Introduction
Prostate cancer (PCa) is the second leading cause of
mortality in the western world, but the single most common
non-cutaneous malignancy in the United States, with ∼241,740 and
∼28,170, morbidities and mortalities, respectively, in 2012
(1,2). Despite an increase in available
reagents for PCa treatment (3–5), the
prognosis for advanced-stage patients remains discouraging, with a
median life expectancy of ∼2.5 years (6). Long-term complete regression of PCa is
uncommon and the complex mechanisms involved in advanced PCa are
not yet understood. The current report presents the case of a
patient with stage IV PCa, with rare clinical features, indicating
a role for the androgen-receptor in PCa. Written informed consent
was obtained from the patient.
Case report
Patient presentation and diagnosis
A 51-year-old male with progressive weakness, dull
shoulder and back pain and low-grade fevers in the afternoon
(range, 37.7–38.3°C) was referred to Shanghai Changzheng Hospital
(Shanghai, China) in November, 2002. Two months previously, the
individual detected a mass in the right groin, which was pliable in
texture with no pain upon the addition of pressure. A physical
examination revealed a 2×2-cm mass in the right groin. Blood
pressure, pulse and body temperature values were all within the
normal range. ECG results were normal, as were results from blood
and fecal tests. Prostate-specific antigen (PSA) tumor marker
levels were >500 ng/ml (reference value, 0–35 ng/ml), however,
other tumor markers, including α-fetoprotein (AFP),
carcinoembryonic antigen (CEA), carbohydrate antigen (CA)19-9,
CA12-5 and neuron-specific enolase (NSE) remained within the normal
ranges. The erythrocyte sedimentation rate (ESR) was 45 mm/h and
the anti-streptolysin ‘O’ and anti-rheumatoid factor test results
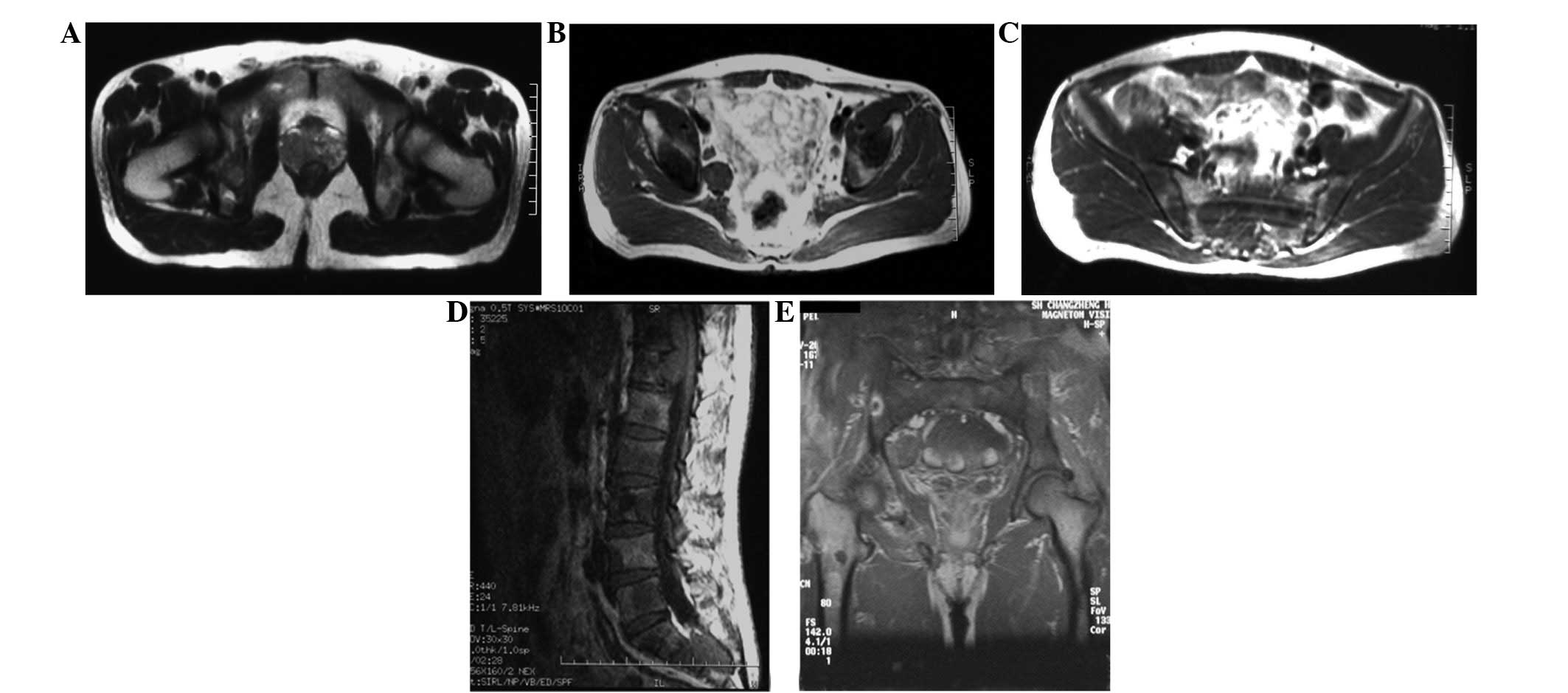
