Introduction
Renal cell carcinoma (RCC) is the most common
malignant tumor of the kidney and its incidence has been increasing
worldwide (1). Despite extensive
clinical trials, the pathogenesis and determination of prognoses
for patients with RCC have remained poor. Although the pathogenesis
of RCC is not completely understood, the dysfunction of immune
regulation mechanisms, such as abnormalities of the immune
homeostasis-maintaining genes, appears to be an important
contributor to the development of RCC (2,3).
Two classes of immune homeostasis-maintaining genes
have been identified (4). The first
class have functions in limiting the strength of immune cell
activation and expansion. The second class controls cell death and
includes Fas, Bim, Bax and caspases 8 and 10 (5). The majority of genes that maintain
immune homeostasis by controlling cell death are also involved in
the pathogenesis of RCC. Tumor necrosis factor-α-induced protein
(TNFAIP) 8 like-2 (TNFAIP8L2 or TIPE2), a negative regulator of
innate immunity and cellular immunity, shares considerable sequence
homology with members of the TNFAIP8 family (6). It is preferentially expressed in
lymphoid- and marrow-derived cells and its deletion in mice leads
to multi-organ inflammation. TNFAIP8 is also aberrantly expressed
in various human cancer cell lines, with higher levels in K562
chronic myelogenous leukemia cells, MOLT 4 lymphoblastic leukemia
cells and A549 lung carcinoma and lower levels in SW480 colorectal
adenocarcinoma cells (7). In
addition, TNFAIP8 expression is induced by TNF-α, high glucose
stimulation and activation of nuclear factor (NF)-κB in various
cells (7–11). At present, little is known about the
mechanism behind the role of TNFAIP8 in carcinogenesis. The common
hypothesis is that TNFAIP8 may be an inhibitor of caspase-mediated
apoptosis. The overexpression of TNFAIP8 in cancer cells may
inhibit TNF-induced apoptosis by inhibiting caspase-8 and caspase-3
activity (7,9), while the depletion of TNFAIP8 enhances
cell death (10,12). Considering that normal expression
levels of the TIPE2 gene in the immune system are essential for
maintaining immune homeostasis, we hypothesized that the expression
of the TIPE2 gene in RCC patients may be different from that of
normal individuals and thus may be involved in the pathogenesis of
RCC. Therefore, in the present study, the TIPE2 mRNA expression
levels in cancer tissue samples from RCC patients were compared
with controls, and the correlations between TIPE2 mRNA expression
levels and the TNM staging of RCC and the mRNA expression levels of
myxoma resistance protein (MX1), an interferon (IFN)-I-inducible
gene, were analyzed (13). The
upregulation of TIPE2 mRNA expression was observed in the RCC
patients and was markedly associated with the IFN-I levels and TNM
staging of RCC. These findings suggest that TIPE2 may be involved
in the pathogenesis of RCC.
Materials and methods
Human subjects
A total of 46 patients with clear cell RCC
fulfilling the RCC criteria of the World Health Organization (WHO;
revised in 2004) (14) were
enrolled in the present study. As controls, 39 gender- and
age-matched renal contusion patients who required surgical
extractions were recruited. None of the control patients had any
tumors or other abnormal conditions. The RCC staging was assessed
with the TNM staging system for kidney cancer revised by the
American Joint Committee On Cancer (AJCC) (15). Tumor and control renal tissues were
sampled from all patients by surgery. All 46 patients were
confirmed to have RCC through pathological examinations. This study
was approved by the ethics committee of Qingdao Municipal Hospital,
Qingdao, China. All patients gave informed written consent prior to
the initiation of the study. The characteristics of the patients
are shown in Table I.
 | Table I.Characteristics of the studied
subjects. |
Table I.
