Introduction
The accurate staging of lymph node (LN) metastases
(LNM) is critical for determining the optimal treatment strategy
for patients with non-small cell lung cancer (NSCLC). Computed
tomography (CT), or positron emission tomography (PET), is the most
commonly used non-invasive staging method of LNM. The CT imaging
criteria for tumor involvement rely on the size and shape of the
LNs. However, even when this size is <1 cm, the rate of LNM is
10% (1). Certain studies have
demonstrated that vascular endothelial growth factor (VEGF)-C is a
major factor associated with the growth of lymphatic endothelial
cells (2–3). It has also been observed that the
expression of VEGF-C in tumor tissue is significantly associated
with LNM, lymphatic vessel invasion and, furthermore, nodal
microdissemination (4–5). However, compared with examining
surgically obtained tissue specimens, serum assays may be performed
easily and frequently due to their minimal invasiveness. In the
present study, the correlation between circulating VEGF-C levels
and pathologically proven LNM was analyzed and an evaluation was
made as to whether circulating VEGF-C was able to provide
additional information for discriminating between the absence and
presence of LNM in patients with lung cancer.
Materials and methods
Patients
Between January 2007 and October 2009, 66 patients
underwent surgery for primary tumors of the lung at Shandong Cancer
Hospital (Shandong Academy of Medical Sciences, Jinan, Shandong,
China). Peripheral venous blood samples were obtained prior to
surgery from 56 patients with primary NSCLC and 10 patients with
benign tumors of the lung. All patients underwent diagnostic
procedures prior to surgery using brain and body CT scans and bone
scintiscans. The present study was conducted according to the
institutional and ethics rules concerning research on tissue
specimens and the study was approved by the ethics committee of
Shandong Cancer Hospital, Shandong, China. Written informed consent
was obtained from all patients. A total of 56 patients with NSCLC
received curative surgery with routine systematic nodal dissection
of the hilar and mediastinal LNs. The pathological stage was
classified as stage I in 16 patients, stage II in 17 patients and
stage III in 23 patients. The histopathological types included 26
adenocarcinomas, 24 squamous cell carcinomas and 6 adenosquamous
cell and large cell carcinomas. The characteristics of the 56
patients are shown in Table I. No
patients received blood transfusions, radiotherapy or chemotherapy
prior to the study.
 | Table I.Associations between
clinicopathological findings and expression of VEGF-C in patients
with primary lung carcinoma. |
Table I.
Associations between
clinicopathological findings and expression of VEGF-C in patients
with primary lung carcinoma.
| Characteristics | No. | Serum VEGF-C levels
(pg/ml) | LN VEGF-C levels
(mRNA) | Tumor VEGF-C levels
(mRNA) |
|---|
|
|
|
|---|
| Concentration | P-value | Concentration | P-value | Concentration | P-value |
|---|
| Age (years) | | | 0.600 | | 0.452 | | 0.302 |
| <60 | 20 | 661.5±110.1 | | 59.2±15.9 | | 58.1±16.6 | |
| ≥60 | 36 | 653.2±99.9 | | 56.2±14.3 | | 52.3±17.4 | |
| Gender | | | 0.660 | | 0.345 | | 0.409 |
| Male | 46 | 653.2±102.8 | | 58.2±14.6 | | 54.8±16.2 | |
| Female | 10 | 676.5±111.5 | | 53.4±13.7 | | 50.2±11.8 | |
| Tumor histology | | | 0.793 | | 0.562 | | 0.566 |
| Adenocarcinoma | 26 | 679.4±124.1 | | 46.3±11.6 | | 47.9±12.6 | |
| Squamous cell
carcinoma | 24 | 672.8±110.6 | | 52.5±13.7 | | 53.4±13.7 | |
| Other | 6 | 645.2±139.5 | | 51.5±19.8 | | 50.9±19.8 | |
| Histological
grade | | | 0.512 | | 0.213 | | 0.205 |
|