were negative. MRI of the pelvis and the lumbar spine detected an
enlarged prostate with non-uniform signals at the bottom of the
peripheral ribbon, multiple infiltrating lesions in the lumbar,
sacrum, pelvis and bilateral thighbone, a T11–12 intra-spinal tumor
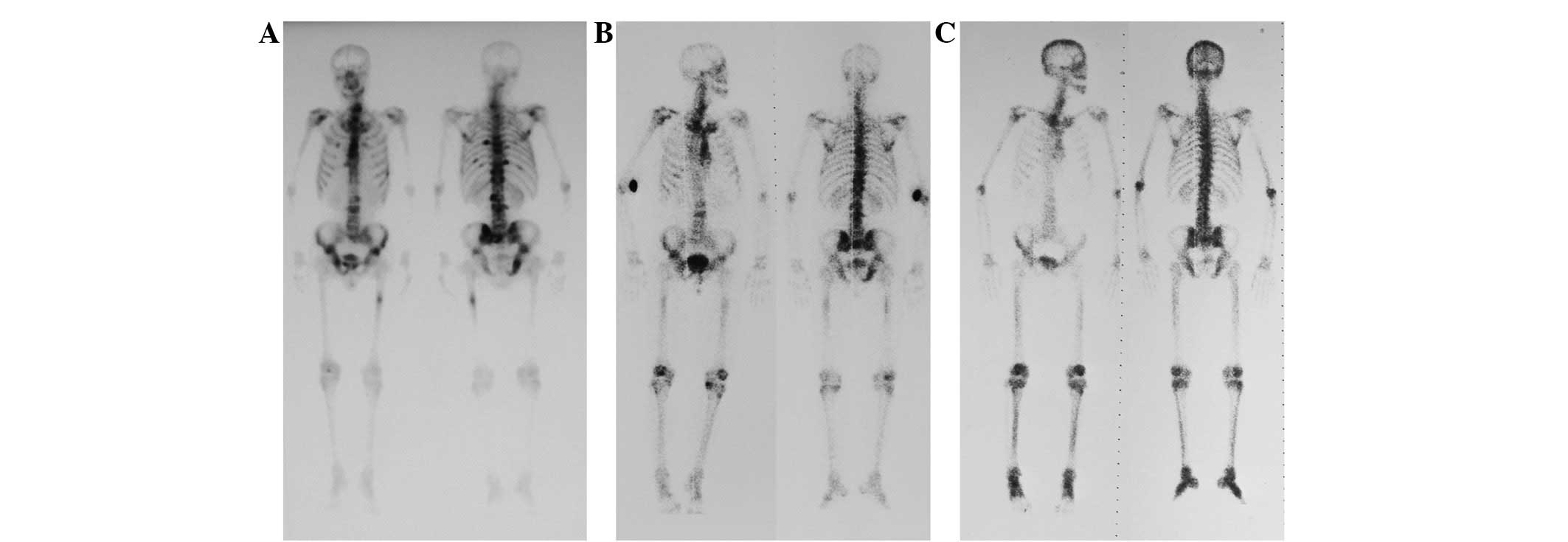
and soft tissue nodules in the right groin (Fig. 1). A bone scan revealed multiple
skeletal metastases (Fig. 2A) and a
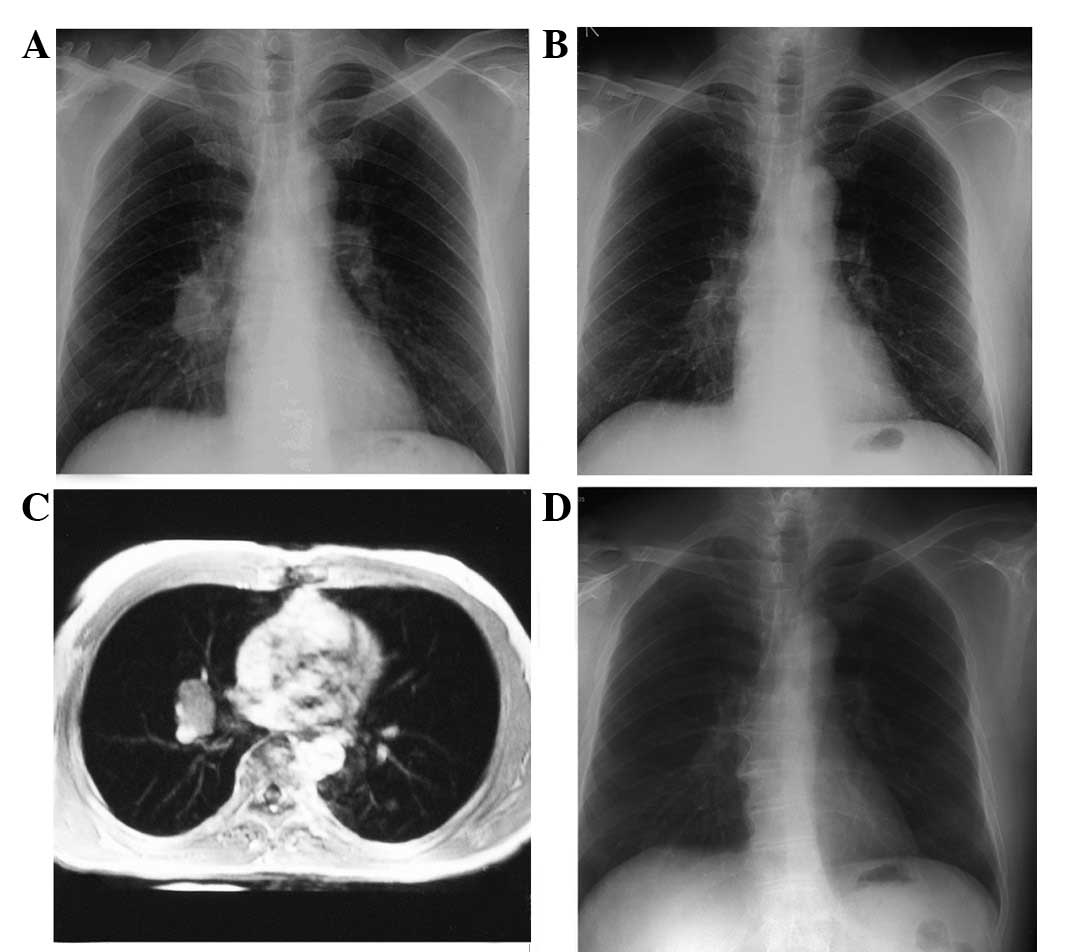
chest radiograph and lung MRI identified a 3×2-cm lobulated node in
the right hilum (Fig. 3A and B). A
review of the patient’s medical history showed the individual had
suffered from lumbar disk disease (T5-S1) for 8 years, in addition
to a long-term history of smoking and alcohol use. The patient was
diagnosed with advanced prostatic cancer (IV, cT4N2M1c) following
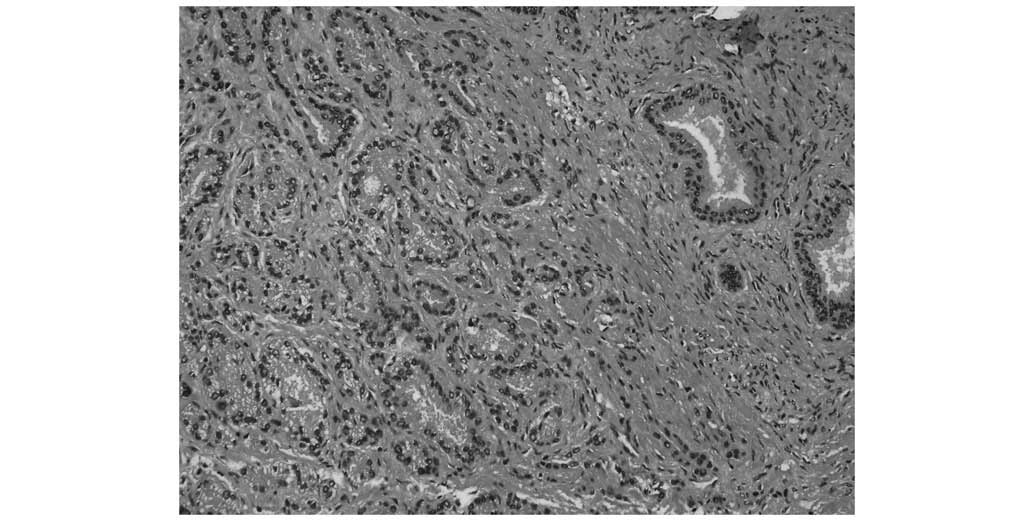
an ultrasonographic-guided biopsy performed in November, 2002.