Characteristics of the studied
subjects.
| Characteristic | RCC patients
(n=46) | Healthy controls
(n=39) |
|---|
| Female, n (%) | 20 (43.47) | 16 (41.03) |
| Male, n (%) | 26 (56.53) | 23 (58.97) |
| Age (years) | 52.4±13.1 | 49.2±12.8 |
| LDH (U/l) | 129±30 | 115±32 |
| Ca (mmol/l) | 2.47±0.68 | 2.29±0.67 |
| BUN (mmol/l) | 6.2±1.3 | 5.9±1.4 |
| Cr
(μmol/l) | 81±24 | 74±19 |
| RBC (1012
cells/l) | 3.46±1.23 | 4.42±1.17 |
| WBC (109
cells/l) | 5.63±1.99 | 5.87±2.16 |
| HGB (g/l) | 120±25 | 118±23 |
| PLT (109
cells/l) | 201±72 | 197±90 |
| PT (sec) | 12.30±2.40 | 11.59±2.63 |
| APTT (sec) | 26.40±3.30 | 24.40±3.79 |
| ESR (mm/h) | 18±6 | 14±4 |
| TNM staging, n
(%) | | |
| I | 12 (26.08) | - |
| II | 10 (21.73) | - |
| III | 18 (39.13) | - |
| IV | 6 (13.04) | - |
Laboratory assessments
For the RCC patients, the biochemical parameters of
the serum, including lactate dehydrogenase (LDH), calcium (Ca),
blood urea nitrogen (BUN) and creatinine (Cr), were analyzed using
an automatic biochemical analyzer (Hitachi P7600; Hitachi, Tokyo,
Japan). The hematological indices of the blood, including levels of
red blood cells (RBC), white blood cells (WBC), hemoglobin (HGB)
and platelets (PLT), were analyzed by an automatic hematological
analyzer (Sysmex XS-800i; Sysmex, Kobe, Japan). The coagulation
parameters of prothrombin time (PT) and activated partial
thromboplastin time (APTT) were analyzed by an automatic
coagulation analyzer (Sysmex CA7000, Sysmex, Kobe, Japan).
Erythrocyte sedimentation rates (ESR) were analyzed with a
MONITOR-J+ analyzer (Electa Lab Srl, Forli, Italy).
RNA and cDNA preparation from RCC tissues
and controls
Tumor and control renal tissues were sampled from
all the subjects through surgery. The samples were pulverized using
liquid nitrogen. Total RNA was extracted from the samples using
TRIzol (Invitrogen, Carlsbad, CA, USA) and treated with RNase-free
DNase (Sangon Biotech, Shanghai, China) to remove genomic DNA
contamination. The RNA (1 μg) was reverse transcribed to
cDNA using a reverse transcription system kit (Sangon Biotech).
Quantitative polymerase chain reaction
(qPCR) analysis
The expression of the TIPE2 gene was evaluated by
qPCR. The serum level of IFN-1 was quantified by qPCR measurements
of the mRNA expression of the IFN-I inducible gene MX1. Primers
were synthetized as described by Li et al (4). The primers for TIPE2 were as follows:
forward, 5′-GGAACATCCAAGGCAAGACTG-3′ and reverse,
5′-AGCACCTCACTGCTTGTCTCATC-3′. The level of β-actin mRNA was also
detected as an internal control for each sample. The primers for
MX1 were as follows: forward, 5′-TGCTTATCCGTTAGCCGTGG-3′ and
reverse, 5′-CGCCAGCTCATGTGCATCT-3′. For β-actin, the following
primers were used: forward 5′-GACTACCTCATGAAGATCCTCACC-3′ and
reverse, 5′-TCTCCTTAATGTCACGCACGATT-3′. qPCR was performed using
the SYBR Green I Real-Time PCR kit in accordance with the
instructions of the manufacturer (Takara, Dalian, China) in an ABI
PRISM 7500 Sequence Detection System (Perkin-Elmer, Norwalk, CT,
USA). The amplification conditions were as follows: 95°C for 10
sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 41 sec.
Each sample was run in triplicate. The PCR products were run on an
agarose gel and all cases were confined to a single band of the
expected size. A melting curve analysis was also performed to
ensure the specificity of the products. The relative mRNA
expression levels of TIPE2 and MX1 were determined using the
comparative (2−ΔΔCt) method.
Statistical analysis
Statistical analysis was performed using the SPSS
13.0 software (SPSS, Inc., Chicago, IL, USA). The data are
expressed as the mean ± standard deviation. The differences in
TIPE2 or MX1 mRNA levels between the subject groups were analyzed
independently using Student’s t-test. A correlation analysis was
performed using Spearman’s rank test. P<0.05 was considered to
indicate a statistically significant difference. All figures were
created with GraphPad Prism software, version 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA).
