Introduction
The Rho GTPases, which are from a distinct branch of
the Ras-like low molecular weight GTP-binding protein super-family,
are involved in actin cytoskeleton organization (1) and have been associated with invasion
and metastasis (2). RhoGTPases
alternate between an inactive GDP-bound state and an active
GTP-bound state. The regulators of this GDP/GTP cycle include GDP
dissociation inhibitors (GDIs), which bind to the inactive form,
thereby blocking further activation. RhoGDI1 was first identified
on the basis of its ability to inhibit GDP dissociation from RhoA
(3), CDC42Hs (4) and Rac1 (5). RhoGDI2, also known as D4-GDI or
Ly-GDI, shares a 67% amino acid analogy with RhoGDI1 (6–8).
However, in contrast with the ubiquitous property of RhoGDI1, the
protein is believed to be expressed exclusively on cells of
hematopoietic lineage (6,7). However, Seraj et al (9) and Gildea et al (10) suggested that the RhoGDI2 gene is
also expressed in non-hematopoietic neoplasms. Furthermore, Gildea
et al (11) have shown using
an animal model of human bladder cancer metastasis and DNA
microarray technology that RhoGDI2 is a putative metastasis
suppressor gene in human cancer. By analyzing patient
immunohistochemistry (IHC), Theodorescu et al reported that
RhoGDI2 is an independent predictor of prognosis for patients with
bladder cancer (12). Also, Hu
et al (13) demonstrated
that the reduced expression of RhoGDI2 in breast cancer was
associated with lymph node metastasis. To date, there have been no
studies on the impact of RhoGDI2 mRNA expression for the survival
of patients with gastric carcinoma. Hence, the present study
investigated the expression of RhoGDI2 in human gastric cancer
specimens and evaluated the significance of RhoGDI2 expression on
the outcome and clinicopathological parameters of the patients.
Patients and methods
Patients and tumor samples
A total of 46 patients who had undergone a
gastrectomy with lymph node dissection for primary gastric
carcinoma at Jikei University Daisan Hospital (Tokyo, Japan) and
Shiomidai Prefectural Hospital (Kanagawa, Japan) between March 2006
and January 2010 were studied. Pre-operative informed consent was
obtained from each patient in accordance with institutional
guidance. Tumor specimens and normal mucosal tissues were stored at
4°C in an RNA preserving reagent (RNA-later, Ambion, Austin, TX,
USA). The extraction of total RNA from the samples was performed
within three months of tissue extraction. The total RNA was
immediately transcribed to first-strand cDNA (1st Strand cDNA
Synthesis kit; Roche, Basel, Switzerland), which was stored at
−80°C until the reverse transcription-polymerase chain reaction
(RT-PCR). RT-PCR for RhoGDI2 and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was performed between July 2011 and August
2011. The RhoGDI2 and GAPDH mRNA levels were quantified using NIH
Image 1.63 computer software (Wayne Rasband, NIH, Bethesda, MD,
USA), and the intensity of RhoGDI2 for GAPDH was calculated.
Pathological and clinical records were reviewed and the disease
stage was determined according to the classification by the
Japanese Research Society for gastric cancer (14). The histological grading and depth of
invasion of the tumors in all the cases were available and are
summarized in Table I. The presence
of liver metastasis and peritoneal dissemination was determined
pre-operatively using radiographic examinations or
intra-operatively. Based on the histological grade, the tumor
specimens were classified into two groups consisting of the
differentiated group (well- to moderately-differentiated
adenocarcinoma and papillary adenocarcinoma) and the
undifferentiated group (poorly-differentiated adenocarcinoma and
signet-ring cell or mucinous carcinoma). This study was approved by
the ethics committee of Jikei University School of Medicine, Tokyo,
Japan.
 | Table I.Clinical and pathological
characteristics of the patients and tumors. |
Table I.
Clinical and pathological
characteristics of the patients and tumors.