Well-differentiated | 11 | 627.2±121.0 | | 51.1±13.8 | | 52.1±13.8 | |
|
Moderately-differentiated | 24 | 685.2±113.5 | | 53.3±14.7 | | 54.8±14.2 | |
|
Poorly-differentiated | 21 | 719.3±111.0 | | 54.9±13.4 | | 56.7±13.5 | |
| Tumor size | | | 0.334 | | 0.589 | | 0.561 |
| Diameter ≤3 cm | 13 | 652.2±84.5 | | 55.4±12.0 | | 53.7±12.8 | |
| Diameter >3
cm | 43 | 684.1±108.5 | | 57.9±15.2 | | 59.8±16.5 | |
| LNM | | | 0.026 | | 0.004 | | 0.001 |
| Positive | 38 | 697.7±96.9 | | 61.1±14.2 | | 62.3±15.3 | |
| Negative | 18 | 532.5±95.9 | | 49.5±12.1 | | 48.2±12.6 | |
| Stage | | | 0.017 | | 0.621 | | 0.632 |
| I | 16 | 623.2±109.6 | | 53.2±11.8 | | 54.2±12.8 | |
| II | 17 | 632.1±126.5 | | 55.7±12.8 | | 56.8±13.8 | |
| III | 23 | 712.2±107.4 | | 59.3±15.3 | | 58.1±16.3 | |
Measurement of VEGF-C levels in blood
samples
Blood samples were drawn pre-operatively by venous
puncture and divided into plain tubes without anticoagulant for the
serum. Within 1 h of collection, the blood samples were centrifuged
at 778 × g for 10 min within 1 h of collection and the aliquots
were frozen at −80°C for later analysis. The VEGF-C kit was
provided by Adlitteram Diagnostic Laboratories (San Diego, CA,
USA). VEGF was assayed using commercially available sandwich
enzyme-linked immunosorbent assay kits (Adlitteram Diagnostic
Laboratories) according to the manufacturer’s instructions. The
sensitivity limit of the VEGF-C assays was 0.1 ng/ml. The
coefficient of variation was <5.0%.
Measurement of VEGF-C levels in tumor and
LN samples
Fresh tissues were snap frozen in liquid nitrogen
and stored at −80°C until use. During the analyses, extracts were
made from the tissue and LN samples and the mRNA levels of the
extracts were analyzed. The extraction method involved the
following: Total RNA was extracted using the TRIzol method
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s
instructions. The purity and concentration of the total RNA was
then determined with spectrophotometry at 260 and 280 nm. Total RNA
was reverse transcribed to cDNA using a First-Strand cDNA Synthesis
kit (Takara, Otsu, Japan). To confirm RNA quality, the RT products
were checked with PCR using a pair of primers specific for GAPDH.
No significant degradation was observed in any of the RNA
samples.
The quantitative polymerase chain reaction (qPCR)
was performed with a QuantiTect SYBR Green PCR kit (Takara). A cDNA
pool serially diluted from 1:10 to 1:1,000 was used to generate
standard curves. The data were normalized to the housekeeping GAPDH
gene. The protocol of the PCR was as follows: Incubation at 95°C
for 10 min followed by 40 cycles, which included preliminary
denaturing at 95°C for 10 sec, annealing at 55°C for 10 sec and
extension at 72°C for 15 sec. All reactions were performed in
triplicate. The PCR was evaluated by melting curve analysis and the
calculations for determining the relative level of gene expression
were performed using the cycle threshold (Ct) method. The mean Ct
values from the triplicate measurements were used to calculate the
relative expression of the target genes with normalization to GAPDH
(used as an internal control) via the 2−ΔΔCt method.
Primers were synthesized by Shanghai Sangon Biological Engineering
Technology & Services Co., Ltd. (Shanghai, China) as follows:
VEGF-C forward, 5′-TCAAGGACAGAA GAGACTATAAAATTTGC-3′ and reverse,
5′-ACTCCAAAC TCCTTCCCCACAT-3′; GAPDH forward, 5′-CAACAGCCT
CAAGATCATCAGC-3′ and reverse, 5′-TTCTAGACGGCA GGTCAGGTC-3′.
Statistical analysis
Statistical analysis was performed with the SPSS
statistical software package, version 10.0 (SPSS Inc., Chicago, IL,
USA). Differences in distribution were determined using t-tests.