Results
General condition of patients
The clinical manifestations, demographic
characteristics, TNM staging and laboratory measurements are shown
in Table I.
The median hematological indices and biochemical
parameters, including LDH, Ca, BUN and Cr levels of the RCC
patients and controls all remained similar. No significant
differences in general condition were observed between the RCC
patients and controls.
Quantification of TIPE2 mRNA expression
in RCC tissues and controls by qPCR
The expression levels of TIPE2 mRNA in the renal
tumor tissue from 46 RCC patients and 39 gender- and age-matched
controls were examined using qPCR. The results showed that the
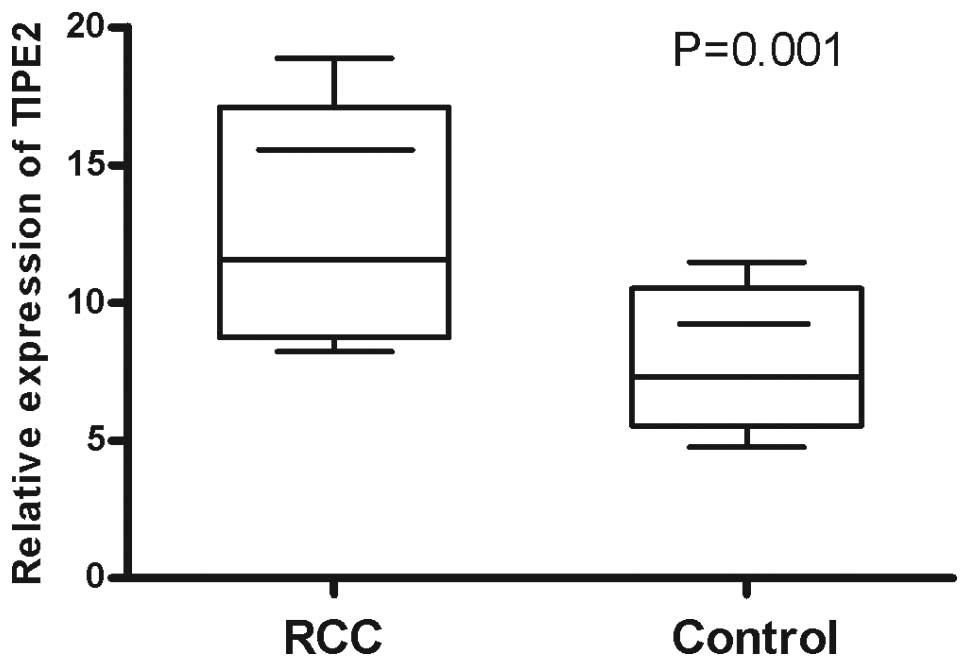
relative expression levels of TIPE2 mRNA (11.58±3.75) in the RCC
patients were significantly higher compared with the controls
(7.32±3.93; P=0.001; Fig. 1). The
correlations between TIPE2 expression and demographic
characteristics, clinical manifestations and laboratory parameters