| Characteristic | Value |
|---|
| Median age,
years | 73 |
| Gender, n
(male:female) | 31:15 |
| Median size, mm | 40 |
| Histological type, n
(%) | |
| Differentiated | 27 (59) |
|
Undifferentiated | 19 (41) |
| Location of tumor, n
(%) | |
| U | 10 (22) |
| Mid | 25 (54) |
| L | 11 (24) |
| Gross form, n
(%) | |
| 0-I | 1 (2) |
| 0-IIa | 7 (15) |
| 0-IIb | 1 (2) |
| 0-IIc | 8 (17) |
| 0-III | 0 (0) |
| Type 1 | 2 (4) |
| Type 2 | 12 (26) |
| Type 3 | 14 (30) |
| Type 4 | 1 (2) |
| Depth of invasion, n
(%) | |
| pT1 (M, SM) | 15 (32) |
| pT2 (MP, SS) | 20 (43) |
| pT3 (SE) | 11 (24) |
| pT4 (SI) | 0 (0) |
| Venous
invasion+, n (%) | 20 (43) |
| Lymphatic
invasion+, n (%) | 36 (78) |
| Lymph node
metastasis+, n (%) | 25 (54) |
| Peritoneal
dissemination+, n (%) | 3 (7) |
| Synchronous liver
metastasis+, n (%) | 1 (2) |
| Synchronous lung
metastasis+, n (%) | 0 (0) |
Semi-quantitative RT-PCR
Total RNA was extracted from the resected gastric
carcinoma and normal tissue specimens using the RNeasy Mini kit
(Qiagen, Valencia, CA, USA), according to the manufacturer’s
instructions. The first-strand cDNA was synthesized from total RNA
by reverse transcriptase (1st Strand cDNA Synthesis kit; Roche,
Basel, Switzerland). The PCR analysis was performed using the
following pairs of primers: RhoGDI2 forward,
5′-agtacgacgtgatcgtgctg-3′ and reverse, 5′-gcgagcaatttctccttcag-3′;
and GAPDH forward, 5′-atcatccctgcctctactgg-3′ and reverse,
5′-ccctccgacgcctgcttcac-3′. Following this, 1 μg/l of the
