Introduction
Oral squamous cell carcinoma (OSCC) is the most
prevalent pathological oral cancer, accounting for >80% of head
and neck malignancies (1). The
carcinogenesis of OSCC is a multistage process involving the
activation of oncogenes and the inactivation of tumor suppressor
genes, with a cellular imbalance between cell death and growth.
Regulators of apoptosis and the cell cycle may be important for the
cellular balance of cell death and growth in cancer.
p21, p27 and survivin proteins have been identified
to be abnormally expressed in the majority of human forms of
cancer, including oral carcinoma. These expression levels usually
correlate with cell proliferation and apoptosis, which contribute
to the molecular carcinogenesis of cancer (2–5). The
overexpression of p21 and p27 enhances the binding to cyclin-CDK
complexes, which inhibits cell proliferation (6). Previous studies have shown that, in
conjunction with survivin, p21 and p27 may also inhibit cell
apoptosis (7,8). The correlation between patient
prognosis and biomarker expression has received considerable
interest, however, the clinical implications and prognostic value
of this correlation remain controversial due to the complex
mechanisms of carcinogenesis and limitations with regard to small
sample sizes and short follow-up periods (9–13). In
the current study, the long-term follow-up of p21, p27 and survivin
immunoexpression was extensively monitored in 110 patients with
OSCC to examine the association with prognosis.
Material and methods
Study participants
p21, p27 and survivin expression levels were
identified in formalin-fixed and paraffin-embedded specimens from
110 OSCC patients admitted to the Department of Oral and
Maxillofacial Surgery (The Ninth People’s Hospital, Shanghai Jiao
Tong University School of Medicine, Shanghai, China) between 1989
and 1993. Oral mucosa samples from 20 healthy participants were
included as controls. All individuals were treated by standard
radical surgery with negative margins, and patients who were
classified with T3, T4 or lymph node metastasis, according to the
International Union Against Cancer TNM classification (14), were treated with 50–65 Gy
radiotherapy post-operatively. Patients were followed up for >5
years. The mean age and range of the patients was 58 and 37–78
years-old, respectively. Gender, pathological grade and clinical
stage are shown in Table I.
Pathological grade was independently evaluated by three experienced
pathologists. This study was approved by the ethics committee of
The Ninth People’s Hospital, Shanghai Jiao Tong University School
of Medicine, Shanghai, China. Written informed consent was obtained
from the patient’s family.
 | Table I.Baseline characteristics of OSCC
patients. |
Table I.
Baseline characteristics of OSCC
patients.
| Clinical
observations | n | Percentage |
|---|
| Gender | | |
| Male | 59 | 53.64 |
| Female | 51 | 46.36 |
| Age, years | | |
| ≤60 | 75 | 68.18 |
| >60 | 35 | 31.82 |
| Tumor size | | |
| T1 | 14 | 12.73 |
| T2 | 53 | 48.18 |
| T3 | 16 | 14.55 |
| T4 | 27 | 24.54 |
| Lymph node
metastasis | | |
| N0 | 82 | 74.55 |
| N1 | 25 | 22.73 |
| N2 | 3 | 2.72 |
| Pathological
grading | | |
| I–II | 94 | 85.45 |
| III | 16 | 14.55 |
Immunohistochemical staining
Immunostaining for p21 (ZM-0206), p27 (ZM-0340) and
survivin (ZA-O530) was performed using reagents from Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing, China)
according to the manufacturer’s instructions. Sections were dewaxed
in xylene and rehydrated in graded alcohol prior to pretreatment
with 0.3% hydrogen peroxide in phosphate-buffered saline (PBS) for
15 min to block endogenous peroxidase. Following this, a further 3
PBS washes were performed. The dewaxed sections were heated in a
microwave oven in 10 mM citric acid buffer (pH 6.0) for 15 min and
gradually cooled down to room temperature. The sections were
incubated with appropriate antibodies (mouse anti-human p21, p27
and rabbit anti-human survivin) overnight in a humidified chamber
at 4°C. The sections were then washed 3 times with PBS and
incubated for 1 h at room temperature in a humidified chamber with
a corresponding second antibody (goat anti-mouse and goat
anti-rabbit), followed by being washed with PBS. Next, the sections
were washed with a developing solution containing 0.06%
diaminobenzidine and 0.1% hydrogen peroxide and then counterstained
with hematoxylin and mounted. The sections without primary
antibodies or with non-immunized rabbit serum for p21, p27 and
survivin were included as the negative controls.