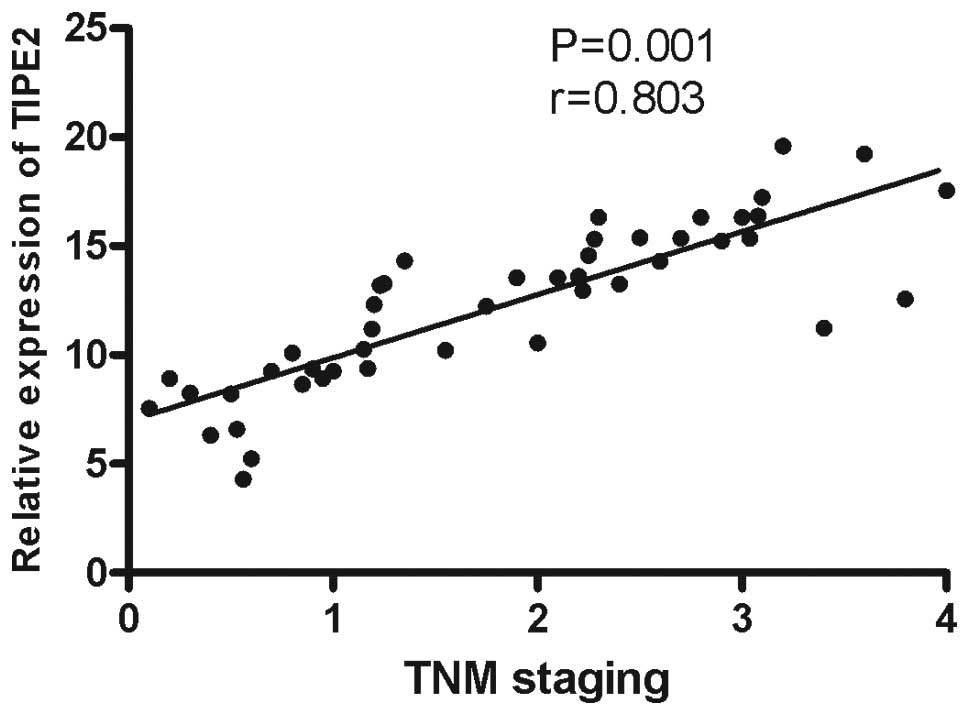
were also analyzed. The results demonstrated that the TIPE2 mRNA
expression levels were positively correlated with TNM staging
(r=0.803, P=0.001; Fig. 2). No
statistically significant associations were observed between the
TIPE2 mRNA expression levels and other characteristics, clinical
manifestations or laboratory parameters among the RCC patients.
Quantification of MX1 mRNA expression in
RCC tissues and controls by qPCR
Since all members of the IFN-I family bind to the
same receptor complex, measuring the expression of IFN-I-inducible
genes, such as MX1, by qPCR permits total IFN-I production (all
isoforms) to be estimated (2). MX1
mRNA expression levels were examined in the renal tumor tissue
samples from 46 RCC patients and 39 controls using qPCR. It was
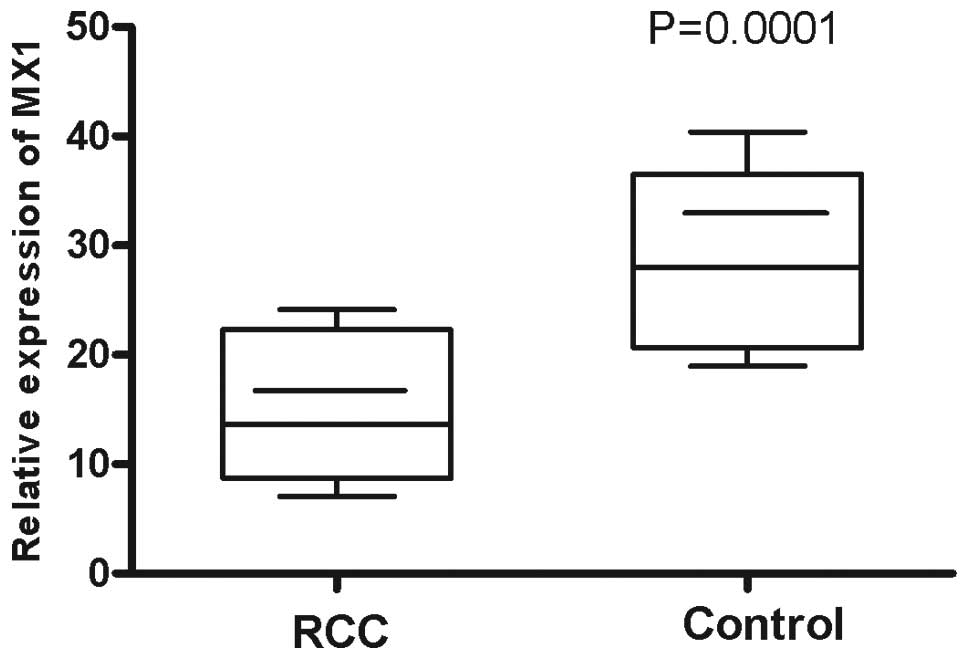
observed that the mean relative MX1 mRNA expression level
(13.65±7.34) of the RCC patients was lower compared with the
controls (27.96±9.98; P=0.0001; Fig.