The associations between the levels of VEGF-C and the
clinicopathological features were evaluated using the Spearman’s
rank correlation coefficient of Fisher’s exact probability test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
The median serum VEGF-C concentration (25–75th
quartile) was 655.7±103.6 pg/ml (612.7–762.4 pg/ml) in patients
with lung carcinoma and 577.5±44.2 pg/ml (547.5–586.8 pg/ml) in
patients with benign tumors. These concentrations were
significantly different (P=0.012). The correlation between the
clinicopathological findings and the expression of VEGF-C at the
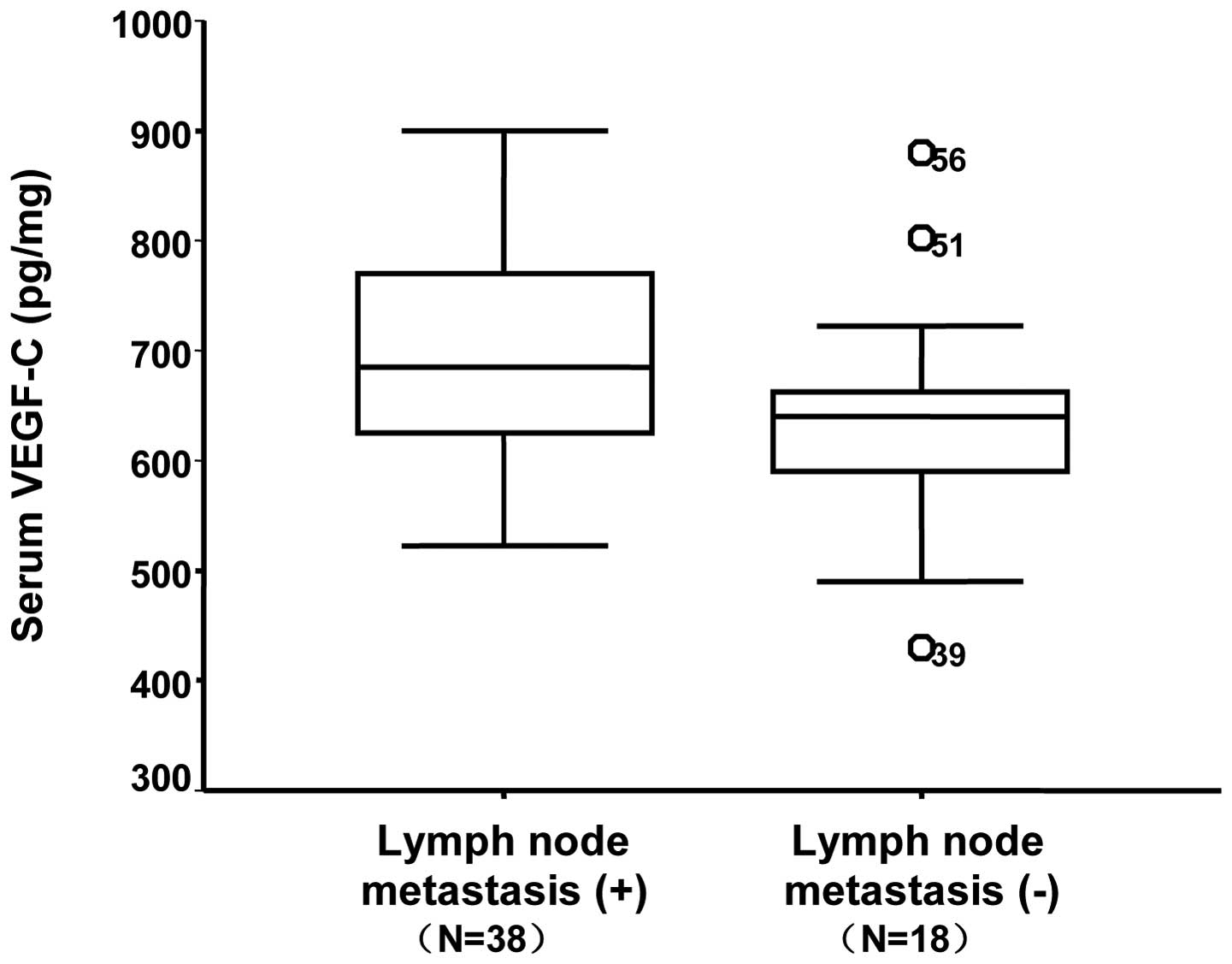
serum, tumor tissue and LN levels are shown in Table I. Of the 56 patients with NSCLC, 38
patients had metastatic LNs while 18 had no LNM. Patients with LNM
exhibited higher serum VEGF-C levels compared with those without
(697.7±96.9 pg/ml vs. 532.5±95.9 pg/ml, respectively; P=0.026).