The sections were examined microscopically by three
pathologists and scored according to the fraction of stained tumor
cells and the staining intensity (Table
II). The mean of each protein expression level was used as a
cut-off value to define high and low expression (15), for example, p21 expression was high
if the p21 score was >2.7 and low if ≤2.7. Similarly, the p27
and survivin cut-off values were 2.8 and 2.0, respectively.
 | Table II.Classification standard for
immunohistochemical staining of p21, p27 and survivin, evaluation
and expression score calculation. |
Table II.
Classification standard for
immunohistochemical staining of p21, p27 and survivin, evaluation
and expression score calculation.
| Standard | Score |
|---|
| Percentage | |
| 0 | 0 |
| 5 | 1 |
| ≤25 | 2 |
| ≤50 | 3 |
| >50 | 4 |
| Intensity | |
| Negative | 0 |
| Weak | 1 |
| Moderate | 2 |
| Intense | 3 |
Statistical analysis
The results were analysed using SAS package version
9.2 (SAS Institute Inc., Cary, NC, USA). Prognostic factors were
evaluated by univariable and multivariable analyses in the Cox
proportional hazards model, and the correlation between all the
parameters was analyzed using Pearson’s correlation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of p21 and p27 in OSCC
cells
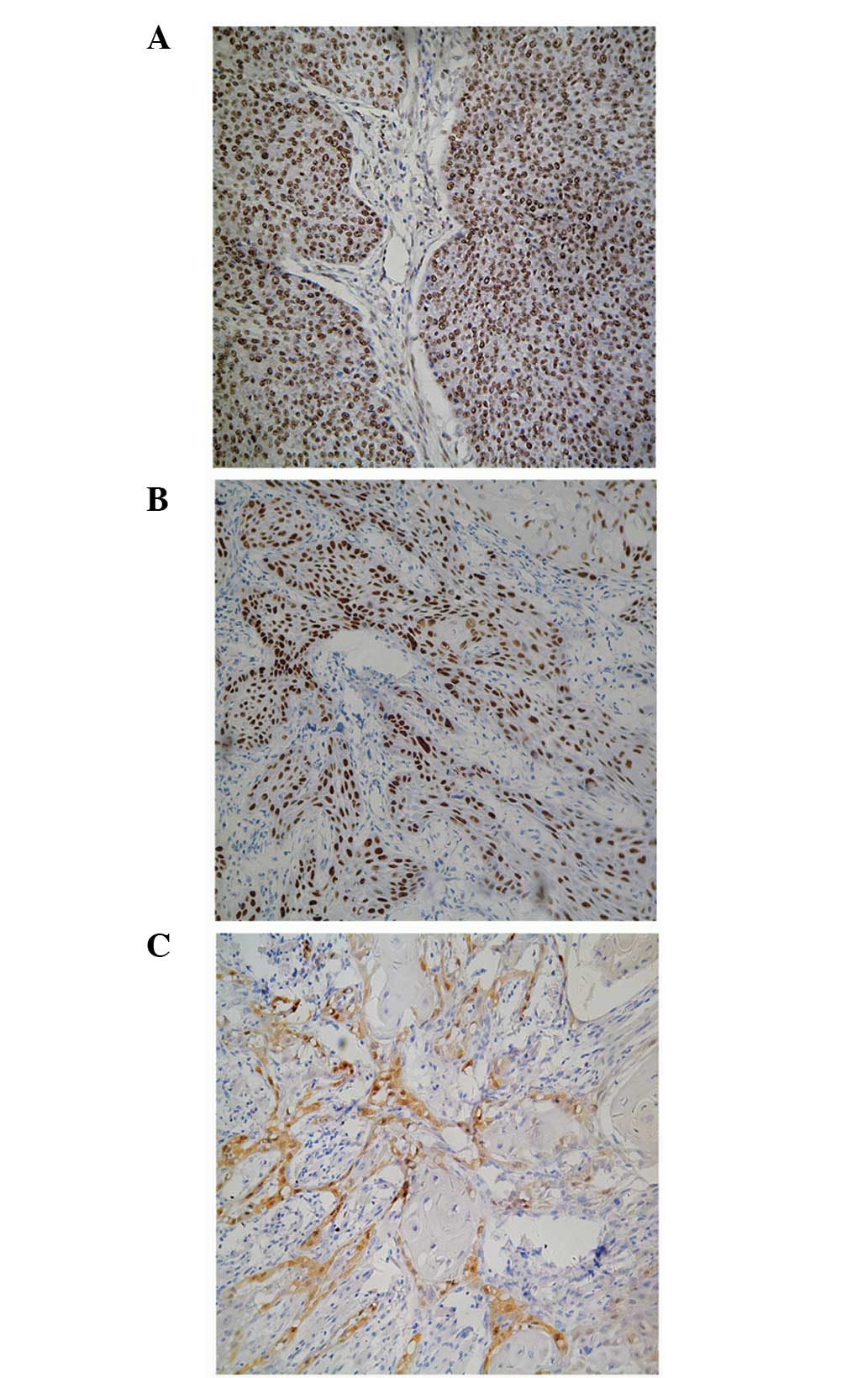
The majority of p21 expression was localized in the
nucleus of the OSCC cells and was identified by dark staining
(Fig. 1A). The percentage of cells
with positive p21 expression was 64.55% with a mean score of