Pathology results identified rounded cells with enlarged nuclei and
an irregular gland shape, which were deeply stained and infiltrated
the normal tissue (Fig. 4).
Treatment and clinical course
Flutamide (0.25 g) was administered (p.o.) 3 times a
day prior to surgical castration in December 2002. In addition,
3.75 mg enantone was injected (i.h.) once every month, for 3
months, without suspending the flutamide treatment. A traditional
Chinese herbal medicine (TCHM) was administrated immediately
following surgery and at follow-up appointments (Table I). In January 2003, strontium-89
radiotherapy for multiple bone metastases was performed. Laboratory
tests at that time indicated a significant decrease in PSA levels
to 0.32 ng/ml, which had reached 0.03 ng/ml at the end of the
month. In addition, a chest radiograph identified that the lung
lesion had gone (Fig. 3C). In March
2003, a repeat chest radiograph, which detected no abnormalities,
was performed and a bone scan demonstrated a marked reduction of
bone metastasis (Fig. 2B). Upon
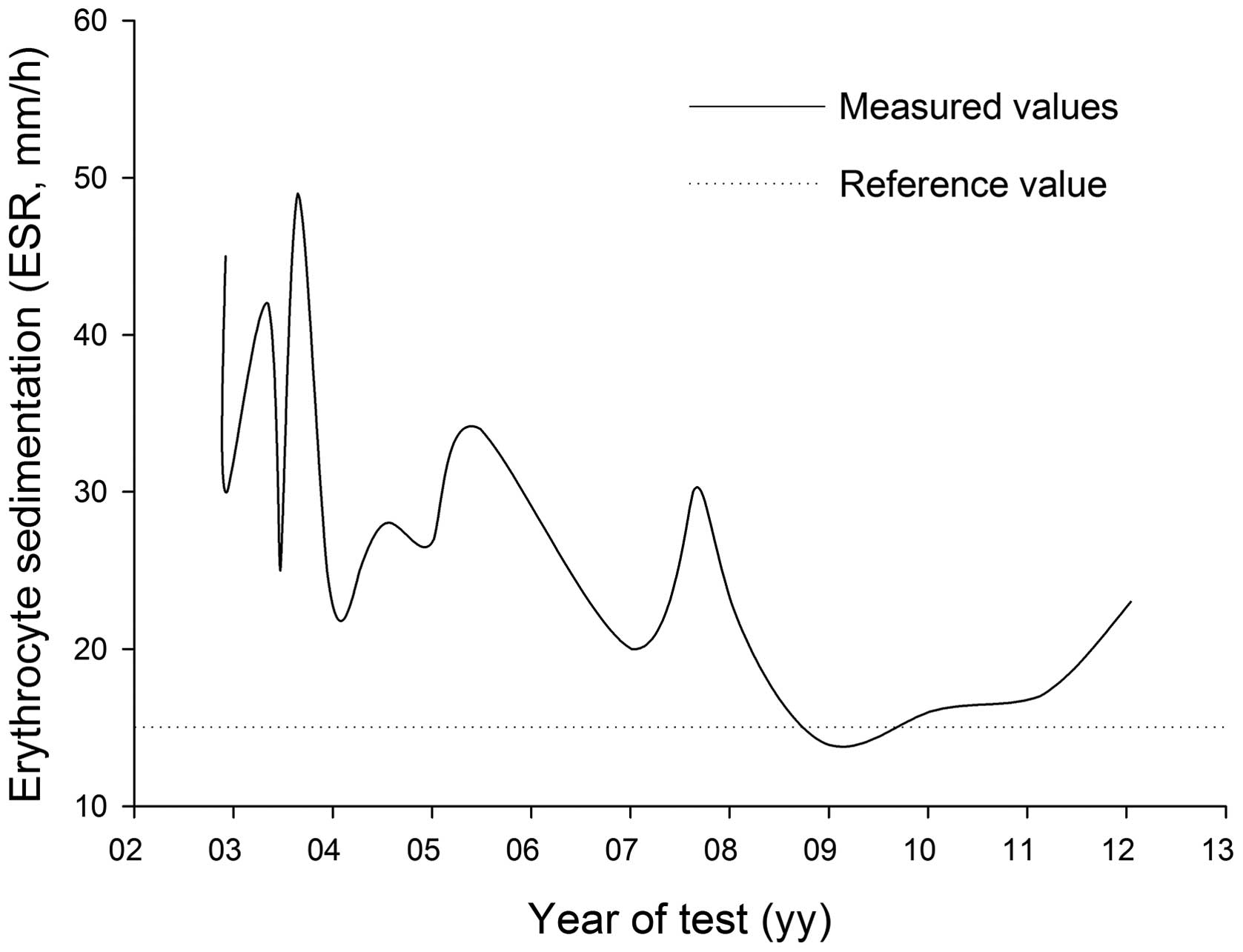
first admission, the patient exhibited levels of ESR that
fluctuated above normal (Fig. 5),
while the PSA levels remained at <0.1 ng/ml. In June 2003, an
additional bone scan revealed complete remission of the bone
metastasis (Fig. 2C). Annual bone
scans continued to confirm this result until the scans were stopped
in June 2005.
 | Table I.Ingredients of the traditional Chinese
herbal medicine. |
Table I.
Ingredients of the traditional Chinese
herbal medicine.
| Chinese name | Latin name | % |
|---|
| Huangqi | Astragali
Radix | 13.51 |
| Taizishen | Pseudostellariae
Radix | 13.51 |
| Nvzhenzi | Ligustri Lucidi
Fructus | 13.51 |
| Gouqi | Lycii
Fructus | 6.75 |
| Biejia | Trionycis
Carapax | 4.50 |
| QuanXie | Scorpio | 2.70 |
| Wugong |
Scolopendra | 4.50 |