Similarly, significant differences were detected between the
different clinical stages (stage I, 623.2±109.6 pg/ml vs. stage II,
632.1±126.5 pg/ml vs. stage III, 712.2±107.4 pg/ml, respectively;
P=0.017). Serum VEGF-C concentration exhibited a trend of gradually
increasing with histological grade, but a statistically significant
difference was not detected (P=0.512). There were no associations
between the expression of VEGF-C in serum and the patient age,
gender, histology, histological grade or tumor size
(P>0.05).
In addition, the median mRNA level of VEGF-C in the
cytoplasm of tumor cells was 59.6±12.5 in patients with lung
carcinoma and 42.8±8.5 in patients with benign tumors. These
concentrations were statistically significantly different
(P=0.001). Among the 56 patients with NSCLC, high VEGF-C mRNA
levels in the lung tumor tissue cells were associated with a
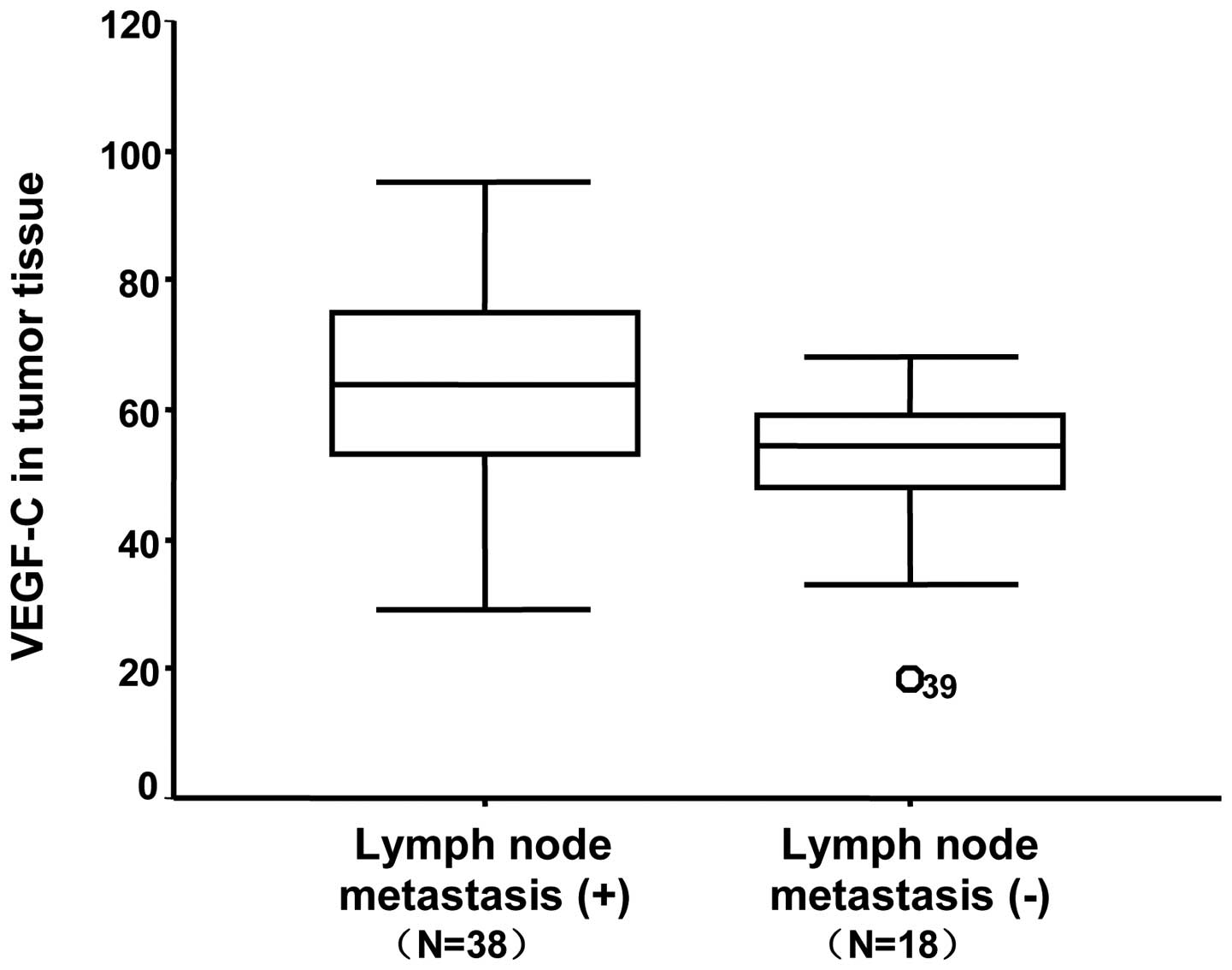
significantly higher incidence of LNM. The median mRNA levels of
VEGF-C in primary tumor tissues with and without LNM were 62.3±15.3
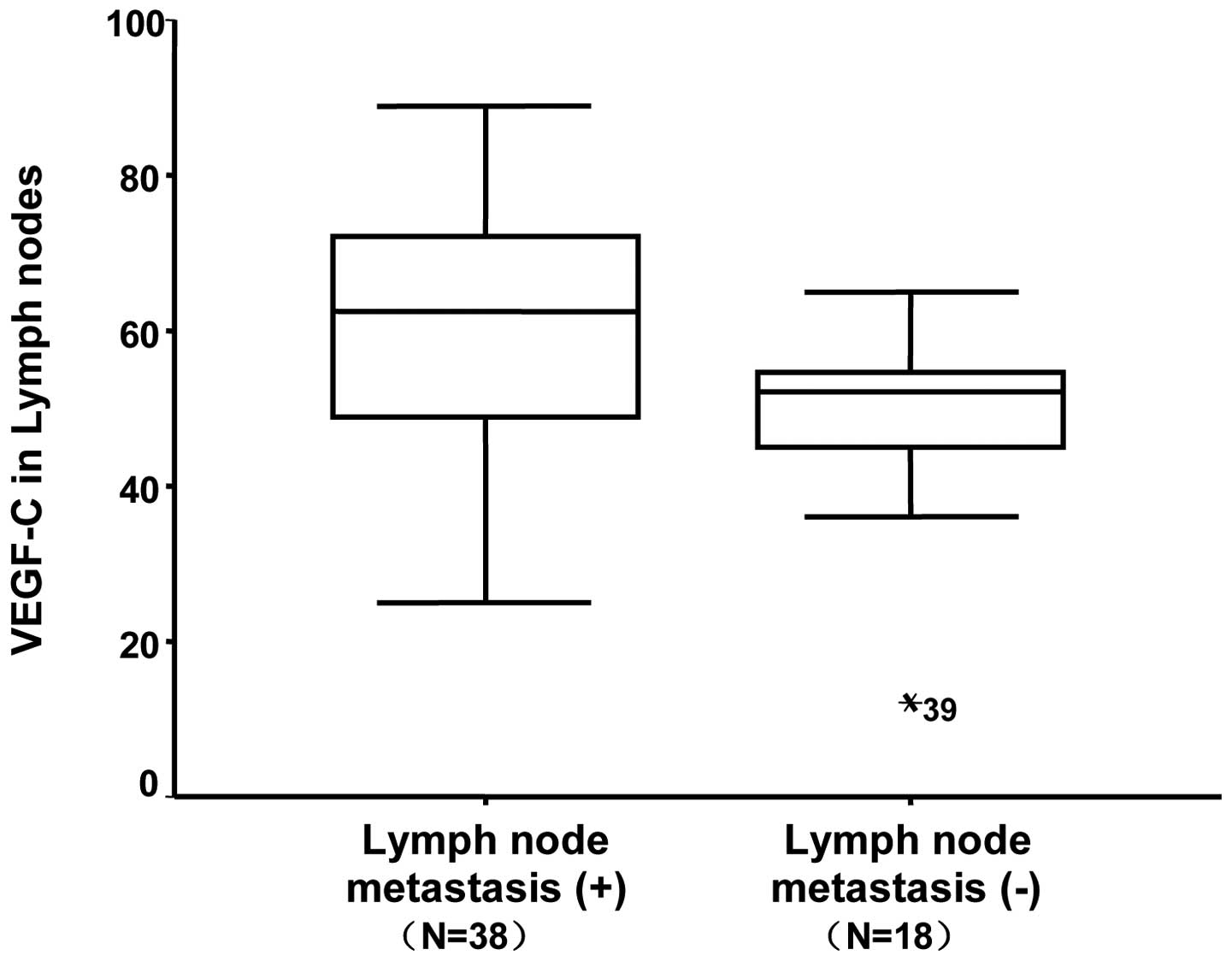
and 48.2±12.6, respectively (P=0.001). Similarly, the median VEGF-C
mRNA level in LNM was 61.1±14.2, while it was 49.5±12.1 in patients
without LNM (P=0.004). There was no association between VEGF-C mRNA
levels in tumor tissue or LNs and the patient age, gender,
histology, histological grade and tumor size (P>0.05).