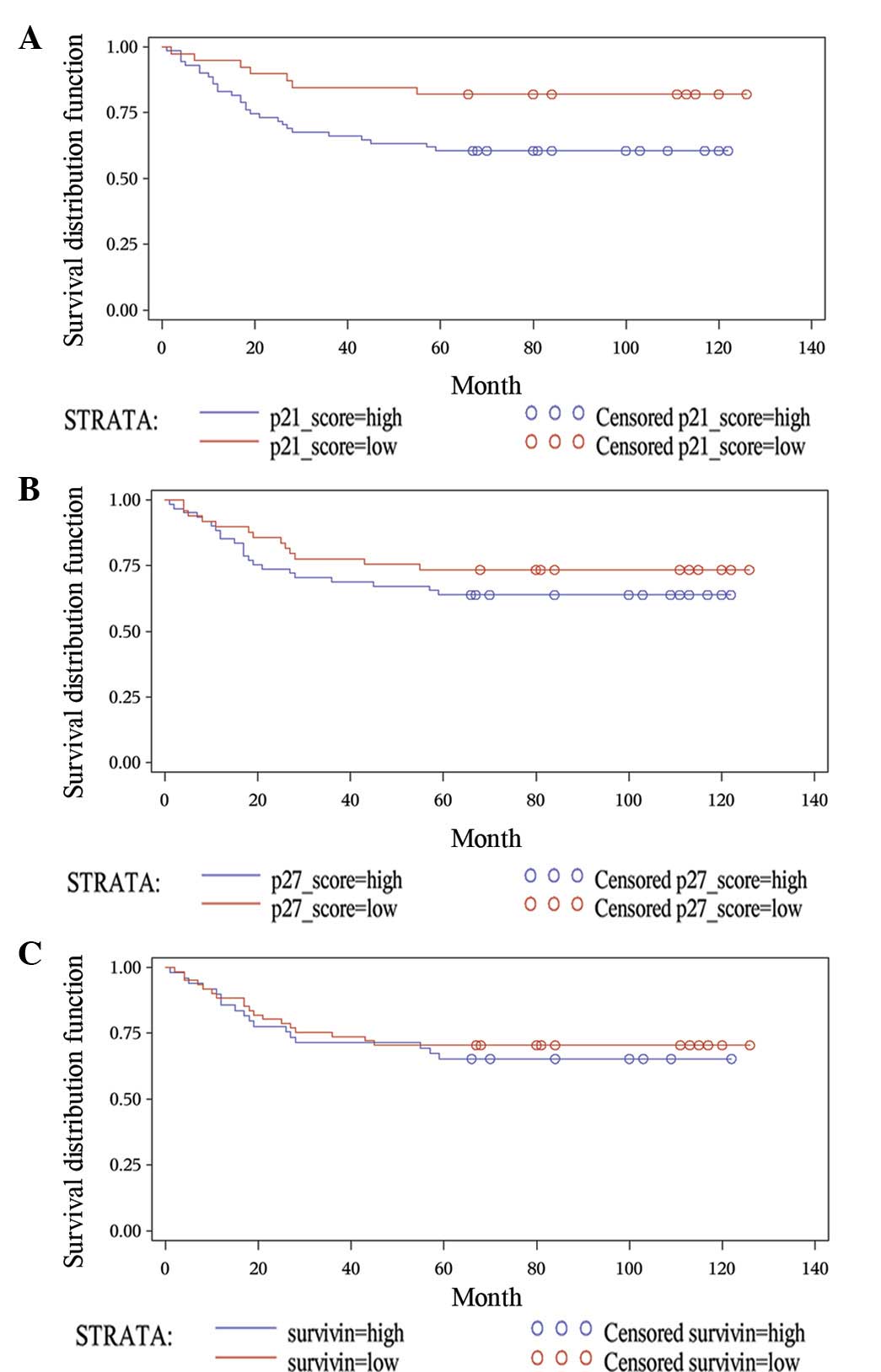
2.7±0.8. A log-rank test showed that the five-year survival rate of
patients with elevated p21 expression levels was significantly
lower when compared with that of patients with low p21 expression
levels (60.56±5.80 vs. 82.05±6.15%; P=0.0222; Fig. 2A). Similarly, the majority of p27
expression was localized in the nucleus of the OSCC cells, however,
the staining intensity was moderate (Fig. 1B). The percentage of cells with
positive p27 expression was 55.46%, with a mean score of 2.8±0.9.
Patients with an elevated p27 level exhibited a lower five-year
survival rate when compared with that of patients with lower p27
expression levels (63.93±6.15 vs. 73.47±6.31%; P=0.2777), however,
this was not statistically significant (Fig. 2B).
Expression of survivin in OSCC cells
By contrast, the majority of survivin was expressed
in the cytoplasm of the OSCC cells, with negligible detection in
the nucleus (Fig. 1C). The
percentage of cells with positive survivin expression was 64.55%,
with a mean score of 2.0±1.0. No significant difference was
identified between the five-year survival rate of patients with an
elevated level of survivin expression when compared with that of
patients with lower survivin expression levels (65.31±6.80 vs.
70.49±5.84%; P=0.5843; Fig.
2C).
Correlation between p21, p27 and survivin
and various parameters, and the univariate analysis results
The Pearson’s correlation analysis (Table III) revealed that p21 expression
significantly correlated with p27 (r=0.210; P=0.026) and survivin