| Tianlong | Gekko
Chinensis | 6.75 |
| Dilong | Pheretima | 6.75 |
| Banbianlian | Lobeliae Chinensis
Herba | 6.75 |
| Banzhilian | Scutellariae
Barbatae Herba | 6.75 |
| Jineijin | Galli Gigerii
Endothelium Corneum | 6.75 |
| Dazao | Jujubae
Fructus | 4.50 |
| Gancao | Glycyrrhizae Radix
Et Rhizoma | 2.70 |
The administration of flutamide was withdrawn in May
2007, but the use of TCHM was continued; no adverse effects were
identified by the individual, with the exception of controllable
hot flushes. However, no recurrence was detected at the annual
follow-up appointments. During treatment, blood, urine, stool,
electrolyte, biochemistry, tumor marker and hemagglutinin tests
were performed and demonstrated to be within the normal ranges.
When CT or MRI scans were not performed at the patient’s follow up
appointments, a visceral ultrasound examination, including an
examination of the prostate, was arranged and no abnormalities were
detected. The patient’s most recent appointment was in December
2012, where a physical examination and a chest radiograph detected
no abnormalities (Fig. 3D). An
examination of the liver, gall bladder, pancreas, spleen, kidney,
prostate, bladder and the lymph nodes of the bilateral groin was
performed by abdominal ultrasound and were all identified to be
normal. PSA and ESR levels were 0.06 ng/ml and 25 mm/h,
respectively.
Discussion
According to the international system for staging
PCa, the present case was classified as clinical stage IV
(cT4N1M1c) PCa. Few studies of the regression of PCa metastasis
have been published and the majority describe single lesions with
no records of long-term follow-up (Table II) (7–13).
Therefore, in this regard, the present case is unique.
 | Table II.Results of published case reports of
the regression of metastasis in prostate cancer. |
Table II.