cDNA reaction was subjected to 35 PCR cycles (denaturing at 94°C
for 1 min, annealing at 58°C for 50 sec and polymerization at 72°C
for 1 min), in the presence of 0.25 U Taq DNA Polymerase (Roche,
Indianapolis, IN, USA), 1X PCR reaction buffer (Roche), 0.25 mM
dNTPs (Promega, Madison, WI, USA) and 0.5 μM specific
primers for RhoGDI2 and GAPDH in a final reaction volume of 50
μl.
IHC
Using paraffin-embedded specimens from eight
patients with gastric cancer, the RhoGDI2 protein was detected
using the anti-RhoGDI2 rabbit polyclonal antibody (Abcam,
Cambridge, UK). Briefly, subsequent to being microwaved in citrate
buffer solution (pH 6.0), the deparaffinized sections were
incubated with 1% methanol-hydrogen peroxide for 30 min. The slides
were then incubated with the rabbit polyclonal antibody against
RhoGDI2 (undiluted solution) for 60 min. This was followed by
incubation with anti-rabbit secondary antibody
(Envision™/Rabbit/HRP; Dako, Carpinteria, CA, USA) for 30 min. The
staining was visualized using the diaminobenzidine (DAB) method
(Dako) for 5 min. Counter-staining was performed lightly with
hematoxylin. All incubations were performed at room temperature in
a humidified chamber. Control rabbit immunoglobulin G was used for
each staining (Daiichi Fine Chemical, Takaoka, Toyama, Japan).
Statistics
The significance of the data was determined using
the chi-square test or Student’s t-test. The multivariate analysis
for patient prognosis was determined using the Cox proportional