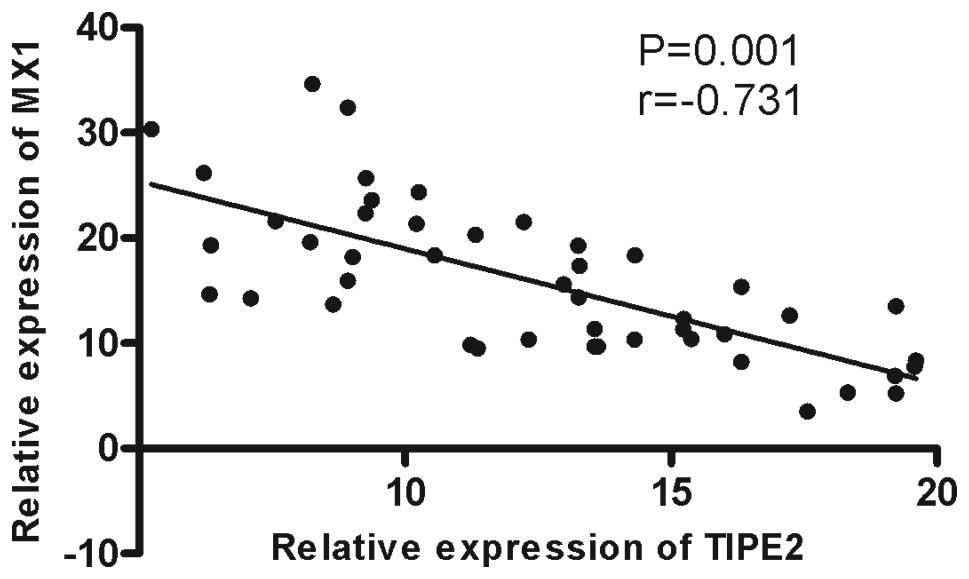
3) and a negative correlation was observed between MX1 mRNA
expression and TNM staging (r=−0.731, P=0.001; Fig. 4).
Correlation between TIPE2 mRNA expression
and MX1 mRNA expression in RCC tissues
Since IFN-I is important in the pathogenesis of RCC
and the MX1 mRNA level represents the level of IFN-I, the
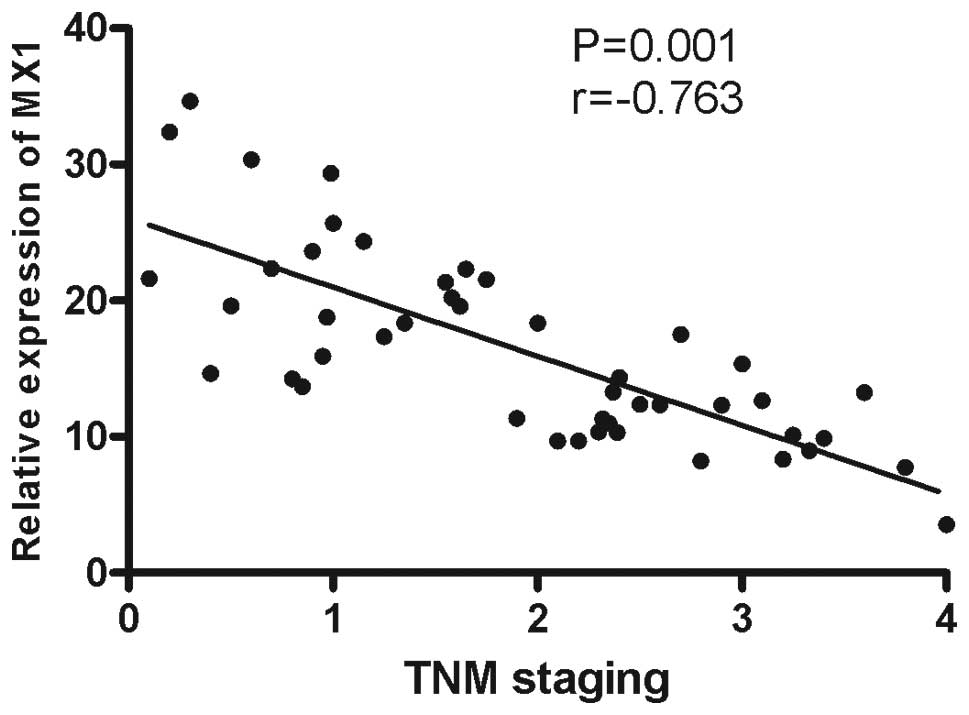
association between TIPE2 and MX1 expression was investigated. The
results showed a negative correlation between TIPE2 and MX1 mRNA
expression (r=−0.763, P=0.001; Fig.