Results of published case reports of
the regression of metastasis in prostate cancer.
| First author/s
(ref) | No. of cases | Location of
metastasis | Evidence of
regression | Management | Follow-up record |
|---|
| Peyrí Rey (7) | 1 | Bone | Bone scan | ADT | NA |
| Kumar et al
(8) | 1 | Eye | Not available | Hormonal therapy | NA |
| Hoshi et al
(9) | 1 | Bone | Bone scan | Cisplatin, UFT,
dexamethasone, diethylstilbestrol diphosphate | NA |
| Weiss et al
(10) | 1 | Bone | Scintigraphy |
Surgery/153Sm-EDTMP | NA |
| Ameur et al
(11) | 1 | Brain | NA | NA | Recurrence |
| Gayet and Curtillet
(12) | NA | Lung | NA | NA | NA |
| Turner and Chaudhary
(13) | 1 | Bone | PSA/Imaging | Alternative
therapies | NA |
The cellular and molecular events underlying the
development of PCa are not yet understood, but it has been
demonstrated that the role that androgens play is significant and,
as a result, anti-androgen therapy is the preferred treatment. For
previously untreated and advanced PCa, anti-androgen monotherapies,
including flutamide therapy, has been reported to be effective
(14,15). However, only single androgen
deprivation therapy (ADT) is recommended by the National
Comprehensive Cancer Network (2011) for M1 patients, based on the
evidence that combined- or triple-androgen blockage represents no
survival benefit over castration alone (16). In the present case report, the
treatment regimens conflicted with the treatment guidelines and
recommendations for PCa, and the reason for the final notable
results remains currently unclear. The majority of advanced PCa
cases are initially sensitive to ADT, however, the magnitude of
castration-induced primary regression does not predict clinical
outcome (17) and patients
generally develop castration resistance within a median time of
12–18 months (18). Treatment of
castration-resistant PCa (CRPC) is challenging since growth of the
cancer at this stage is hypothesized to be regulated by androgens,
and mutations of the androgen-receptor (AR) genes are common
(19,20). However, previous studies have
indicated that the AR remains a significant target in patients with
CRPC (21). Although results of the
current case report are unclear, based on the management and
clinical presentation, it may be hypothesized that androgens play a
significant role in PCa.
Advances in molecular biomarkers have developed
prognostic factors, allowing for improved identification of
patients likely to benefit from a specific reagent and are
therefore essential for selecting treatments. PSA is a
well-established marker for monitoring treatment response and
disease recurrence (22,23). Various parameters of PSA have been
studied (24,25) for example, a nadir PSA of <4
ng/ml within 6–7 months following initial treatment has been
identified to be a significant predictor of the progression time to
CRPC and overall survival (24,26).
Previous studies have demonstrated that 40–50 mm/h ESR at diagnosis
is a marker for low-risk cancer-specific mortality (27). The present patient had a nadir PSA
of 0.1 ng/ml at 2 months after the treatment, which remained low at
the follow-up appointments. In addition, ESR was 45 mm/h at
diagnosis. We hypothesize that these features indicate an improved
prognosis. Other prognostic factors, including circulating tumor
cells, have also been demonstrated as useful for predicting
survival benefit following treatment for metastatic CRPC and
hormone-sensitive PCa (28,29), however, results have yet to be
confirmed and validated by future studies (30).
Alternative medicine is popular among cancer
patients and previous studies have demonstrated that 8.4–26.5% of
PCa patients use herbal remedies (31,32).
TCHMs, including Realgar-Indigo naturalis and PHY906, are some of
the most popular remedies and have been scientifically proven to be
effective for cancer management (33–35).
Results indicating that TCHMs may lead to the complete regression
of cancer have been obtained in lung cancer and hepatocellular
carcinoma (36,37). In the present case report, a TCHM
was taken at the onset of treatment and then consistently for 4
years. Although it is hypothesized that the withdrawal of flutamide
may induce a reduction of PSA in 40% of PCa patients (38), no rebound of PSA or recurrence was
identified, therefore, TCHM may have a certain treatment value. The
efficacy of TCHM cannot be defined in patients based on the current
case report and no conclusive evidence has been obtained from
randomized trials. However, the current study and others have
indicated that TCHM may be an effective option for the future
management of PCa.
Overall, the present case report demonstrates a role
for the androgen-receptor in PCa and indicates that the careful
interpretation of nadir PSA and ESR may effectively predict patient
prognosis in the future.
References
|
1.
|
Siegel R, Naishadham D and Jemal A: Cancer
Statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2.
|
Boyle P and Ferlay J: Cancer incidence and
mortality in Europe, 2004. Ann Oncol. 16:481–488. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Attard G, Reid AH, Yap TA, Raynaud F,
Dowsett M, Settatree S, Barrett M, Parker C, Martins V, Folkerd E,
Clark J, Cooper CS, Kaye SB, Dearnaley D, Lee G and de Bono JS:
Phase I clinical trial of a selective inhibitor of CYP17,
abiraterone acetate, confirms that castration-resistant prostate
cancer commonly remains hormone driven. J Clin Oncol. 26:4563–4571.
