Introduction
Gastric cancer is the fourth most frequently
diagnosed malignancy worldwide, accounting for 12% of all
cancer-related mortalities. In Asia and parts of South America in
particular, gastric cancer is the most common epithelial malignancy
and is a leading cause of cancer-related mortality (1,2).
Proliferation, invasiveness and metastasis are the
predominant biological characteristics of a malignant tumor, and
are closely correlated with factors such as the movement of the
tumor cells, apoptosis and the expression of metastasis-associated
genes. Matrix metalloproteinase-9 (MMP-9), tissue inhibitor of
metalloproteinases type-1 (TIMP-1) (3) and vascular endothelial growth factor
(VEGF) are important angiogenic factors that have a higher
expression level in tumor tissues. These factors induce
angiogenesis in the tumor and are important in the metastasis,
invasion and prognosis of gastric cancer (4–8).
Proliferating cell nuclear antigen (PCNA) (9) is a protein that is widely expressed in
the S phase of the cell cycle, and the levels of PCNA reflect the
proliferative activity of the tumor cells. Studies have
demonstrated that the PCNA proliferation index increases in
correlation with the histological grading and staging of the
malignant degree of progress (10,11).
Levels of PCNA are considered to be a reliable indicator of cell
proliferation, due to the correlation between PCNA levels and the
proliferative activity of tumor cells (10,12).
However, the underlying mechanisms behind this remain unclear
Nuclear factor-κB (NF-κB) has been linked to the
control of cell growth and oncogenesis. The mechanisms of NF-κB in
cancer appear to be complex, but are likely to involve the ability
of this transcription factor to control programmed cell death (PCD)
and cell cycle progression, as well as cell differentiation,
angiogenesis and cell migration. It has been demonstrated that
NF-κB is activated in cancer cells by several types of chemotherapy
and by radiation, and that in a number of instances this response
inhibits the radiotherapy and chemotherapy-induced death of the
cancer cells (13). Therefore, the
inhibition of NF-κB p65 is under investigation as a potentially
useful approach in the treatment of cancer. However, the detailed
mechanisms are poorly understood. The present study investigated
the effects of the nuclear import inhibitor, SN50, on the growth
and invasiveness of implanted SGC7901 cell tumors in nude mice, and
the relative mechanism involved.
Materials and methods
Materials
SGC7901 cells and female Balb/c nude mice (age, 4
weeks; weight, 16–18 g) were purchased from the Chinese Academy of
Sciences (Shanghai, China). RPMI-1640 medium was obtained from
Gibco (Rockville, MD, USA), and fetal bovine serum (FBS) was
provided by Hangzhou Sijiqing Biological Engineering Material Co.,
Ltd. (Hangzhou, China). Anti-MMP-9, -PCNA and -TIMP-1 monoclonal
antibodies and PCNA, TIMP-1 and VEGF were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The
streptavidin-peroxidase kit was purchased from Fuzhou Maixin
Biotechnology Development Co., Ltd. (Fuzhou, China). This study was
approved by the Ethics Committee of The Second Affiliated Hospital,
Soochow University, Suzhou, China.
Drug preparation
The SN50 (Biomol, Plymouth Meeting, PA, USA) was
diluted in sterilized distilled water to create a stock solution
that was stored in accordance with the manufacturer’s instructions.
The final concentration of the SN50 solution used was 18
μmol/l. This concentration was selected on the basis of our
previous experiments on implanted human gastric cancer SGC7901
cells in nude mice (14).
Cell culture
The SGC7901 cells were maintained in RPMI-1640
medium (Gibco) containing 10% heat-inactivated FBS (Hangzhou
Sijiqing Biological Engineering Material Co., Ltd.) and 0.03%
L-glutamine (Sigma, St. Louis, MO, USA), and incubated in a 5%
CO2 atmosphere at 37°C. Cells in the mid-log phase were
used in the experiments.