5). This indicates that TIPE2 mRNA expression levels are
negatively correlated with the levels of IFN-I in the renal tumor
tissues of RCC patients.
Discussion
Immune homeostasis is maintained by multiple immune
regulation genes that are unable to compensate for each other
(4). The breakdown of immune
homeostasis caused by the dysfunction of these genes contributes to
tumor pathogenesis. TIPE2 is a negative regulator of innate and
cellular immunity (5). In the
present study, the upregulation of TIPE2 expression was
demonstrated via qPCR and western blotting in RCC patients, and a
positive correlation between TIPE2 expression and the TNM staging
of RCC was reported for the first time.
Studies have indicated that TNFAIP8 is an apoptosis
regulator which may be involved in oncogenesis (7,12,16–18).
Upregulation of TIPE2 expression in RCC patients may cause an
abnormal resistance to apoptosis, which would ultimately lead to
cancer. Similar to several other regulators of apoptosis, TNFAIP8
contains a death effector domain (DED) and is able to inhibit
caspase-mediated apoptosis (7). The
majority of studies support the hypothesis that TNFAIP8 is able to
inhibit TNF-α-induced caspase activation and apoptosis (7,10,12).
Experimental evidence also supports the theory that TNFAIP8 is an
oncogene in human cancers. TNFAIP8 overexpression has frequently
been observed in several types of cancer tissues, suggesting that
TNFAIP8 may be significant in oncogenesis (12,16,19).
The evaluation of a limited number of clinical specimens has
revealed higher expression levels of TNFAIP8 protein in human
breast cancer and RCC tissues compared with matched normal adjacent
tissues (16). The direct role of
TIPE2 in apoptosis remains unclear. TIPE2 was considered to contain
a DED or DED-like domain, but its structure and topology differ
from that of the DED domains contained in caspase-8 or c-FLIP
(20). It has been shown that TIPE2
binds to the Ras-interacting domain of the RalGDS family of
proteins, which are essential effectors of activated Ras (22). RT-PCR revealed no significant
differences in TIPE2 mRNA between hepatocellular carcinoma (HCC)
and adjacent tissues (21). The
development of human HCC is associated with the downregulation of
TIPE2 (21). This result is
possibly associated with inflammation since chronic HBV infection
is a major cause of HCC. The direct correlation between increased
TIPE2 expression and tumorigenesis in RCC patients requires further
investigation.
The overexpression of the TIPE2 gene may cause the
depression of proinflammatory cytokines, which characterizes RCC
disease. The levels of inflammatory cytokines, including IFN-α,
IL-2 and IFN-γ, are significantly decreased in patients with RCC,
although the mechanisms are not completely understood. TIPE2
expression in mouse macrophages negatively regulates NF-κB
signaling, inhibiting the secretion of certain inflammatory
cytokines. TIPE2-knockout and -knockdown macrophages in mice are
hypersensitive to TLR stimulation, producing more IL-6 and IL-12
compared with wild-type macrophages (5). Therefore, the upregulation of TIPE2
expression in renal cancer tissue from patients with RCC may be
wholly or partially responsible for the reduced levels of
inflammatory cytokines.
Clinical evidence has shown that the administration
of cytokines leads to regression in certain patients with RCCs.
IFNs are glycoproteins that exert antiproliferative effects on
tumor cell growth, as well as immunomodulatory and antiviral
effects (22). IFN-I induces the
unabated activation of peripheral dendritic cells (pDCs), which are
recognized as efficient stimulators of B and T lymphocytes and key
controllers of immunity and tolerance (23–25).
Since the IFN-I family includes 14 IFN-α subtypes and IFN-β, and
all subtypes bind to a single receptor, the serum levels of IFN-I
may be represented by the mRNA expression levels of MX1, an IFN-I
inducible gene (13,26,27).
The present study further analyzed the correlation between MX1 and
TIPE2 expression in RCC patients and consequently identified a
negative correlation between them. This result indicates that there
is a close association between the production of IFN-I and TIPE2
expression. Although the production of IFN-I and proinflammatory
cytokines requires TLR stimulation, the signaling pathway producing
IFN-I downstream of TLR differs from that producing proinflammatory
cytokines. IFN regulatory factors (IRFs) are required for the
production of IFN-I, while NF-κB is required for the production of
proinflammatory cytokines (28,29).