2008. View Article : Google Scholar
|
|
4.
|
Kantoff PW, Higano CS, Shore ND, Berger
ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims
RB, et al IMPACT Study Investigators: Sipuleucel-T immunotherapy
for castration-resistant prostate cancer. N Engl J Med.
363:411–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Pezaro C and Attard G: Prostate cancer in
2011: redefining the therapeutic landscape for CRPC. Nat Rev Urol.
9:63–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Aus G, Robinson D, Rosell J, Sandblom G
and Varenhorst E; South-East Region Prostate Cancer Group: Survival
in prostate cancer outcomes from a prospective, population-based
cohort of 8887 men with up to 15 years of follow-up: results from
three countries in the population-based National Prostate Cancer
Registry of Sweden. Cancer. 103:943–951. 2005.
|
|
7.
|
Peyrí Rey E: Regression bone metastases in
patient with prostatic cancer. Actas Urol Esp. 32:10502008.(In
Spanish).
|
|
8.
|
Kumar P, Duarte J and Pati J: Metastatic
prostate cancer presenting as diplopia with regression of signs
with hormone manipulation. Br J Hosp Med (Lond). 66:6462005.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Hoshi S, Ohyama C, Hagisawa S, Ono K,
Satoh M, Saito S, Fukuzaki A and Arai Y: Complete regression of
bone metastases on super bone scan, by low-dose cisplatin, UFT,
diethylstilbestrol diphosphate, and dexamethasone in a patient with
hormone-refractory prostate cancer. Int J Clin Oncol. 8:118–120.
2003. View Article : Google Scholar
|
|
10.
|
Weiss K, Köck HH, Atefie K and Sinzinger
H: Complete scinti-graphic lesion regression after single
153Sm-EDTMP therapy in prostate cancer. Rev Esp Med Nucl.
20:311–312. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ameur A, Touiti D, el Mostarchid B, el
Alami M, Jira H and Abbar M: Brain metastasis of prostatic cancer:
regression under hormonal treatment. Prog Urol. 11:1298–1301.
2001.(In French).
|
|
12.
|
Gayet R and Curtillet H: Regression of
pulmonary metastases from prostatic cancer after treatment with
estrogens. J Urol Nephrol (Paris). 80:709–715. 1974.(In
French).
|
|
13.
|
Turner J and Chaudhary U: Dramatic
prostate-specific antigen response with activated hemicellulose
compound in metastatic castration-resistant prostate cancer.
Anticancer Drugs. 20:215–216. 2009. View Article : Google Scholar
|
|
14.
|
Lundgren R: Flutamide as primary treatment
for metastatic prostatic cancer. Br J Urol. 59:156–158. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Delaere KP and Van Thillo EL: Flutamide
monotherapy as primary treatment in advanced prostatic carcinoma.
Semin Oncol. 18(5 Suppl 6): 13–18. 1991.PubMed/NCBI
|
|
16.
|
National Comprehensive Cancer Network: The
NCCN clinical practice guidelines in oncology 2011: prostate cancer
version 1: clinical practice guidelines in oncology. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
Accessed Jan 12, 2013.
|
|
17.
|
Ohlson N, Bergh A, Nygren K, Stattin P and
Wikström P: The magnitude of early castration-induced primary
tumour regression in prostate cancer does not predict clinical
outcome. Eur Urol. 49:675–683. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Shelke AR and Mohile SG: Treating prostate
cancer in elderly men: how does aging affect the outcome? Curr
Treat Options Oncol. 12:263–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Feldman BJ and Feldman D: The development
of androgen-independent prostate cancer. Nat Rev Cancer. 1:34–45.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Taplin ME, Bubley GJ, Shuster TD, Frantz
ME, Spooner AE, Ogata GK, Keer HN and Balk SP: Mutation of the
androgen-receptor gene in metastatic androgen-independent prostate
cancer. N Engl J Med. 332:1393–1398. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
de Bono JS, Logothetis CJ, Molina A,
Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F,
et al COU-AA-301 Investigators: Abiraterone and increased survival
in metastatic prostate cancer. N Engl J Med. 364:1995–2005.