hazards model. The survival curves of the patients were compared
using the Kaplan-Meier method and analyzed by the log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of RhoGDI2 mRNA in human
gastric carcinoma tissues
Semi-quantitative RT-PCR
A total of 46 pairs of tissue samples obtained from
the tumors of patients with gastric carcinoma and the adjacent
non-cancerous mucosa were examined for RhoGDI2 gene expression
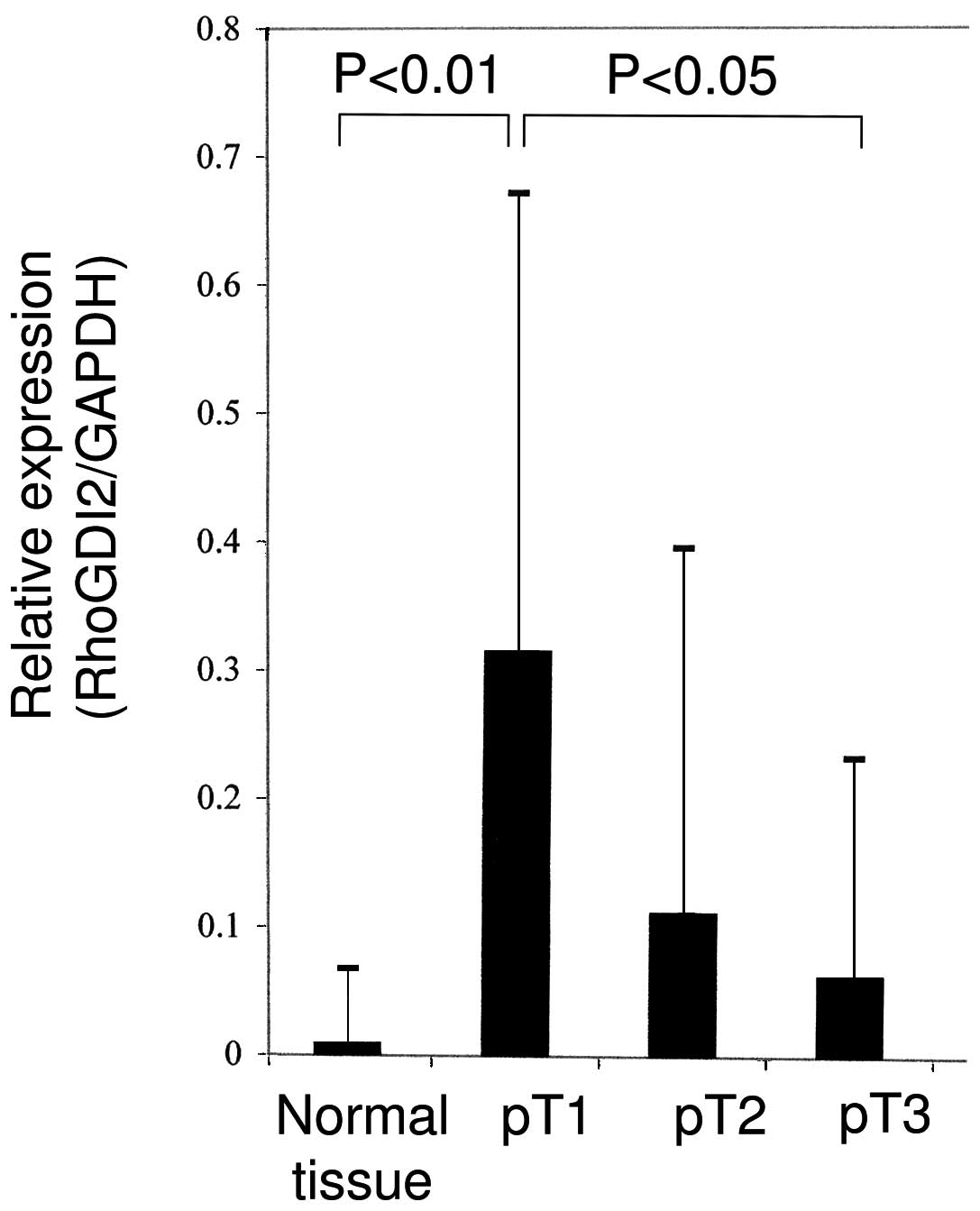
using RT-PCR (Fig. 1).
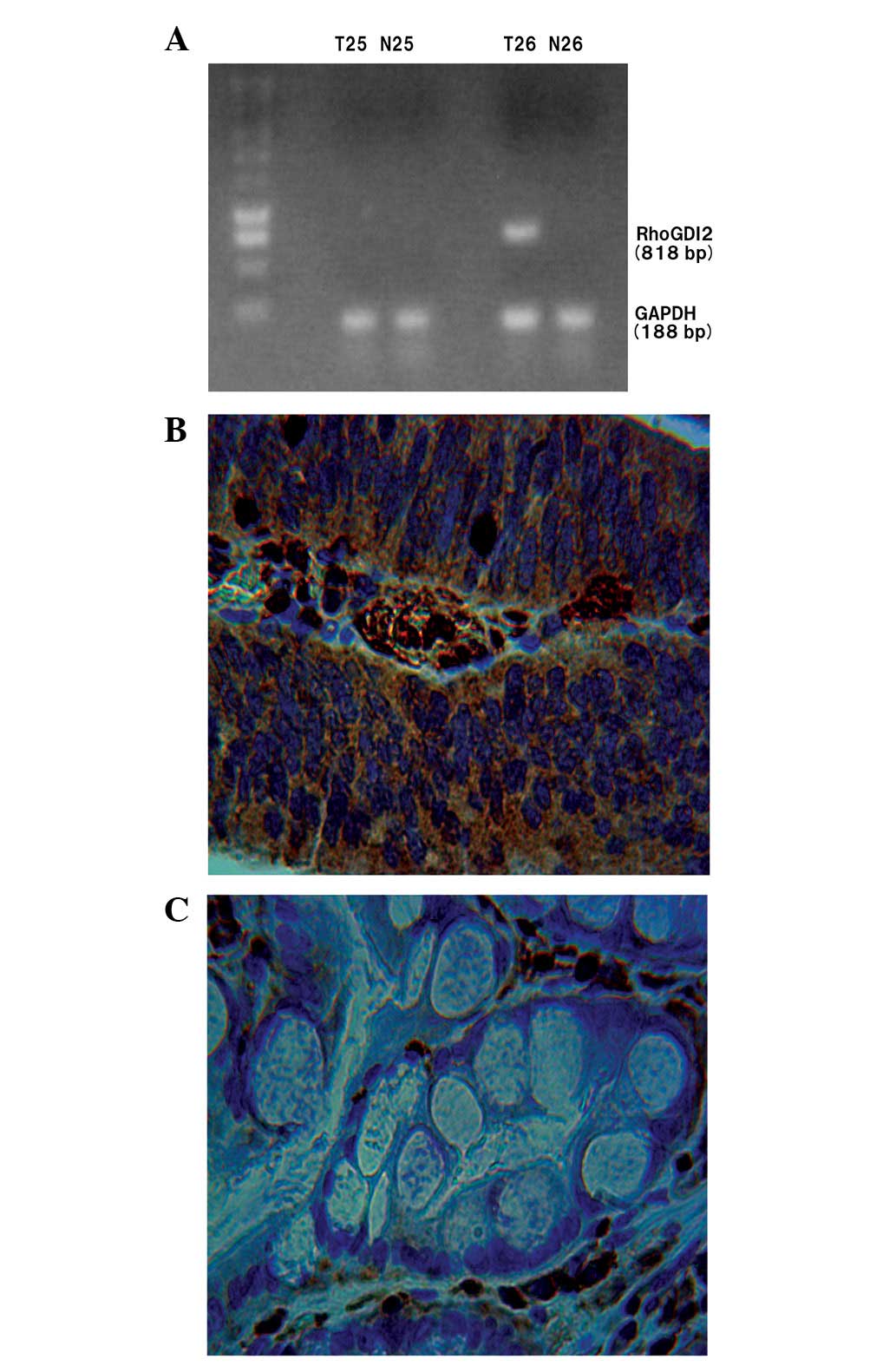 | Figure 1.(A) RT-PCR analysis of RhoGDI2 and
GAPDH mRNA in two human gastric carcinoma patients. In patient 25,
RhoGDI2 mRNA was not expressed in the T or N mucosa. However, in
patient 26, RhoGDI2 mRNA was expressed in the T but not the N
mucosa. (B) Representative IHC staining with RhoGDI2 polyclonal
antibody in gastric cancer, showing strong staining for RhoGDI2 in
the cytoplasm of the tumor tissue (×400). (C) In the N gastric
mucosa, RhoGDI2 was not observed in the epithelial cells (×400).
The samples shown in (B) and (C) are derived from patient 26.
RT-PCR, reverse transcription-polymerase chain reaction; RhoGDI2,
Rho GDP dissociation inhibitor 2; GAPDH, glyceraldehyde-phosphate
dehydrogenase; T, tumor; N, normal; IHC, immunohistochemistry. |
The 95% CI [the average ratio + two standard
deviations (SD)] was used to select a cut-off line. The mean
intensity of RhoGDI2 for GAPDH in 46 normal gastric tissues was