Level of inhibition of tumor growth
A transplanted tumor model was established by
injecting human SGC7901 cells (1x109 ml) into the
subcutaneous tissue of the armpit of nude mice. Ten days later, the
25 nude mice were randomly divided into five groups as follows: i)
control, ii) phosphate-buffered saline (PBS), iii) 5 days after
SN50 treatment, iv) 10 days after SN50 treatment and v) 15 days
after SN50 treatment. Then, 0.2 ml normal saline solution, 1.5
mg/kg PBS or SN50 (18 μmol/l) were directly injected
adjacent to the tumor three times, at 2-day intervals. Changes in
tumor volume were calculated using the following formula: V = (π/6)
x abc, where a is the length of the tumor, b is the width of the
tumor and c is the depth of the tumor). These changes were measured
at 5, 10 and 15 days after the SN50 treatment. The level of
inhibition of tumor growth in each group was calculated as follows:
level of inhibition of tumor growth = [C(V1-V0) − T(V1-V0)] /
C(V1-V0) x 100, where C is the control group, T is the treatment
group, V1 is the volume prior to treatment (mm3) and V0
is the volume following treatment (mm3).
Hematoxylin and eosin (HE), and
immunohistochemical staining
Tumor specimens were taken from areas adjacent to
the margins of the tumors and from central areas. The specimens
were formalin-fixed, paraffin-embedded and pathologically diagnosed
as gastric carcinoma. The specimens were then evaluated by HE
staining for a conventional histological assessment. The
histological characteristics were reviewed by two pathologists.
The tumor samples were cut into 4-μm thick
slices and fixed in acetone. Following washing in PBS, the slices
were incubated in 0.3% H2O2 solution at room
temperature for 5 min. The slices were then incubated with
anti-TIMP-1, -MMP-9, -PCNA or -VEGF monoclonal antibodies at a
1:300 dilution at 4°C overnight. Following further washing in PBS,
a second antibody, biotinylated anti-rat immunoglobulin G (IgG),
was added and the cells were incubated at room temperature for 1 h.
The cells were then washed again in PBS, avidin-biotin complex
(ABC) compound was added and the cells were subsequently incubated
at room temperature for 10 min. 3,3′-Diaminobenzidine (DAB) was
used as the chromogen. Following incubation, the brown color
signifying the presence of antigens binding to antibodies was
detected by light microscopy. The controls were prepared in the
same manner as the experimental group, with the exception of the
incubation with the primary antibody. The positive rate (PR) of
protein expression was calculated as follows: PR = (number of
positive cells / total number of cells) x 100.
Immunohistochemical assessment
The cytoplasm of the cells containing MMP-9, PCNA,
TIMP-1 and VEGF was brown in appearance. The immunohistochemical
staining was independently evaluated by two pathologists, who were
blinded to the clinical data. In total, 200 cells were selected
under the microscope to evaluate the stained cell number against
the total cell number in the field. Based on the positive cell
number, the criteria were set as follows: negative −, <10%
positive cells; +, 11–50% positive cells; ++, 51–75% positive
cells; and +++, >75% positive cells. The staining results for
the presence of MMP-9, PCNA, TIMP-1 and VEGF were classified into
negative (staining of ≤10% of cells) or positive (staining of
>10% of cells) results.
Statistical analysis
Data are presented as mean ± standard deviation. The
statistical analysis was carried out using an ANOVA, followed by
Dunnett’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of SN50 on tumor growth
SGC7901 cells (1x109) were injected
subcutaneously into the armpits of nude mice. Within 1 week,
visible tumors had developed at the injection sites. To determine
the therapeutic effectiveness of the SN50, intratumoral injections
of SN50 were administered once the volume of the implanted tumor
reached 20 mm3, and were repeated every 2 days three
times in total. As shown in Table
I, SN50 suppressed tumor growth compared with the control group
(P<0.01). No gross adverse effects, e.g. the loss of body
weight, were observed during the experimental period. Furthermore,
SN50 inhibited the proliferation of the implanted human gastric
cancer SGC7901 cells in the nude mice, in a time-dependent fashion.
The level of inhibition of the tumors were 8.2±2.1, 19.7±1.6 and
28.3±2.6% following treatment with the SN50 for 5, 10 and 15 days,
respectively (Table I).
 | Table I.Inhibitory effect of SN50 on implanted
tumors in nude mice. |
Table I.