(r=0.292; P=0.002) expression. In addition, there was a significant
correlation between lymph node metastasis and tumor size (r=0.229;
P=0.016). However, statistical correlations were not found between
the immunoexpression of p21, p27 and survivin and age, gender,
tumor size, lymph node metastasis or pathological grade
(P>0.05).
 | Table III.Pearson’s correlation between
parameters observed in patients with OSCC. |
Table III.
Pearson’s correlation between
parameters observed in patients with OSCC.
| Parameter | Gender | Age | Pathological
grade | Tumor size | Node
metastasis | p21 | p27 | Survivin |
|---|
| Gender | 1.000 | −0.002 | −0.030 | 0.182 | −0.023 | −0.022 | 0.128 | 0.190 |
| Age, years | −0.002 | 1.000 | −0.056 | 0.051 | 0.049 | −0.153 | −0.104 | −0.182 |
| Pathological
grade | −0.030 | −0.056 | 1.000 | 0.100 | −0.082 | 0.104 | 0.106 | −0.039 |
| Tumor size | 0.182 | 0.051 | 0.100 | 1.000 | 0.229 | −0.027 | 0.050 | −0.011 |
| Node
metastasis | −0.023 | 0.049 | −0.082 | 0.229 | 1.000 | −0.120 | 0.006 | 0.044 |
| p21 | −0.022 | −0.153 | 0.104 | −0.027 | −0.120 | 1.000 | 0.210 | 0.292 |
| p27 | 0.128 | −0.104 | 0.106 | 0.050 | 0.006 | 0.210 | 1.000 | 0.212 |
| Survivin | 0.190 | −0.182 | −0.039 | −0.011 | 0.044 | 0.292 | 0.212 | 1.000 |
The univariate analysis indicated that among the
observed variables, only p21 expression and the status of lymph
node metastasis were associated with the prognosis of patients with
OSCC (Table IV).
 | Table IV.Univariate analysis of prognostic
factors for survival using the Cox proportional hazards model. |
Table IV.