0.01 and the SD was 0.057. Hence, the cut-off value of RhoGDI2 was
defined as 0.124. According to this cut-off line, RhoGDI2-positive
expression was observed in 14 (30.4%) human gastric carcinoma
samples and in one (2.2%) normal gastric mucosa sample. The
expression of RhoGDI2 mRNA was significantly higher in the
early-stage gastric cancer samples compared with the normal gastric
mucosa or advanced gastric cancer tissues (Fig. 2; Student’s t-test, P<0.01). A
univariate analysis was carried out to determine the correlation
between the conventional pathological prognostic markers and
RhoGDI2 mRNA expression. The results show that a reduced expression
of RhoGDI2 is associated with venous system invasion and lymph node
metastasis (Table II). The tumor
expression of RhoGDI2 was further evaluated as a prognostic
variable in patients with gastric carcinoma. Table III and IV show the multivariate analysis, which
identified the fact that RhoGDI2 expression was not an independent
prognostic factor for relapse-free survival (RFS) or overall
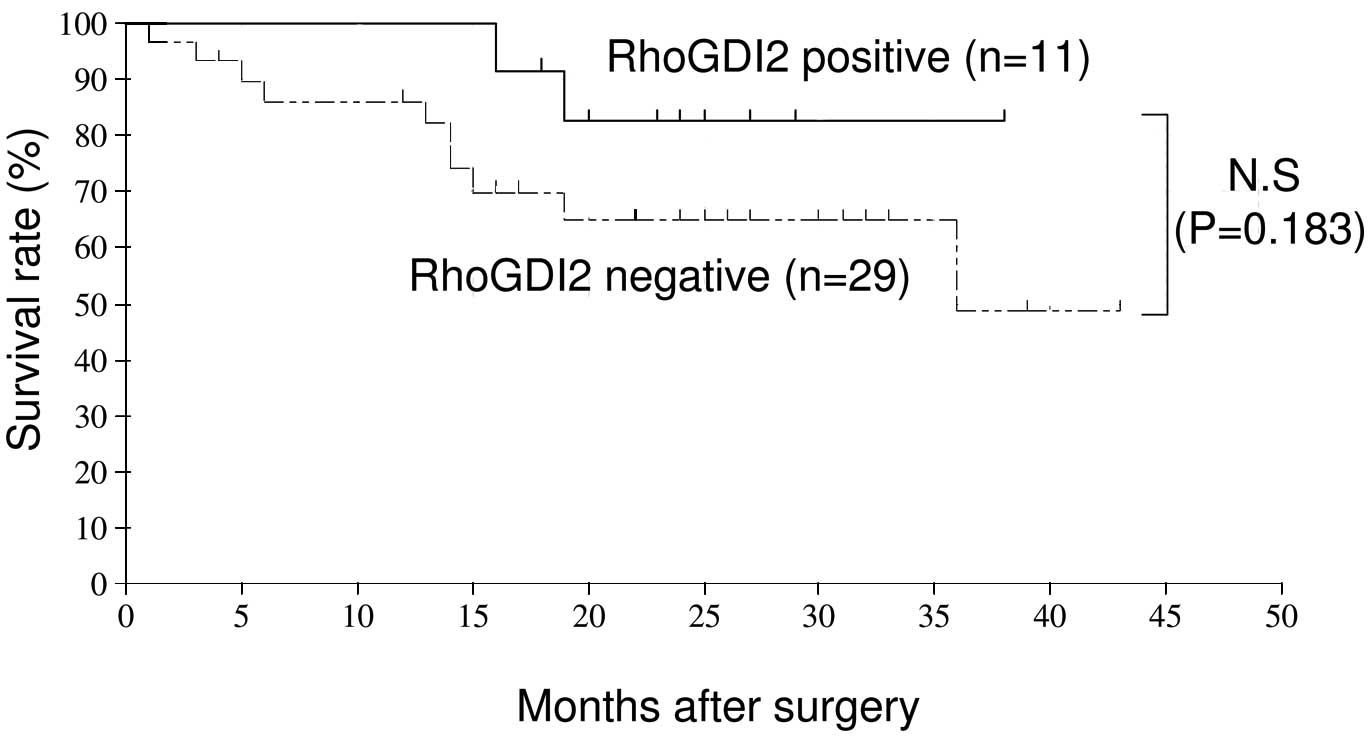
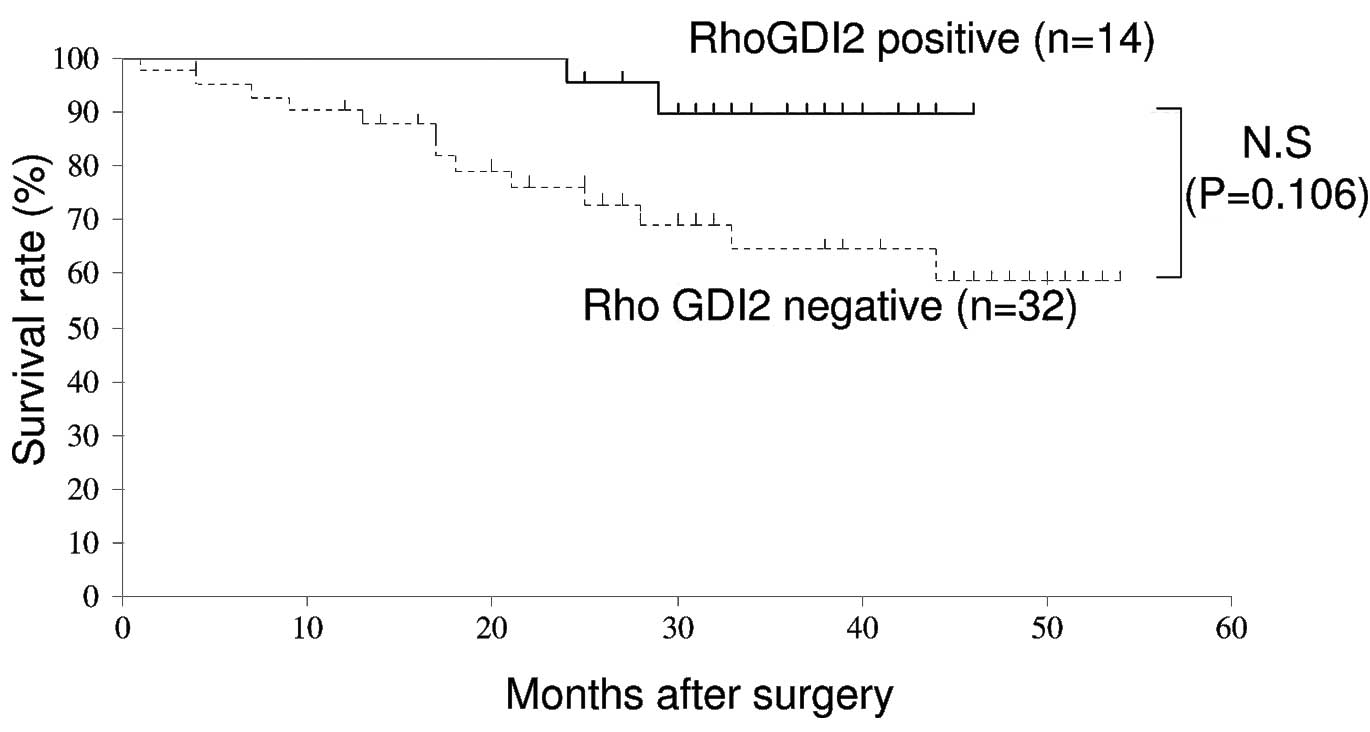
survival (OS). However, the data show that RhoGDI2-positive
patients had a good prognosis compared with those who were
RhoGDI2-negative (Figs. 3 and
4).
 | Table II.Correlation between
clinicopathological observations and RhoGDI2 expression. |
Table II.
Correlation between
clinicopathological observations and RhoGDI2 expression.
| Findings | RhoGDI2 expression
| P-value |
|---|