Inhibitory effect of SN50 on implanted
tumors in nude mice.
| Group | Number of animals
| Level of inhibition
(%) |
|---|
| Start | End |
|---|
| Control | 5 | 5 | 0 |
| PBS | 5 | 5 | 1.65±0.12 |
| SN50 | 15 | 15 | |
| 5 days | 5 | 5 | 8.2±2.1 |
| 10 days | 5 | 5 | 19.7±1.6a |
| 15 days | 5 | 5 | 28.3±2.6a |
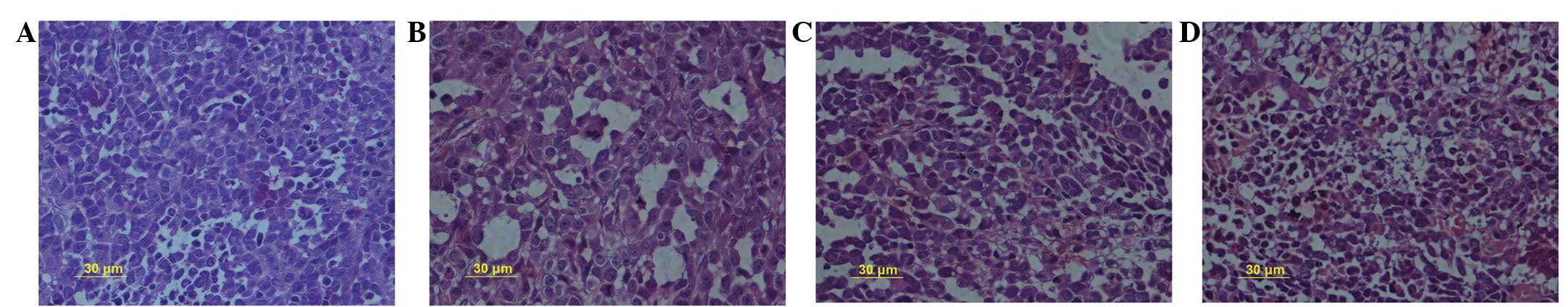
SN50 inhibits cell proliferation and
induces cell death of transplanted SGC7901 tumor cells
Treatment of the SGC7901 cells with SN50 for 5, 10
and 15 days produced intensive HE staining, indicating apoptosis.
An increase in cell death was observed in correlation with the
treatment period of the tumors (Fig.
1). Five days after the SN50 treatment, the level of inhibition
was 13.5±2.3%. The level of inhibition increased as the experiment
progressed, reaching 25.6±3.1% on day 10 and 32.9±2.7% on day 15
following the SN50 treatment. The results indicated that 18
μol/l SN50 induced cell death (Fig. 1). It was observed that SN50