Univariate analysis of prognostic
factors for survival using the Cox proportional hazards model.
| Clinicopathological
parameters | 5-year survival
rate, % | χ2 | P-value |
|---|
| Gender | | | |
| Male | 66.10±6.16 | 0.2087 | 0.6478 |
| Female | 70.59±6.38 | | |
| Age, years | | | |
| ≤60 | 64.00±5.54 | 1.8642 | 0.1721 |
| >60 | 77.14±7.10 | | |
| Tumor size | | | |
| T1–T2 | 73.13±5.42 | 1.8661 | 0.1713 |
| T3–T4 | 60.47±7.46 | | |
| Node
metastasis | | | |
| Positive | 75.61±4.74 | 10.8832 | 0.0011 |
| Negative | 46.43±9.42 | | |
| Pathology
grading | | | |
| I–II | 70.21±4.72 | 1.2445 | 0.2646 |
| III | 56.25±12.40 | | |
| p21 expression | | | |
| ≤2.7 | 82.05±6.15 | 5.2307 | 0.0222 |
| >2.7 | 60.56±5.80 | | |
| p27 expression | | | |
| ≤2.8 | 73.47±6.31 | 1.1783 | 0.2777 |
| >2.8 | 63.93±6.15 | | |
| Survivin
expression | | | |
| ≤2.0 | 70.49±5.84 | 0.2993 | 0.5843 |
| >2.0 | 65.31±6.80 | | |
Discussion
p21 and p27 are members of the Cip/Kip gene family
that inhibit cell cycle progression by binding cyclin/Cdk
complexes, thus functioning as regulators of cell cycle progression
at the G1 stage (16,17).
In addition, the Cip/Kip family has been identified to activate the
cyclin D/Cdk4 complex (18–22). p21 is capable of stabilizing the
Cdk4-cyclin D interaction and promoting the formation of active
complexes in a dosage-dependent manner (19). p21 may also regulate apoptosis
(23,24) through interactions with p27 and
survivin (7,8). p21 functions as an inhibitor of
apoptosis in a number of systems, which may counteract its
tumor-suppressive function as a growth inhibitor (25). p21 has been reported to regulate
apoptosis via p53-dependent and -independent pathways (26–28).
In the present study, the percentage of p21 and
p27-positive cells was ∼64.55 and 55.46%, respectively. The results
showed that the expression of p21 (P=0.0222) and p27 (P=0.2777)
negatively correlated with prognosis, however, this was not
statistically significant for p27. The present study demonstrated
that the five-year survival rate of patients with a p21 expression
score of >2.7 was significantly lower when compared with that of
patients with a score of ≤2.7, inconsistent with the majority of
previous studies in OSCC (10,11)
and other types of cancer (29–34).
However, certain other controversial results have also been
reported (35–37).
Survivin is a member of the inhibitor of apoptosis
protein family, and specific expression levels of survivin have
been identified in embryogenesis and tumor cells (38–40).
Survivin has been shown to be involved in cell division,
anti-apoptosis and cell cycle control (40–44),
and it has been hypothesized that survivin interacts with p21 to
regulate cell apoptosis (7,8). The survivin-p21 axis is important for
the proliferation of normal hematopoietic cells and in the
regulation of apoptosis through the p21WAF1/Cip1-dependent pathway
(45). In the current study, the
percentage of cells with survivin expression was ∼64.55%, similar
to results from previous studies (46). However, inconsistent with these
previous results, survivin was not found to be an independent
prognostic factor of OSCC (47,48).
