inhibited tumor growth compared
with the vehicle-treated group, with inhibitory rates of 39.82±4.98
and 58.33±9.29%, respectively (P<0.05 compared with
vehicle-treated group; Table II).
These data indicate that EEC has the ability to inhibit the growth
of H22 cells in vivo.
 | Table II.Inhibitory effect of EEC on
H22 murine hepatoma cell growth in mice. |
Table II.
Inhibitory effect of EEC on
H22 murine hepatoma cell growth in mice.
| Group | Body weight
(g) | Tumor weight
(g) | Net body weight (g)
(body weight − tumor weight) | Tumor inhibition
rate (%) |
|---|
|
Vehicle-treated | 32.91±5.11 | 1.73±0.32 | 31.18±4.79 | - |
| EEC | 29.00±4.63 | 1.04±0.13 | 27.96±4.52a | 39.82±4.98 |
| Cisplatin | 19.28±2.41 | 0.72±0.12 | 18.56±2.29a | 58.33±9.29 |
The gross toxicity of EEC and cisplatin was then
compared using the body weights of the mice. The body weights were
19–21 g at baseline and were similar among the three groups.
Subsequent to 18 days of treatment the differences in net body
weight per mouse (body weight - tumor weight) were statistically
significant among the three groups (Table II). The net body weight of the EEC
group was significantly higher than that of the cisplatin group,
indicating that EEC has less severe adverse reactions compared with
cisplatin.
EEC enhances immunity in mice
To further investigate the mechanism by which EEC
inhibits tumor growth, lymphocyte proliferative activity was
evaluated using a flow cytometry assay. As shown in Table III, the cell cycle analysis showed
that EEC enhanced lymphocyte proliferation. Moreover, the hemolysis
assay showed that EEC significantly increased the production of RBC
antibody (HC50; Table
III). Compared with the vehicle-treated group, cisplatin
significantly decreased the production of RBC antibody. These data
indicate that EEC inhibits tumor growth partially via enhancing
host immunity.
 | Table III.Antibody production and inhibition of
lymphocyte proliferation. |
Table III.
Antibody production and inhibition of
lymphocyte proliferation.
| Group | Proliferation rate
(%) | RBC antibody
(HC50) |
|---|
| Normal | 63.75±8.93 | 179.80±22.47 |
|
Vehicle-treated | 49.18±19.23 | 110.34±13.79 |
| EEC | 55.20±16.82a |
188.44±23.56b |
| Cisplatin | 39.78±19.79 | 54.32±6.79 |
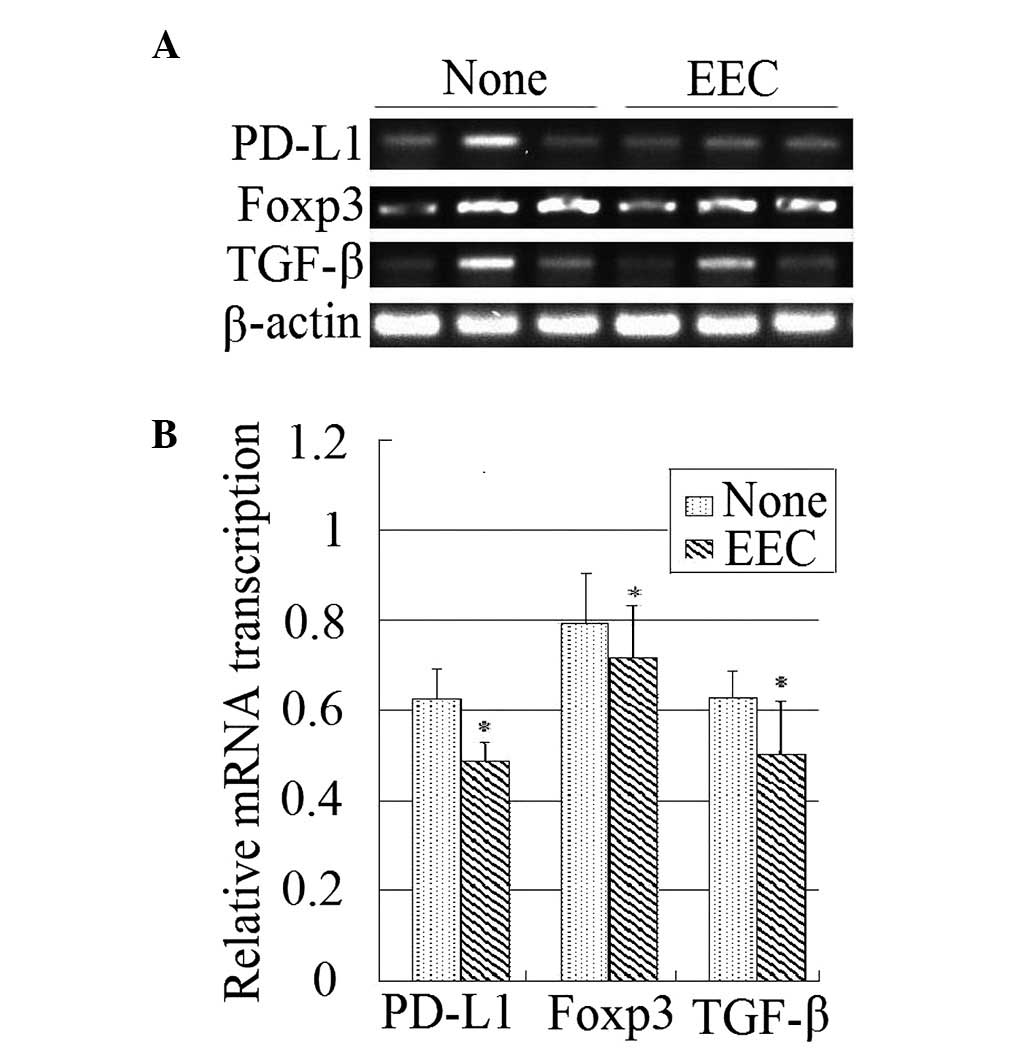
EEC suppresses the expression of PD-L1,