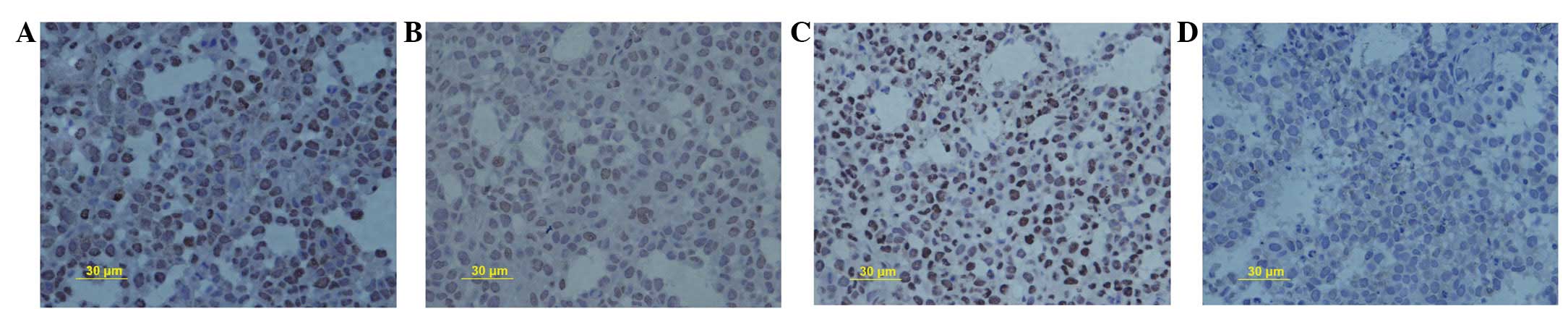
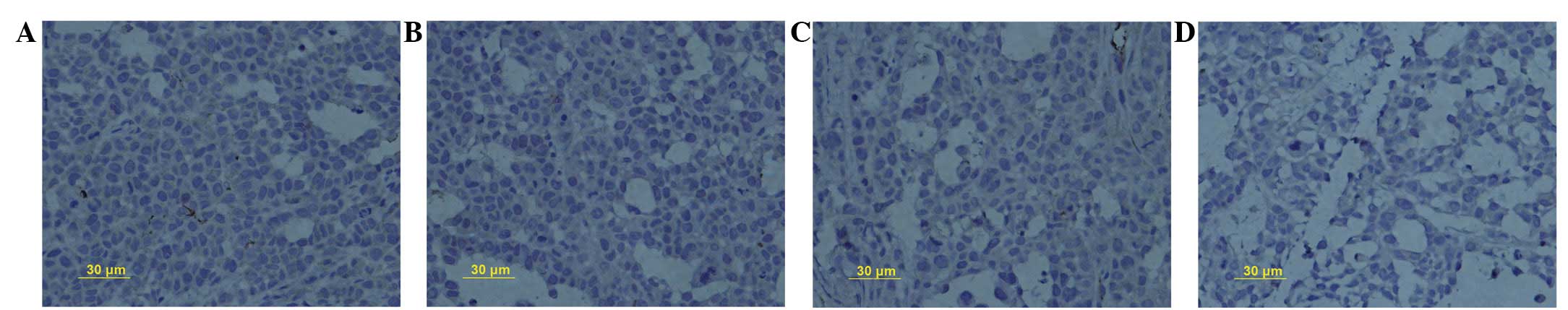
inhibited cell proliferation by decreasing PCNA protein expression
from 59.2±2.4% in the control group to 46.3±1.2, 37.5±1.9 and
28.3±1.6% in the experimental groups, following treatment with 18
μmol/l SN50 for 5, 10 and 15 days, respectively (Fig. 2A–D, respectively).
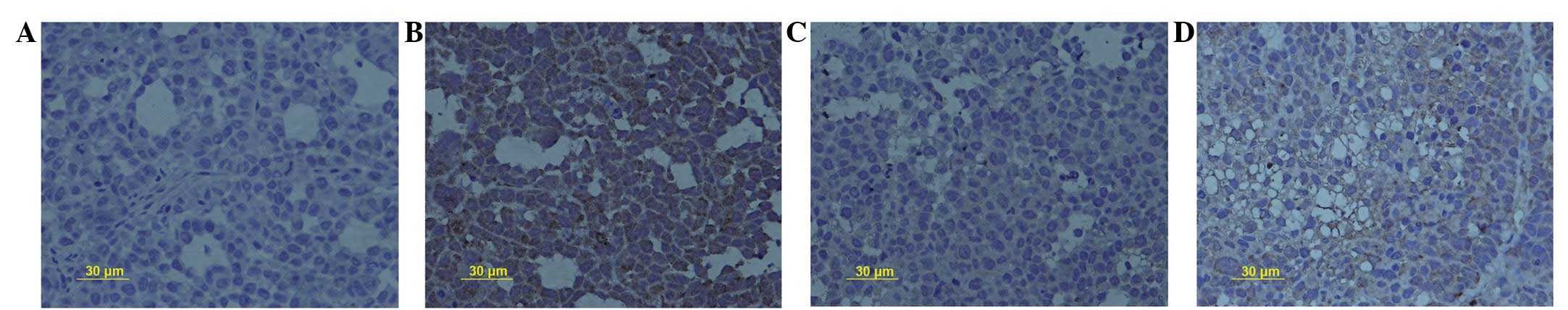
SN50 inhibits the expression of MMP-9
protein
Positive staining for MMP-9 protein was distributed
in the cell membrane and cytoplasm. The PR for MMP-9 protein
expression decreased from 46.2±2.1% in the control group to
33.7±1.3, 21.6±0.7 and 9.3±1.2% in the experimental groups,
following treatment with 18 μmol/l SN50 for 5, 10 and 15
days, respectively (Fig. 3A–D,
respectively). Significant differences were observed in MMP-9
protein expression between the 18 μmol/l SN50 group and the
control group at every time-point (P<0.05).