The expression of p53 and Bcl-2 was also examined in
the samples (data not shown) and, notably, p21 expression was shown
to be correlated with p27, survivin and Bcl-2, but not p53,
indicating that p21 may function in a p53-independent manner in
OSCC. The coexpression patterns among p21, p27, Bcl-2 and survivin
demonstrated that p21 plays a predominant role in inhibiting
apoptosis, likely through interactions with p27 and survivin
(7,8). In addition, p21-expressing cells may
produce antiapoptotic proteins that affect the survival of adjacent
cells through a paracrine effect (49). It has also been hypothesized that by
inhibiting apoptosis in OSCC, p21 may enable tumor cells to
accumulate for cell proliferation and may also present resistance
to therapy by inhibiting treatment-related apoptosis, resulting in
a reduced five-year survival rate (50). This may explain the negative
correlation between the overexpression of p21 and prognosis.
In conclusion, the current study reveals that the
prognosis of OSCC may be affected by a number of clinical
pathological factors and biomarkers, among which, p21 plays an
important role by inhibiting cell apoptosis or resistance to
therapy. To improve overall survival rates, patients with a high
p21 expression level must be administered intensive combined
therapy and provided with follow-ups at an increased frequency.
Acknowledgements
This study was supported by grants
from the Projects of the Shanghai Science and Technology Committee
(nos. 08JC1414400, 10XD1402500, 11DZ2291800 and 10DZ1951300). The
authors would like to thank the participants of the study.
References
|
1.
|
Landis SH, Murray T, Bolden S and Wingo
PA: Cancer statistics, 1999. CA Cancer J Clin. 49:8–31. 1999.
View Article : Google Scholar
|
|
2.
|
Coqueret O: New roles for p21 and p27
cell-cycle inhibitors: a function for each cell compartment? Trends
Cell Biol. 13:65–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Bandoh N, Hayashi T, Takahara M, et al:
Loss of p21 expression is associated with p53 mutations and
increased cell proliferation and p27 expression is associated with
apoptosis in maxillary sinus squamous cell carcinoma. Acta
Otolaryngol. 125:779–785. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Lu Y and Cao W: The role of p21
(wafl/cip1) in human colorectal carcinoma cell apoptosis. Zhonghua
Yi Xue Za Zhi. 81:1330–1332. 2001.(In Chinese).
|
|
5.
|
Bissonnette N and Hunting DJ: p21-induced
cycle arrest in G1 protects cells from apoptosis induced by
UV-irradiation or RNA polymerase II blockage. Oncogene.
16:3461–3469. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ravitz MJ and Wenner CE: Cyclin-dependent
kinase regulation during G1 phase and cell cycle regulation by
TGF-beta. Adv Cancer Res. 71:165–207. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Suzuki A, Ito T, Kawano H, et al: Survivin
initiates procaspase 3/p21 complex formation as a result of
interaction with Cdk4 to resist Fas-mediated cell death. Oncogene.
19:1346–1353. 2000. View Article : Google Scholar
|
|
8.
|
Temme A, Diestelkoetter-Bachert P, Schmitz
M, et al: Increased p21(ras) activity in human fibroblasts
transduced with survivin enhances cell proliferation. Biochem
Biophys Res Commun. 327:765–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Xie X, Clausen OP and Boysen M: Prognostic
significance of p21WAF1/CIP1 expression in tongue squamous cell
carcinomas. Arch Otolaryngol Head Neck Surg. 128:897–902. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Fischer CA, Jung M, Zlobec I, et al:
Co-overexpression of p21 and Ki-67 in head and neck squamous cell
carcinoma relative to a significantly poor prognosis. Head Neck.
33:267–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Nemes JA, Nemes Z and Márton IJ:
p21WAF1/CIP1 expression is a marker of poor prognosis in oral
squamous cell carcinoma. J Oral Pathol Med. 34:274–279. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Zhuang Y, Yin HT, Yin XL, Wang J and Zhang
DP: High p27 expression is associated with a better prognosis in
East Asian non-small cell lung cancer patients. Clin Chim Acta.
412:2228–2231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kapranos N, Stathopoulos GP, Manolopoulos
L, et al: p53, p21 and p27 protein expression in head and neck
cancer and their prognostic value. Anticancer Res. 21:521–528.