| Positive, n | Negative, n |
|---|
| Histological
type | | | |
| Differentiated | 11 | 16 | |
|
Undifferentiated | 3 | 16 | 0.1374 |
| Location of
tumor | | | |
| U | 3 | 7 | |
| M | 10 | 15 | |
| L | 1 | 10 | 0.1555 |
| Gross form | | | |
| 0-I | 1 | 0 | |
| 0-IIa | 3 | 4 | |
| 0-IIb | 1 | 0 | |
| 0-IIc | 3 | 5 | |
| 0-III | 0 | 0 | |
| Type 1 | 2 | 0 | |
| Type 2 | 2 | 10 | |
| Type 3 | 2 | 12 | |
| Type 4 | 0 | 1 | 0.1214 |
| Venous invasion | | | |
| Positive | 3 | 19 | |
| Negative | 11 | 13 | 0.0404 |
| Lymphatic
invasion | | | |
| Positive | 8 | 22 | |
| Negative | 6 | 10 | 0.4469 |
| Lymph node
metastasis | | | |
| Positive | 3 | 23 | |
| Negative | 11 | 9 | 0.0043 |
 | Table III.Risk factors affecting RFS as
determined by the Cox proportional hazards model in 40 patients
with gastric cancer. |
Table III.
Risk factors affecting RFS as
determined by the Cox proportional hazards model in 40 patients
with gastric cancer.
| Variable | Multivariate
analysis for RFS
|
|---|
| Hazard ratio | 95% CI | P-value |
|---|
| pT (T1 vs.
T2/T3/T4) | 0.359 | 0.153–0.843 | 0.019 |
| Venous system
invasion (yes vs. no) | 0.723 | 0.327–1.597 | 0.422 |
| Lymphatic system
invasion (yes vs. no) | 1.174 | 0.431–3.198 | 0.754 |
| Lymph node
metastasis (yes vs. no) | 1.289 | 0.522–3.179 | 0.582 |
| RhoGDI2 (positive
vs. negative) | 0.641 | 0.264–1.556 | 0.326 |
 | Table IV.Risk factors affecting OS rate
determined by Cox proportional Hazards model in 46 patients with
gastric cancer. |
Table IV.