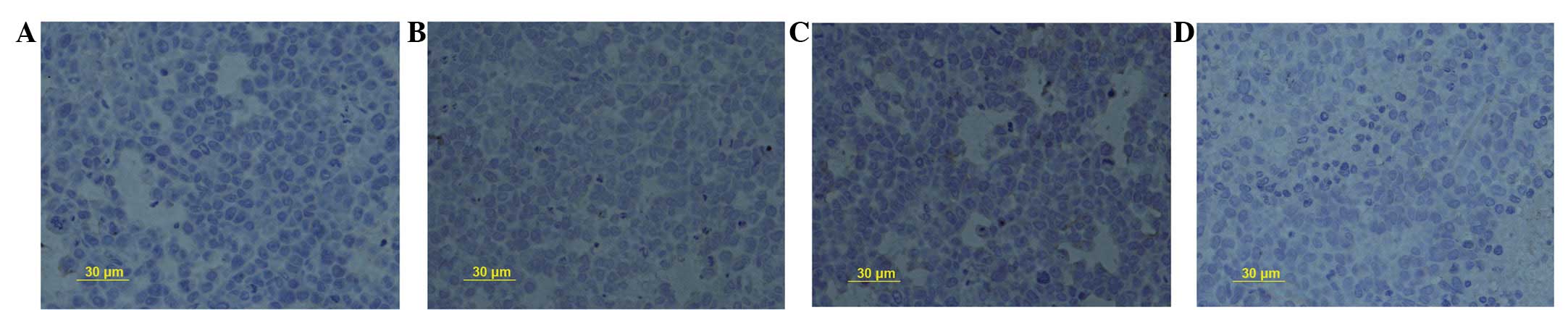
SN50 upregulates the expression of TIMP-1
protein
Positive staining was observed for TIMP-1 protein in
the cytoplasm in the healthy mucosa adjacent to the cancer cells.
The PR for TIMP-1 protein was upregulated from 23.2±2.1% in the
control group to 35.4±2.0, 47.9±1.7 and 31.9±2.3% following
treatment with SN50 for 5, 10 and 15 days, respectively (Fig. 4A–D, respectively). Significant
differences were observed in TIMP-1 protein expression between the
18 μmol/l SN50 group and the control group at every
time-point (P<0.05).
SN50 inhibits the expression of VEGF
The PR for VEGF protein expression indicated that
the expression of VEGF was downregulated from 46.2±2.3% in the
control group to 28.7±1.2, 16.3±1.4 and 12.1±2.6% in the
experimental groups, following treatment with 18 μmol/l SN50
for 5, 10 and 15 days, respectively (Figs. 5A–D, respectively). A significant
difference in positive expression was observed between the 18
μmol/l SN50 group and the control group at every time-point
(P<0.05).
Discussion
At present, there are relatively few
chemotherapeutic drugs that are effective in the treatment of human
gastric carcinoma (15). As a
result, there is an increasing body of interest in the use of drugs
that prevent the invasion of cancer cells. NF-κB signaling pathways
are important in a variety of physiological and pathological
processes. One of the functions of NF-κB is the promotion of cell
survival through the induction of target genes, whose products
inhibit components of the apoptotic machinery in normal and
cancerous cells. Regardless of the mechanism, numerous cancer
cells, of either epithelial or hematopoietic origin, use NF-κB to
achieve a resistance to anticancer drugs, radiation and death
cytokines. Hence, the inhibition of NF-κB activation offers a
future potential strategy for the treatment of different
malignancies, and may induce cell death in gastric cancer SGC7901
cells (16–18).
Tumor metastasis involves a series of complex
processes in which numerous gene products feature. MMPs, which are
important in the breakdown of the extracellular matrix (ECM), are
overexpressed in malignant tumors and have been demonstrated to
contribute to tumor proliferation, invasion and metastasis
(19). Among the MMPs, MMP-9 has a
close association with tumor metastasis, and is considered, in
particular, to be an important factor in facilitating invasion and
metastasis in gastric carcinoma (20–22).
TIMPs (TIMP-1, -2, -3 and -4) have been demonstrated to be the key
regulators of MMP activity and ECM degradation (23). The MMP inhibitors, TIMP-1 and
TIMP-2, have been implicated in several tumorigenic processes,
including the development, invasion and metastasis of bronchial
cancer (24–28). Additionally, the level of PCNA
reflects the proliferative activity of the tumor cells, and is
considered to be a reliable indicator of cell proliferation.
VEGF acts to accelerate the formation of blood
vessels, and also plays a vital role in tumor-associated
microvascular invasion (29–31).
It has been demonstrated that tumor metastasis is accelerated by
the presence of VEGF, which is highly expressed in gastric
carcinomas. VEGF may therefore be used as a marker of a poor
prognosis in gastric carcinoma patients (32–34).