2001.PubMed/NCBI
|
|
14.
|
O’Sullivan B and Shah J: New TNM staging
criteria for head and neck tumors. Seminars Surg Oncol. 21:30–42.
2003.
|
|
15.
|
Zhang M, Zhang P, Zhang C, et al:
Prognostic significance of Bcl-2 and Bax protein expression in the
patients with oral squamous cell carcinoma. J Oral Pathol Med.
38:307–313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Harper JW, Adami GR, Wei N, Keyomarsi K
and Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent
inhibitor of G1 cyclin-dependent kinases. Cell. 75:805–816. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Gu Y, Turck CW and Morgan DO: Inhibition
of CDK2 activity in vivo by an associated 20K regulatory subunit.
Nature. 366:707–710. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Cheng M, Olivier P, Diehl JA, et al: The
p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators
of cyclin D-dependent kinases in murine fibroblasts. EMBO J.
18:1571–1583. 1999.
|
|
19.
|
Zhang H, Hannon GJ and Beach D:
p21-containing cyclin kinases exist in both active and inactive
states. Genes Dev. 8:1750–1758. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Soos TJ, Kiyokawa H, Yan JS, et al:
Formation of p27-CDK complexes during the human mitotic cell cycle.
Cell Growth Differ. 7:135–146. 1996.PubMed/NCBI
|
|
21.
|
Blain SW, Montalvo E and Massagué J:
Differential interaction of the cyclin-dependent kinase (Cdk)
inhibitor p27Kip1 with cyclin A-Cdk2 and cyclin D2-Cdk4. J Biol
Chem. 272:25863–25872. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
LaBaer J, Garrett MD, Stevenson LF, et al:
New functional activities for the p21 family of CDK inhibitors.
Genes Dev. 11:847–862. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Zhang Y, Fujita N and Tsuruo T:
Caspase-mediated cleavage of p21Waf1/Cip1 converts cancer cells
from growth arrest to undergoing apoptosis. Oncogene. 18:1131–1138.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Wang Z, Su ZZ, Fisher PB, Wang S, VanTuyle
G and Grant S: Evidence of a functional role for the
cyclin-dependent kinase inhibitor p21(WAF1/CIP1/MDA6) in the
reciprocal regulation of PKC activator-induced apoptosis and
differentiation in human myelomonocytic leukemia cells. Exp Cell
Res. 244:105–116. 1998. View Article : Google Scholar
|
|
25.
|
Gartel AL and Tyner AL: The role of the
cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer
Ther. 1:639–649. 2002.PubMed/NCBI
|
|
26.
|
Narayanan BA, Geoffroy O, Willingham MC,
Re GG and Nixon DW: p53/p21(WAF1/CIP1) expression and its possible
role in G1 arrest and apoptosis in ellagic acid treated cancer
cells. Cancer Lett. 136:215–221. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Okaichi K, Wang LH, Sasaki J, Saya H, Tada
M and Okumura Y: A point mutation of human p53, which was not
detected as a mutation by a yeast functional assay, led to
apoptosis but not p21Waf1/Cip1/Sdi1 expression in response to
ionizing radiation in a human osteosarcoma cell line, Saos-2. Int J
Radiat Oncol Biol Phys. 45:975–980. 1999. View Article : Google Scholar
|
|
28.
|
Yan Q and Sage EH: Transforming growth
factor-beta1 induces apoptotic cell death in cultured retinal
endothelial cells but not pericytes: association with decreased
expression of p21waf1/cip1. J Cell Biochem. 70:70–83. 1998.
View Article : Google Scholar
|
|
29.
|
Gunia S, Kakies C, Erbersdobler A,
Hakenberg OW, Koch S and May M: Expression of p53, p21 and cyclin
D1 in penile cancer: p53 predicts poor prognosis. J Clin Pathol.
65:232–236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Taghavi N, Biramijamal F, Sotoudeh M, et
al: Association of p53/p21 expression with cigarette smoking and
prognosis in esophageal squamous cell carcinoma patients. World J
Gastroenterol. 16:4958–4967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Siu MK, Chan HY, Kong DS, et al:
p21-activated kinase 4 regulates ovarian cancer cell proliferation,
migration, and invasion and contributes to poor prognosis in
patients. Proc Natl Acad Sci USA. 107:18622–18627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Mineo TC, Ambrogi V, Cufari ME and Pompeo
E: May cyclooxygenase-2 (COX-2), p21 and p27 expression affect
prognosis and therapeutic strategy of patients with malignant
pleural mesothelioma? Eur J Cardiothorac Surg. 38:245–252. 2010.