By linear correlation analysis, there was a positive
correlation between the VEGF-C level of peripheral blood and that
of tumor tissue with NSCLC (r=0.629, P<0.001). An association
was observed between the VEGF-C levels in the LNs and peripheral
blood of patients with NSCLC (r=0.755, P<0.001), as well as
between the levels in the LNs and tumor tissue, which were
positively correlated (r=0.838, P<0.001). All cases were
classified into three groups according to the VEGF-C levels in the
serum, LNs and tumor tissue, and the median VEGF-C concentrations
in the LNM group were 697.7±96.9, 61.1±14.2 and 62.3±15.3 pg/ml in
the serum (Fig. 1), LNs (Fig. 2) and tumor tissue (Fig. 3), respectively. With regard to the
diagnosis of LNM using VEGF-C levels, the VEGF-C serum levels
reached a sensitivity of 65.0% and a specificity of 72.2% when a
cutoff value of 655.65 pg/ml was applied.
Discussion
The present study used VEGF-C levels in serum, tumor
tissue and LNs to determine the correlation between circulating
VEGF-C levels and LNM. In the present study, a clear and
significant correlation was observed between VEGF-C levels and LNM.
These results were in keeping with those of Masaya et al
(6). Moreover, NSCLC patients with
LNM had higher VEGF-C levels in the cytoplasm of tumor cells
compared with those without metastasis. The VEGF-C concentrations
in LNM were significantly higher compared with those without LNM.
There were positive correlations between the VEGF-C levels of
peripheral blood and tumor tissues and LNM from the NSCLC samples.
Notably, the present study revealed that although significant
differences were detected between the different clinical stages,
the serum VEGF-C levels were not significantly associated with
tumor size.
Accurate tumor staging is essential for selecting
the appropriate treatment strategy for patients with cancer in
general, but in particular for patients with lung carcinoma. The
involvement of the mediastinal LNs is a significant prognostic
factor in patients with potentially resectable NSCLC. Surgical
techniques, such as mediastinoscopy or endobronchial ultrasound
guided transbronchial needle aspiration (EBUS), are widely regarded
as the most useful methods for mediastinal staging (7). Non-invasive imaging studies, such as
CT and magnetic resonance imaging (MRI) scans, are less reliable
since the imaging criteria for tumor involvement are morphological,
relying on the size and shape of the LNs. With regard to
identifying the presence or absence of LNM, the accuracy of CT has
been reported to be between 51.4 and 83.0% (8,9).
Several studies have described 18F-fluorodeoxyglucose
(FDG)-PET as being advantageous for diagnosing the LN staging of
NSCLC. The sensitivity and specificity of PET have been reported to
be 67–89% and 82–99%, respectively (10–12). A
non-invasive, accurate and easily performed technique for LN
staging is urgently required as surgical techniques are invasive
and PET is performed only at a limited number of facilities. The
evaluation of serum VEGF-C concentrations would be useful for
hospitals where there is no access to PET scanning; furthermore, it
is a non-invasive and inexpensive examination technique. In the
present study, with regard to the diagnosis of LNM using VEGF-C
levels in serum, a cutoff value of 655.65 pg/ml was applied
The predictive value of CT in diagnosing LNM is
affected by biases due to LN size and shape. Using a combination of
VEGF-C assays and CT or PET may result in suitable positive and
negative predictive values for this diagnosis. Serum VEGF-C levels
may be used as an excellent complementary approach for obtaining a
high sensitivity and specificity. This is likely to contribute to
the selection of patients and avoid unnecessary surgery. It should
be noted that a combined diagnosis by serum VEGF-C levels and CT or
PET is relatively accurate, although there are false-positive as
well as false-negative cases. It may be dangerous to start
induction chemotherapy relying solely on VEGF-C levels. Invasive
staging such as mediastinoscopy or EBUS cannot be omitted, but we
propose that the presented combined diagnosis provides useful
information for selecting patients who require or do not require
mediastinoscopy. In the future, in order to examine the diagnostic
value of a combined assay using other markers, a more detailed
study is required.