In the present study, there was a significant
difference in the protein expression of MMP-9, VEGF, TIMP-1 and
PCNA between the experimental treatment and control groups,
respectively (P<0.05), indicating that SN50 may have inhibited
the expression of MMP-9, PCNA and VEGF and upregulated the
expression of TIMP-1. It was also demonstrated that SN50 inhibited
cell proliferation and induced apoptosis in the implanted human
SGC7901 gastric cancer cells, thus demonstrating the cytotoxic
effects of SN50. In vitro invasion assays and in vivo
nude mice assays suggested that SN50 had the potential to inhibit
the invasion and metastasis of gastric cancer. This may have been
due to the decrease in the protein expression of MMP-9, PCNA and
VEGF, and the increase in the TIMP-1 protein expression induced by
SN50, in combination with the cytotoxicity of SN50 towards the
tumor cells. Furthermore, no gross adverse effects, e.g. the loss
of body weight, were observed during the experimental period. These
results indicate that the inhibition of NF-KB p65 is a potent and
safe strategy for treating gastric cancer, and thus suggest that
the development of SN50-based therapeutics may be an approach for
the next generation of gastric cancer treatment.
References
|
1.
|
Xing CG, Zhu BS, Liu HH, Lin F, Yao HH,
Liang ZQ and Qin ZH: LY294002 induces p53-dependent apoptosis of
SGC7901 gastric cancer cells. Acta Pharmacol Sin. 29:489–498. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Wu CY, Wang CJ, Tseng CC, Chen HP, Wu MS,
Lin JT, Inoue H and Chen GH: Helicobacter pylori promote
gastric cancer cells invasion through a NF-kappaB and
COX-2-mediated pathway. World J Gastroenterol. 11:3197–3203. 2005.
View Article : Google Scholar
|
|
3.
|
Fernandez HA, Kallenbach K, Seghezzi G,
Grossi E, Colvin S, Schneider R, Mignatti P and Galloway A:
Inhibition of endothelial cell migration by gene transfer of tissue
inhibitor of metalloproteinases-1. J Surg Res. 82:156–162. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Lazăr D, Tăban S, Raica M, Sporea I,
Cornianu M, Goldiş A and Vernic C: Immunohistochemical evaluation
of the tumor neoangiogenesis as a prognostic factor for gastric
cancers. Rom J Morphol Embryol. 49:137–48. 2008.PubMed/NCBI
|
|
5.
|
Wang J, Tian XF, Wang S, Ma LF and Yao JH:
Correlation between expression of matrix metalloproteinase-2,
matrix metal-loproteinase-9 and angiogenesis in gastric cancer.
Chin J Cancer Res. 17:283–287. 2005. View Article : Google Scholar
|
|
6.
|
Sun WH, Sun YL, Fang RN, Shao Y, Xu HC,
Xue QP, Ding GX and Cheng YL: Expression of cyclooxygenase-2 and
matrix metalloproteinase-9 in gastric carcinoma and its correlation
with angiogenesis. Jpn J Clin Oncol. 35:707–713. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Gerber HP and Ferrara N: The role of VEGF
in normal and neoplastic hematopoiesis. J Mol Med (Berl). 81:20–31.
2003.PubMed/NCBI
|
|
8.
|
Vacca A, Ria R, Ribatti D, Semeraro F,
Djonov V, Di Raimondo F and Dammacco F: A paracrine loop in the
vascular endothelial growth factor pathway triggers tumor
angiogenesis and growth in multiple myeloma. Haematologica.
88:176–185. 2003.PubMed/NCBI
|
|
9.
|
Hall PA, Levison DA, Woods AL, et al:
Proliferating cell nuclear antigen (PCNA) immunolocalization in
paraffin sections: an index of cell proliferation with evidence of
deregulated expression in some neoplasms. J Pathol. 162:285–294.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Luque I and Gélinas C: Rel/NF-kappa B and
I kappa B factors in oncogenesis. Semin Cancer Biol. 8:103–111.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Haerslev T and Jacobsen GK: Proliferating
cell nuclear antigen in breast carcinomas. An immunohistochemical
study with correlation to histopathological features and prognostic
factors. Virchows Arch. 424:39–46. 1994.PubMed/NCBI
|
|
12.
|
Jónsson ZO and Hübscher U: Proliferating
cell nuclear antigen: more than a clamp for DNA polymerases.