2011.
|
|
22.
|
Scher HI, Halabi S, Tannock I, Morris M,
Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ,
Dreicer R, et al Prostate Cancer Clinical Trials Working Group:
Design and end points of clinical trials for patients with
progressive prostate cancer and castrate levels of testosterone:
recommendations of the Prostate Cancer Clinical Trials Working
Group. J Clin Oncol. 26:1148–1159. 2008. View Article : Google Scholar
|
|
23.
|
Bubley GJ, Carducci M, Dahut W, Dawson N,
Daliani D, Eisenberger M, Figg WD, Freidlin B, Halabi S, Hudes G,
et al: Eligibility and response guidelines for phase II clinical
trials in androgen-independent prostate cancer: recommendations
from the Prostate-Specific Antigen Working Group. J Clin Oncol.
17:3461–3467. 1999.
|
|
24.
|
Nayyar R, Sharma N and Gupta NP:
Prognostic factors affecting progression and survival in metastatic
prostate cancer. Urol Int. 84:159–163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Collette L, de Reijke TM and Schröder FH;
EORTC Genito-Urinary Group: Prostate specific antigen: a prognostic
marker of survival in good prognosis metastatic prostate cancer?
(EORTC 30892). Eur Urol. 44:182–189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Hussain M, Tangen CM, Higano C,
Schelhammer PF, Faulkner J, Crawford ED, Wilding G, Akdas A, Small
EJ, Donnelly B, et al Southwest Oncology Group Trial 9346
(INT-0162): Absolute prostate-specific antigen value after androgen
deprivation is a strong independent predictor of survival in new
metastatic prostate cancer: data from Southwest Oncology Group
Trial 9346 (INT-0162). J Clin Oncol. 24:3984–3990. 2006. View Article : Google Scholar
|
|
27.
|
Johansson JE, Sigurdsson T, Holmberg L and
Bergström R: Erythrocyte sedimentation rate as a tumor marker in
human prostatic cancer. An analysis of prognostic factors in 300
population-based consecutive cases. Cancer. 70:1556–1563. 1992.
View Article : Google Scholar
|
|
28.
|
Scher HI, Jia X, de Bono JS, Fleisher M,
Pienta KJ, Raghavan D and Heller G: Circulating tumour cells as
prognostic markers in progressive, castration-resistant prostate
cancer: a reanalysis of IMMC38 trial data. Lancet Oncol.
10:233–239. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Goodman OB Jr, Symanowski JT, Loudyi A,
Fink LM, Ward C and Vogelzang NJ: Circulating tumor cells as a
predictive biomarker in patients with hormone-sensitive prostate
cancer. Clin Genitourin Cancer. 9:31–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Vishnu P and Tan WW: Update on options for
treatment of meta-static castration-resistant prostate cancer. Onco
Targets Ther. 3:39–51. 2010.PubMed/NCBI
|
|
31.
|
Molassiotis A, Fernadez-Ortega P, Pud D,
Ozden G, Scott JA, Panteli V, Margulies A, Browall M, Magri M,
Selvekerova S, et al: Use of complementary and alternative medicine
in cancer patients: a European survey. Ann Oncol. 16:655–663. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Lin YH, Chen KK and Chiu JH:
Coprescription of chinese herbal medicine and Western medications
among prostate cancer patients: a population-based study in Taiwan.
Evid Based Complement Alternat Med. 2012:1470152012.PubMed/NCBI
|
|
33.
|
Lin YH, Chen KK and Chiu JH: Use of
Chinese medicine among prostate cancer patients in Taiwan: a
retrospective longitudinal cohort study. Int J Urol. 18:383–386.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Wang L, Zhou GB, Liu P, Song JH, Liang Y,
Yan XJ, Xu F, Wang BS, Mao JH, Shen ZX, et al: Dissection of
mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as
an effective treatment for promyelocytic leukemia. Proc Natl Acad
Sci USA. 105:4826–4831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Lam W, Bussom S, Guan F, Jiang Z, Zhang W,
Gullen EA, Liu SH and Cheng YC: The four-herb Chinese medicine
PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci
Transl Med. 2:45–59. 2010.PubMed/NCBI
|
|
36.
|
Liang HL, Xue CC and Li CG: Regression of
squamous cell carcinoma of the lung by Chinese herbal medicine: a
case with an 8-year follow-up. Lung Cancer. 43:355–360.
2004.PubMed/NCBI
|
|
37.
|
Cheng HM and Tsai MC: Regression of
hepatocellular carcinoma spontaneous or herbal medicine related? Am
J Chin Med. 32:579–585. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Scher HI and Kelly WK: Flutamide
withdrawal syndrome: its impact on clinical trials in
hormone-refractory prostate cancer. J Clin Oncol. 11:1566–1572.
1993.PubMed/NCBI
|