Risk factors affecting OS rate
determined by Cox proportional Hazards model in 46 patients with
gastric cancer.
| Variable | Multivariate
analysis for OS
|
|---|
| Hazard ratio | 95% CI | P-value |
|---|
| pT (T1 vs.
T2/T3/T4) | 0.399 | 0.166–0.960 | 0.040 |
| Venous system
invasion (yes vs. no) | 0.826 | 0.374–1.824 | 0.637 |
| Lymphatic system
invasion (yes vs. no) | 1.229 | 0.441–3.426 | 0.692 |
| Lymph node
metastasis (yes vs. no) | 1.188 | 0.453–3.117 | 0.726 |
| RhoGDI2 (positive
vs. negative) | 0.516 | 0.211–1.263 | 0.147 |
IHC
The expression of the RhoGDI2 protein was assessed
in eight cases of surgically removed gastric tissues using a
polyclonal antibody that was specific to RhoGDI2. In several cases,
strong staining for RhoGDI2 was observed in the cytoplasm of the
tumor tissues, even though negative or very weak RhoGDI2 was
observed in the normal gastric tissues.
These IHC examinations corresponded with the results
from the RT-PCR (Fig. 1).
Discussion
RhoGDIs are known to inhibit the activation of
RhoGTPases (15). RhoGDI2 has been
established as a metastasis suppressor gene in bladder cancer.
Using a gene array analysis, Theodorescu et al reported that
decreased RhoGDI2 gene/protein expression was associated with a
more invasive variant of the HRAS mutation-positive T24 bladder
cancer cell line (12). Ectopic
restoration of RhoGDI2 expression in the invasive T24 variant has
also been shown to decrease metastasis, as determined by tail-vein
lung tumor colonization in mice (10). Finally, the IHC analysis of 51
bladder tumors revealed that RhoGDI2 overexpression correlated with
a poor survival time to disease-specific mortality (12). Hu et al identified a biphasic
pattern of increased RhoGDI2 expression with breast hyperplasia,
but decreased expression with progression and lymph node metastasis
using IHC staining in 71 patients (13). In addition, Stevens et al
showed that a stable suppression of RhoGDI2 protein expression in
ovarian cancer cells increased anchorage-independent growth and
Matrigel invasion in vitro, and in tail-vein lung colony
metastatic growth in vivo (16). These data show that RhoGDI2
suppresses cancer progression.
Although an increasing number of studies on the role
of RhoGDI2 are appearing, the function of RhoGDI2 remains
controversial. Tapper et al demonstrated that the
upregulation of RhoGDI2 was associated with the malignant potential
of ovarian carcinoma by cDNA array analysis (17). An increased motility of murine
cancer has been reported to correlate with an overexpression of
RhoGDI2 (18). In addition, Cho
et al observed that the ectopic overexpression of RhoGDI2 in
poorly-invasive gastric carcinoma cell lines significantly
increased Matrigel invasiveness in vitro. Conversely, the
depletion of endogenous RhoGDI2 in RhoGDI2-overexpressing gastric
carcinoma cells suppressed invasion in vitro. A forced
expression of RhoGDI2 in these cells lines increased tumor growth,
angiogenesis and lung metastasis in mice (19). The findings indicate that RhoGDI2 is
involved in tumorigenesis and cancer progression.
According to the present data, the reduced
expression of RhoGDI2 is associated with venous system invasion and
lymph node metastasis. The data suggest that RhoGDI2 suppresses
cancer metastasis and that the detection of RhoGDI2 mRNA expression
may be an useful clinical modality for detecting lymph node
metastasis in gastric carcinoma. Dransart et al (20) and Stevens et al (16) found that RhoGDI2 preferentially
bound and activated Rac1, and that Rac1 activation antagonized
metastasis. Since RhoGDIs are able to modulate Rho GTPase
interaction with Rho guanine nucleotide exchange factor (RhoGEFs)
and Rho GTPase-activating proteins (RhoGAPs), RhoGDI2 may activate
Rac through the altered regulation of GDP/GTP cycling and then Rac
activation may be able to antagonize tumor invasion and metastasis
(15).
In summary, the RhoGDI2 mRNA expression that is
usually decreased in advanced-stage gastric cancer was
significantly increased in the early-stage gastric cancer patients
of the present study. Such expression was almost non-existent in
the normal epithelial samples. A reduced expression of RhoGDI2 is
associated with venous system invasion and lymph node metastasis,
which may lead to the formation of clinical applications for the
evaluation of lymph node metastasis in patients with gastric
carcinoma.