View Article : Google Scholar
|
|
33.
|
Ogino S, Nosho K, Shima K, et al: p21
expression in colon cancer and modifying effects of patient age and
body mass index on prognosis. Cancer Epidemiol Biomarkers Prev.
18:2513–2521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Hu TH, Tai MH, Chuah SK, et al: Elevated
p21 expression is associated with poor prognosis of rectal stromal
tumors after resection. J Surg Oncol. 98:117–123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Hafkamp HC, Mooren JJ, Claessen SM, et al:
P21 Cip1/WAF1 expression is strongly associated with HPV-positive
tonsillar carcinoma and a favorable prognosis. Mod Pathol.
22:686–698. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Pasz-Walczak G, Kordek R and Faflik M: P21
(WAF1) expression in colorectal cancer: correlation with P53 and
cyclin D1 expression, clinicopathological parameters and prognosis.
Pathol Res Pract. 197:683–689. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Fillies T, Woltering M, Brandt B, et al:
Cell cycle regulating proteins p21 and p27 in prognosis of oral
squamous cell carcinomas. Oncol Rep. 17:355–359. 2007.PubMed/NCBI
|
|
38.
|
Adida C, Crotty PL, McGrath J, Berrebi D,
Diebold J and Altieri DC: Developmentally regulated expression of
the novel cancer anti-apoptosis gene survivin in human and mouse
differentiation. Am J Pathol. 152:43–49. 1998.PubMed/NCBI
|
|
39.
|
Ambrosini G, Adida C, Sirugo G and Altieri
DC: Induction of apoptosis and inhibition of cell proliferation by
survivin gene targeting. J Biol Chem. 273:11177–11182. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Li F, Ackermann EJ, Bennett CF, et al:
Pleiotropic cell-division defects and apoptosis induced by
interference with survivin function. Nat Cell Biol. 1:461–466.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Kobayashi K, Hatano M, Otaki M, Ogasawara
T and Tokuhisa T: Expression of a murine homologue of the inhibitor
of apoptosis protein is related to cell proliferation. Proc Natl
Acad Sci USA. 96:1457–1462. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Li F, Ambrosini G, Chu EY, et al: Control
of apoptosis and mitotic spindle checkpoint by survivin. Nature.
396:580–584. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Suzuki A and Shiraki K: Tumor cell ‘dead
or alive’: caspase and survivin regulate cell death, cell cycle and
cell survival. Histol Histopathol. 16:583–593. 2001.
|
|
45.
|
Fukuda S, Mantel CR and Pelus LM: Survivin
regulates hematopoietic progenitor cell proliferation through
p21WAF1/Cip1-dependent and -independent pathways. Blood.
103:120–127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Tanaka C, Uzawa K, Shibahara T, Yokoe H,
Noma H and Tanzawa H: Expression of an inhibitor of apoptosis,
survivin, in oral carcinogenesis. J Dent Res. 82:607–611. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Li C, Li Z, Zhu M, et al:
Clinicopathological and prognostic significance of survivin
over-expression in patients with esophageal squamous cell
carcinoma: a meta-analysis. PloS One. 7:e447642012. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Ekeblad S, Lejonklou MH, Stálberg P and
Skogseid B: Prognostic relevance of survivin in pancreatic
endocrine tumors. World J Surg. 36:1411–1418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Chang BD, Watanabe K, Broude EV, et al:
Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression:
implications for carcinogenesis, senescence, and age-related
diseases. Proc Natl Acad Sci USA. 97:4291–4296. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Schmidt M and Fan Z: Protection against
chemotherapy-induced cytotoxicity by cyclin-dependent kinase
inhibitors (CKI) in CKI-responsive cells compared with
CKI-unresponsive cells. Oncogene. 20:6164–6171. 2001. View Article : Google Scholar
|