In conclusion, the present study demonstrated that
serum VEGF-C levels provide additional and useful information for
discriminating between the absence and presence of LNM in patients
with lung carcinoma. These findings suggest that VEGF-C may be an
ideal target for diagnosis or therapy to improve the prognosis of
patients with this deadly disease. The pre-operative evaluation of
serum VEGF-C concentrations in patients with primary NSCLC is
non-invasive, easily performed and inexpensive. Making a combined
diagnosis with serum VEGF-C evaluation is a more reliable marker.
Further investigation is necessary to determine and understand the
role of VEGF-C in patients with NSCLC. In the future, to examine
the diagnostic value of serum VEGF-C levels for predicting LNM
microdissemination and to compare its diagnostic utility with that
of commonly used tools such as CT, MRI and FDG-PET scans, a more
detailed study is required.
Acknowledgements
The present study was supported by the
Technology Research and Development Program of Medicine and Drugs
in Shandong Province (Grant 2007HW137).
References
|
1.
|
Miller DL, Rowland CM, Deschamps C, et al:
Surgical treatment of non-small cell lung cancer 1 cm or less in
diameter. Ann Thorac Surg. 73:1545–1550. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Nishida N, Yano H, Komai K, et al:
Vascular endothelial growth factor C and vascular endothelial
growth factor receptor 2 are related closely to the prognosis of
patients with ovarian carcinoma. Cancer. 101:1364–1374. 2004.
View Article : Google Scholar
|
|
3.
|
Cianfarani F, Mastroeni S, Odorisio T, et
al: Expression of vascular endothelial growth factor-C in primary
cutaneous melanoma predicts sentinel lymph node positivity. J Cutan
Pathol. 39:826–834. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Wang TB, Chen ZG, Wei XQ, et al: Serum
vascular endothelial growth factor-C and lymphoangiogenesis are
associated with the lymph node metastasis and prognosis of patients
with colorectal cancer. ANZ J Surg. 81:694–699. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Acs G, Paragh G, Rakosy Z, et al: The
extent of retraction clefts correlates with lymphatic vessel
density and VEGF-C expression and predicts nodal metastasis and
poor prognosis in early-stage breast carcinoma. Mod Pathol.
25:163–177. 2012.PubMed/NCBI
|
|
6.
|
Tamura M and Ohta Y: Serum vascular
endothelial growth factor-C level in patients with primary nonsmall
cell lung carcinoma: a possible diagnostic tool for lymph node
metastasis. Cancer. 98:1217–1222. 2003. View Article : Google Scholar
|
|
7.
|
McNeil TM and Chamberlain JM: Diagnostic
anterior mediastinotomy. Ann Thorac Surg. 2:532–539. 1966.
View Article : Google Scholar
|
|
8.
|
Kitajima K, Yamasaki E, Kaji Y, et al:
Comparison of DWI and PET/CT in evaluation of lymph node metastasis
in uterine cancer. World J Radiol. 4:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Zhang L, Xi M, Deng XW, et al:
Four-dimensional CT-based evaluation of volumetric modulated arc
therapy for abdominal lymph node metastasis from hepatocellular
carcinoma. J Radiat Res. 53:769–776. 2012.PubMed/NCBI
|
|
10.
|
Takenaka T, Yano T, Morodomi Y, et al:
Prediction of true-negative lymph node metastasis in clinical IA
non-small cell lung cancer by measuring standardized uptake values
on positron emission tomography. Surg Today. 42:934–939. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Geraldson CT, Stephenson JE, Lagrew JP, et
al: Use of positron emission tomography in initial staging of
nonsmall cell lung carcinoma: a regional teaching hospital
experience. Am Surg. 78:305–308. 2012.PubMed/NCBI
|
|
12.
|
Kernstine KH, Mclaughlin KA, Menda Y, et
al: Can FDG-PET reduce the need for mediastinoscopy in potentially
resectable nonsmall cell lung cancer? Ann Thorac Surg. 73:394–401.
2002. View Article : Google Scholar : PubMed/NCBI
|