References
|
1.
|
Sahai E and Marshall CJ: RHO-GTPases and
cancer. Nat Rev Cancer. 2:133–142. 2002. View Article : Google Scholar
|
|
2.
|
Clark EA, Golub TR, Lander ES and Hynes
RO: Genomic analysis of metastasis reveals an essential role for
RhoC. Nature. 406:532–535. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ueda T, Kikuchi A, Ohga N, Yamamoto J and
Takai Y: Purification and characterization from bovine brain
cytosol of a novel regulatory protein inhibiting the dissociation
of GDP from and the subsequent binding of GTP to rhoB p20, a ras
p21-like GTP-binding protein. J Biol Chem. 265:9373–9380. 1990.
|
|
4.
|
Leonard D, Hart MJ, Platko JV, et al: The
identification and characterization of a GDP-dissociation inhibitor
(GDI) for the CDC42Hs protein. J Biol Chem. 267:22860–22868.
1992.PubMed/NCBI
|
|
5.
|
Abo A, Pick E, Hall A, Totty N, Teahan CG
and Segal AW: Activation of the NADPH oxidase involves the small
GTP-binding protein p21rac1. Nature. 353:668–670. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Scherle P, Behrens T and Staudt LM:
Ly-GDI, a GDP-dissociation inhibitor of the RhoA GTP-binding
protein, is expressed preferentially in lymphocytes. Proc Natl Acad
Sci USA. 90:7568–7572. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lelias JM, Adra CN, Wulf GM, et al: cDNA
cloning of a human mRNA preferentially expressed in hematopoietic
cells and with homology to a GDP-dissociation inhibitor for the rho
GTP-binding proteins. Proc Natl Acad Sci USA. 90:1479–1483. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Leffers H, Nielsen MS, Andersen AH, et al:
Identification of two human Rho GDP dissociation inhibitor proteins
whose over expression leads to disruption of the actin
cytoskeleton. Exp Cell Res. 209:165–174. 1993. View Article : Google Scholar
|
|
9.
|
Seraj MJ, Harding MA, Gildea JJ, Welch DR
and Theodorescu D: The relationship of BRMS1 and RhoGDI2 gene
expression to metastatic potential in lineage related human bladder
cancer cell lines. Clin Exp Metastasis. 18:519–525. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Gildea JJ, Seraj MJ, Oxford G, et al:
RhoGDI2 is an invasion and metastasis suppressor gene in human
cancer. Cancer Res. 62:6418–6423. 2002.PubMed/NCBI
|
|
11.
|
Gildea JJ, Golden WL, Harding MA and
Theodorescu D: Genetic and phenotypic changes associated with the
acquisition of tumorigenicity in human bladder cancer. Genes
Chromosomes Cancer. 27:252–263. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Theodorescu D, Sapinoso LM, Conaway MR, et
al: Reduced expression of metastasis suppressor RhoGDI2 is
associated with decreased survival for patients with bladder
cancer. Clin Cancer Res. 10:3800–3806. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hu LD, Zou HF, Zhan SX and Cao KM:
Biphasic expression of RhoGDI2 in the progression of breast cancer
and its negative relation with lymph node metastasis. Oncol Rep.
17:1383–1389. 2007.PubMed/NCBI
|
|
14.
|
Japanese Gastric Cancer Association:
Japanese Classification of Gastric Carcinoma - 2nd English Edition.
Gastric Cancer. 1:10–24. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Olofsson B: Rho guanine dissociation
inhibitors: pivotal molecules in cellular signaling. Cell Signal.
11:545–554. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Stevens EV, Banet N, Onesto C, et al:
RhoGDI2 antagonizes ovarian carcinoma growth, invasion and
metastasis. Small GTPases. 2:202–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Tapper J, Kettunen E, El-Rifai W, et al:
Changes in gene expression during progression of ovarian carcinoma.
Cancer Genet Cytogenet. 128:1–6. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Yanagawa T, Watanabe H, Takeuchi T, et al:
Overexpression of autocrine motility factor in metastatic tumor
cells: possible association with augmented expression of KIF3A and
GDI-beta. Lab Invest. 84:513–522. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Cho HJ, Baek KE, Park SM, et al: RhoGDI2
expression is associated with tumor growth and malignant
progression of gastric cancer. Clin Cancer Res. 15:2612–2619. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Dransart E, Olofsson B and Cherfils J:
RhoGDIs revisited: novel roles in Rho regulation. Traffic.
6:957–966. 2005. View Article : Google Scholar : PubMed/NCBI
|