Further study is required in the future with regard to whether
TIPE2 is able to affect the signaling pathway producing IFN-I.
A question remains concerning the mechanisms
involved in the overexpression of TIPE2 mRNA in RCC patients. The
etiology of RCC involves environmental and genetic factors. The
altered expression of certain immune homeostasis-maintaining genes
is ascribed to genetic polymorphisms (4). As for the TIPE2 gene, the upregulation
of TIPE2 mRNA expression in renal tumor tissues from RCC patients
may also be due to gene polymorphisms. Therefore, investigations
into the association between the SNPs of the TIPE2 genes and RCC
pathogenesis are eagerly anticipated.
In the present study, the TIPE2 mRNA expression of
renal tumor tissues from RCC patients was analyzed prior to
treatment with any anti-tumor drugs. The emphasis of our next study
will be on investigating the potential effects of anti-tumor drugs
and surgical intervention on TIPE2 expression, and assessing the
stability of the TIPE2 expression profile over time in individual
patients.
In conclusion, the presence of one or several gene
amplifications may have prognostic significance (30). The present study indicates that the
mRNA expression levels of TIPE2 are increased in renal tumor tissue
from RCC patients compared with controls and positively correlated
with TNM staging, but negatively correlated with the levels of
IFN-I. These findings suggest a significant role for the TIPE2 gene
in the pathogenesis of RCC.
References
|
1.
|
Ku JH, Park YH, Myung JK, Moon KC, Kwak C
and Kim HH: Expression of hypoxia inducible factor-1α and 2α in
conventional renal cell carcinoma with or without sarcomatoid
differentiation. Urol Oncol. 29:731–737. 2011.
|
|
2.
|
Cavalcanti E, Gigante M, Mancini V, et al:
JAK3/STAT5/6 pathway alterations are associated with immune
deviation in CD8 T cells in renal cell carcinoma patients. J Biomed
Biotechnol. 2010:9357642010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Atkins D, Ferrone S, Schmahl GE, et al:
Down-regulation of HLA class I antigen processing molecules: an
immune escape mechanism of renal cell carcinoma? J Urol.
171:885–889. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Li D, Song L, Fan Y, et al:
Down-regulation of TIPE2 mRNA expression in peripheral blood
mononuclear cells from patients with systemic lupus erythematosus.
Clin Immunol. 133:422–427. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Sun H, Gong S, Carmody RJ, et al: TIPE2, a
negative regulator of innate and adaptive immunity that maintains
immune homeostasis. Cell. 133:415–426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Luan YY, Yao YM, Zhang L, et al:
Expression of tumor necrosis factor-α induced protein 8 like-2
contributes to the immunosuppressive property of CD4(+)CD25(+)
regulatory T cells in mice. Mol Immunol. 49:219–226. 2011.
|
|
7.
|
Kumar D, Whiteside TL and Kasid U:
Identification of a novel tumor necrosis factor-alpha-inducible
gene SCC-S2, containing the consensus sequence of a death effector
domain of fas-associated death domain-like interleukin-1
beta-converting enzyme-inhibitory protein. J Biol Chem.
275:2973–2978. 2000. View Article : Google Scholar
|
|
8.
|
Horrevoets AJ, Fontijn RD, van Zonneveld
AJ, de Vries CJ, ten Cate JW and Pannekoek H: Vascular endothelial
genes that are responsive to tumor necrosis factor-alpha in vitro
are expressed in atherosclerotic lesions, including inhibitor of
apoptosis protein-1, stannin, and two novel genes. Blood.
93:3418–3431. 1999.
|
|
9.
|
You Z, Ouyang H, Lopatin D, Polver PJ and
Wang CY: Nuclear factor-kappa B inducible death effector
domain-containing protein suppresses tumor necrosis factor-mediated
apoptosis by inhibiting caspase-8 activity. J Biol Chem.