Foxp3 and TGF-β
PD-L1 is a ligand of programmed death 1 (PD-1),
which is expressed on activated lymphocytes and negatively
regulates the immune response. Foxp3 is specifically expressed on
regulatory T (Treg) cells and suppresses effective lymphocyte
activity. TGF-β is a cytokine that activates the Treg cells. All of
these factors are negative immunomodulatory factors. The present
study observed that EEC decreases the expression levels of these
three genes in the tumor tissues, as indicated by RT-PCR (Fig. 2).
Discussion
The present study demonstrated that EEC inhibits the
growth of H22 cells in vivo and causes the
apoptosis of cells in vitro. Furthermore, tumors isolated
from the inoculated mice expressed more PD-L1, Foxp3 and TGF-β in
the vehicle-treated group compared with the EEC group, suggesting
that EEC is able to inhibit the expression of negative
immnoregulatory genes.
Despite advances in the understanding of the
carcinogenic processes of cancer, the relatively low remission rate
of chemotherapy, radiotherapy and immunotherapy have encouraged the
scientific community to identify active medicinal compounds from
herbal/natural sources. In the present study, it was demonstrated
that EEC was able to suppress cancer cell growth through the
induction of apoptosis, the inhibition of cell proliferation and
the promotion of host immune function. This is the first study that
clearly characterizes the antitumor properties of EEC in cancer
cells and tumor xenografts.
It is not clear which compounds are responsible for
the anti-tumor effects of EEC. Triterpenoids, flavonoids and
organic acids are the main classes of compounds identified from
C. speciosa Nakai (15–20).
The major triterpenoids include 3-O-acetylursolic acid,
3-O-acetylpomolic acid, oleanolic acid and betulinic acid.
Betulinic acid was initially characterized as a highly selective
inhibitor of human melanoma cells and tumor growth through the
induction of apoptosis (21).
Subsequent research has shown that betulinic acid is an effective
inhibitor of cell proliferation and that it induces apoptosis in
numerous types of cancer cells (22). Oleanolic acid has also been shown to
induce apoptosis in leukemia cells (23–26).
Therefore, the antitumor effects of EEC may involve contributions
by oleanolic acid and betulinic acid.
In order to further clarify the mechanism involved
in the EEC-induced growth suppression of tumor xenografts, the
immune function of tumor-bearing mice was investigate in the
present study. EEC was observed to promote the production of
immunoglobulin. Further investigation demonstrated that EEC was
able to suppress the expression of PD-L1, Foxp3 and TGF-β. The
transcription factor Foxp3 has a major role in the development of
Treg cells and is critical for their suppressive function (27). Naive CD4+ T cells
selected by Foxp3 during development in the thymus have the
potential to be converted into functional Foxp3+ Treg
[adaptive or induced Treg (iTreg)] cells in peripheral lymphoid
organs or tissue culture. Thus, the decreased expression of Foxp3
in the EEC-treated tumors may indicate that there are fewer Treg
cells in EEC-treated tumors compared with vehicle-treated tumors.
In accordance with this finding, the expression of TGF-β, a factor
that is critical in the induction of Foxp3 expression in
vitro and in vivo, was also suppressed. Furthermore, the
expression of PD-L1, the ligand of PD-1, was also suppressed by
EEC. PD-1 is an inhibitory receptor that is expressed on activated
lymphocytes and that regulates tolerance and autoimmunity. These
findings suggest that the enhancement of immunity in EEC-treated
tumor-bearing mice may be associated with the suppression of immune
tolerance.
In addition, the promising antitumor effect of EEC
was achieved without the toxicity and side-effects anticipated with
cisplatin (e.g. a significant drop in body weight and
immunosuppression), which further suggests that EEC has the
potential to be established as novel adjuvant agent in cancer
chemotherapy. At present, the fractionation of EEC is being
performed to identify the fractions that contain the bioactive
constituents responsible for the growth-inhibitory effects.
In summary, the present study demonstrated that EEC
was able to inhibit cancer cell growth in vitro and in
vivo. In contrast to orthodox chemotherapy using cytotoxic
drugs, the use of this herbal extract imposes less toxicity while
retaining antitumor effects. This indicated the possibility of
further developing EEC as an adjuvant chemotherapeutic agent in
cancer therapy.
References
|
1.
|
Han B, Peng H, Yao Q, Zhou Y, Cheng M and
Wang D: Analysis of genetic relationships in germplasms of Mugua in
China revealed by internal transcribed spacer and its taxonomic
significance. Z Naturforsch C. 65:495–500. 2010.PubMed/NCBI
|
|
2.
|
Liu S, Bai Z and Li J: Comprehensive
evaluation of multi-quality characteristic indexes of
Chaenomeles speciosa and C. sinensis fruits. Zhongguo
Zhong Yao Za Zhi. 37:901–907. 2012.(In Chinese).
|
|
3.
|
Dai M, Wei W, Shen Y and Zheng Y: Effect
of total saponin of Haenomeles speciosa on hemorrheology of
immunity arthritis in rats. Zhongguo Zhong Yi Yao Xin Xi Za Zhi.
12:20–21. 2002.(In Chinese).
|
|
4.
|
Song YL, Zhang L, Gao JM, Du GH and Cheng
YX: Speciosaperoxide, a new triterpene acid, and other terpenoids
from Chaenomeles speciosa. J Asian Nat Prod Res. 10:217–222.
2008.PubMed/NCBI
|
|
5.
|
Dai M, Wei W, Wang N and Chen Q:
Therapeutic effect of glucosides of Chaenomeles speciosa on
adjuvant arthritis in rats. Zhongguo Yao Li Xue Tong Bao.
3:340–344. 2003.(In Chinese).
|
|
6.
|
Sawai R, Kuroda K, Shibata T, Gomyou R,
Osawa K and Shimizu K: Anti-nfluenza virus activity of
Chaenomeles sinensis. J Ethnopharmacol. 118:108–112. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Yang Y, Li X, Yang Q, Wu Z and Sun L:
Studies on chemical constituents of Chaenomeles
speciosa(Sweet) Nakai (II). Di 2 Jun Yi Da Xue Xue Bao.
10:1195–1198. 2009.(In Chinese).
|
|
8.
|
Thomas MB, Jaffe D, Choti M, et al:
Hepatocellular carcinoma: consensus recommendations of the National
Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol.
28:3994–4005. 2010. View Article : Google Scholar
|
|
9.
|
Kumarnsit E, Keawpradub N and Nuankaew W:
Acute and long-term effects of alkaloid extract of Mitragyna
speciosa on food and water intake and body weight in rats.
Fitoterapia. 77:339–345. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Shimojo RY and Iwaoka WT: A rapid
hemolysis assay for the detection of sodium channel-specific marine
toxins. Toxicology. 154:1–7. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Guo B, Romero J, Kim BJ and Lee H: High
levels of Cdc7 and Dbf4 proteins can arrest cell-cycle progression.