276:26398–26404. 2001. View Article : Google Scholar
|
|
10.
|
Zhang HG, Hyde K, Page GP, et al: Novel
tumor necrosis factor alpha-regulated genes in rheumatoid
arthritis. Arthritis Rheum. 50:420–431. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Zhang S, Zhang Y, Wei X, et al: Expression
and regulation of a novel identified TNFAIP8 family is associated
with diabetic nephropathy. Biochim Biophys Acta. 1802:1078–1086.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Zhang C, Chakravarty D, Sakabe I, et al:
Role of SCC-S2 in experimental metastasis and modulation of
VEGFR-2, MMP-1, and MMP-9 expression. Mol Ther. 13:947–955. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lee PY, Li Y, Richards HB, et al: Type I
interferon as a novel risk factor for endothelial progenitor cell
depletion and endothelial dysfunction in systemic lupus
erythematosus. Arthritis Rheum. 56:3759–3769. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Ebele JN, Sauter G, Epstein JI and
Sesterhenn IA: Pathology and Genetics of Tumours of the Urinary
System and Male Genital Organs. IARC; Lyon: 2004
|
|
15.
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A III: AJCC Cancer Staging Manual. 2010, 7th
edition. Springer; New York:
|
|
16.
|
Kumar D, Gokhale P, Broustas C,
Chakravarty D, Ahmad I and Kasid U: Expression of SCC-S2, an
antiapoptotic molecule, correlates with enhanced proliferation and
tumorigenicity of MDA-MB 435 cells. Oncogene. 23:612–616. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Patel S, Wang FH, Whiteside TL and Kasid
U: Identification of seven differentially displayed transcripts in
human primary and matched metastatic head and neck squamous cell
carcinoma cell lines: implications in metastasis and/or radiation
response. Oral Oncol. 33:197–203. 1997. View Article : Google Scholar
|
|
18.
|
Yoshinaga-Hirabayashi T and Yoneda Y:
Expression of SCC in ovarian granulosa cells and cultured cells,
induced rapid structural changes in mitochondria. Ital J Anat
Embryol. 106(2 Suppl 1): 51–57. 2001.PubMed/NCBI
|
|
19.
|
Dong QZ, Zhao Y, Liu Y, Wang Y, et al:
Overexpression of SCC-S2 correlates with lymph node metastasis and
poor prognosis in patients with non-small-cell lung cancer. Cancer
Sci. 101:1562–1569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Zhang X, Wang J, Fan C, et al: Crystal
structure of TIPE2 provides insights into immune homeostasis. Nat
Struct Mol Biol. 16:89–90. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Gus-Brautbar Y, Johnson D, Zhang L, et al:
The anti-inflammatory TIPE2 is an inhibitor of the oncogenic Ras.
Mol Cell. 45:610–618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Stark GR, Kerr IM, Williams BR, Silverman
RH and Schreiber RD: How cells respond to interferons. Annu Rev
Biochem. 67:227–264. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Pascual V, Farkas L and Banchereau J:
Systemic lupus erythematosus: all roads lead to type I interferons.
Curr Opin Immunol. 18:676–682. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Koutouzov S, Mathian A and Dalloul A:
Type-I interferons and systemic lupus erythematosus. Autoimmun Rev.
5:554–562. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Hawiger D, Inaba K, Dorsett Y, et al:
Dendritic cells induce peripheral T cell unresponsiveness under
steady state conditions in vivo. J Exp Med. 194:769–779. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Feng X, Wu H, Grossman JM, et al:
Association of increased interferon-inducible gene expression with
disease activity and lupus nephritis in patients with systemic
lupus erythematosus. Arthritis Rheum. 54:2951–2962. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Zhuang H, Narain S, Sobel E, et al:
Association of anti-nucleoprotein autoantibodies with upregulation
of Type I interferon-inducible gene transcripts and dendritic cell
maturation in systemic lupus erythematosus. Clin Immunol.
117:238–250. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Colonna M: TLR pathways and IFN-regulatory
factors: to each its own. Eur J Immunol. 37:306–309. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Honda K, Yanai H, Negishi H, et al: IRF-7
is the master regulator of type-I interferon-dependent immune
responses. Nature. 434:772–777. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Lacunza E, Baudis M, Colussi AG,
Segal-Eiras A, Croce MV and Abba MC: MUC1 oncogene amplification
correlates with protein overexpression in invasive breast carcinoma
cells. Cancer Genet Cytogenet. 201:102–110. 2010. View Article : Google Scholar : PubMed/NCBI
|