Eur J Cell Biol. 84:927–938. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Zhang L, Xu H and Li S: Effects of
micronization on properties of Chaenomeles sinensis (Thouin)
Koehne fruit powder. Innov Food Sci Emerg Technol. 10:633–637.
2009.
|
|
13.
|
Ros JM, Laencina J, Hellin P, Jordan MJ,
Vila R and Rumpunen K: Characterization of juice in fruits of
different Chaenomeles species. Lebenson Wiss Technol.
37:301–307. 2004. View Article : Google Scholar
|
|
14.
|
Moongkarndi P, Kosem N, Kaslungka S,
Luanratana O, Pongpan N and Neungton N: Antiproliferation,
antioxidation and induction of apoptosis by Garcinia
mangostana (mangosteen) on SKBR3 human breast cancer cell line.
J Ethnopharmacol. 90:161–166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Chen XZ, Cao ZY, Chen TS, Zhang YQ, Liu
ZZ, Su YT, Liao LM and Du J: Water extract of Hedyotis
Diffusa Willd suppresses proliferation of human HepG2 cells and
potentiates the anticancer efficacy of low-dose 5-fluorouracil by
inhibiting the CDK2-E2F1 pathway. Oncol Rep. 28:742–748. 2012.
|
|
16.
|
Hou XJ, Zhang N, Xiong SY, Li SG and Yang
BQ: Extraction of BaChu mushroom polysaccharides and preparation of
a compound beverage. Carbohydr Polym. 73:289–294. 2008. View Article : Google Scholar
|
|
17.
|
Yamahara J, Yamada T, Kitani T, Naitoh Y
and Fujimura H: Antianoxic action and active constituents of
evodiae fructus. Chem Pharm Bull (Tokyo). 37:1820–1822. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Hong YF, Sun LN and Guo XM: GC-MS analysis
of ether extracts from three species of Chaenomeles fruits.
Di 2 Jun Yi Da Xue Xue Bao. 21:749–752. 2000.(In Chinese).
|
|
19.
|
Talapatra SK, Sarkar AC and Talapatra B:
Two pentacyclic triterpenes from Rubia cordifolia.
Phytochemistry. 20:1923–1927. 1981. View Article : Google Scholar
|
|
20.
|
Liang GY, Gray AI and Waterman PG:
Pentacyclic triterpenes from the fruits of Rosa sterilis. J
Nat Prod. 52:162–166. 1989. View Article : Google Scholar
|
|
21.
|
Pisha E, Chai H, Lee IS, et al: Discovery
of betulinic acid as a selective inhibitor of human melanoma that
functions by induction of apoptosis. Nat Med. 1:1046–1051. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Zou Q, Deng W, Jiang S, Zhang L, Peng S
and Ding L: Studies on chemical constituents from fruits of
Forsythia suspense. Zhongguo Zhong Yao Za Zhi. 37:57–60.
2012.(In Chinese).
|
|
23.
|
Chintharlapalli S, Papineni S, Ramaiah SK
and Safe S: Betulinic acid inhibits prostate cancer growth through
inhibition of specificity protein transcription factors. Cancer
Res. 67:2816–2823. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
George VC, Kumar DR, Suresh PK and Kumar
RA: Apoptosis-induced cell death due to oleanolic acid in HaCaT
keratinocyte cells - a proof-of-principle approach for
chemo-preventive drug development. Asian Pac J Cancer Prev.
13:2015–2020. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Tan Y, Yu R and Pezzuto JM: Betulinic
acid-induced programmed cell death in human melanoma cells involves
mitogen-activated protein kinase activation. Clin Cancer Res.
9:2866–2875. 2003.PubMed/NCBI
|
|
26.
|
Zhang P, Li H, Chen D, Ni J, Kang Y and
Wang S: Oleanolic acid induces apoptosis in human leukemia cells
through caspase activation and poly(ADP-ribose) polymerase
cleavage. Acta Biochim Biophys Sin (Shanghai). 39:803–809. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Zheng Y and Rudensky AY: Foxp3 in control
of the regulatory T cell lineage. Nat Immunol. 8:457–462. 2007.
View Article : Google Scholar : PubMed/NCBI
|