Bioessays. 19:967–975. 1997.PubMed/NCBI
|
|
13.
|
Baldwin AS: Control of oncogenesis and
cancer therapy resistance by the transcription factor NF-kappaB. J
Clin Invest. 107:241–246. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Zhu B, Xing C, Lin F, Fan X, Zhao K and
Qin Z: Blocking NF-κB nuclear translocation leads to p53-related
autophagy activation and cell apoptosis. World J Gastroenterol.
17:478–487. 2011.
|
|
15.
|
Zhou HB, Chen JM, Cai JT, Du Q and Wu CN:
Anticancer activity of genistein on implanted tumor of human SG7901
cells in nude mice. World J Gastroenterol. 14:627–631. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Wang CY, Guttridge DC, Mayo MW and Baldwin
AS Jr: NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1
to preferentially suppress chemotherapy-induced apoptosis. Mol Cell
Biol. 19:5923–5929. 1999.PubMed/NCBI
|
|
17.
|
Wang CY, Mayo MW, Korneluk RG, Goeddel DV
and Baldwin AS Jr: NF-kappaB antiapoptosis: induction of TRAF1 and
TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation.
Science. 281:1680–1683. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Mitsiades N, Mitsiades CS, Poulaki V,
Anderson KC and Treon SP: Intracellular regulation of tumor
necrosis factor-related apoptosis-inducing ligand-induced apoptosis
in human multiple myeloma cells. Blood. 99:2162–2171. 2002.
View Article : Google Scholar
|
|
19.
|
Egeblad M and Werb Z: New functions for
the matrix metal-loproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kabashima A, Yao T, Sugimachi K and
Tsuneyoshi M: Relationship between biologic behavior and phenotypic
expression in intramucosal gastric carcinomas. Hum Pathol.
33:80–86. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Cai H, Kong ZR and Chen HM: Matrix
metalloproteinase-2 and angiogenesis in gastric cancer. Ai Zheng.
21:25–28. 2002.(In Chinese).
|
|
22.
|
Takahashi Y, Kitadai Y, Ellis LM, Bucana
CD, Fidler IJ and Mai M: Multiparametric in situ mRNA hybridization
analysis of gastric biopsies predicts lymph node metastasis in
patients with gastric carcinoma. Jpn J Cancer Res. 93:1258–1265.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Gomez DE, Alonso DF, Yoshiji H and
Thorgeirsson UP: Tissue inhibitors of metalloproteinases:
structure, regulation and biological functions. Eur J Cell Biol.
74:111–122. 1997.PubMed/NCBI
|
|
24.
|
Brand K: Cancer gene therapy with tissue
inhibitors of metal-loproteinases (TIMPs). Curr Gene Ther.
2:255–271. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Jiang Y, Goldberg ID and Shi YE: Complex
roles of tissue inhibitors of metalloproteinases in cancer.
Oncogene. 21:2245–2252. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Chang C and Werb Z: The many faces of
metalloproteases: cell growth, invasion, angiogenesis and
metastasis. Trends Cell Biol. 11:S37–S43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Giannelli G and Antonaci S: Gelatinases
and their inhibitors in tumor metastasis: from biological research
to medical applications. Histol Histopathol. 17:339–345.
2002.PubMed/NCBI
|
|
28.
|
Yoon SO, Park SJ, Yun CH and Chung AS:
Roles of matrix metal-loproteinases in tumor metastasis and
angiogenesis. J Biochem Mol Biol. 36:128–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Giavazzi R, Sennino B, Coltrini D,
Garofalo A, Dossi R, Ronca R, Tosatti MP and Presta M: Distinct
role of fibroblast growth factor-2 and vascular endothelial growth
factor on tumor growth and angiogenesis. Am J Pathol.
162:1913–1926. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Ferrara N: Role of vascular endothelial
growth factor in physiologic and pathologic angiogenesis:
therapeutic implications. Semin Oncol. 29:10–14. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Bellamy WT: Expression of vascular
endothelial growth factor and its receptors in multiple myeloma and
other hematopoietic malignancies. Semin Oncol. 28:551–559. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Tian X, Song S, Wu J, Meng L, Dong Z and
Shou C: Vascular endothelial growth factor: acting as an autocrine
growth factor for human gastric adenocarcinoma cell MGC803. Biochem
Biophys Res Commun. 286:505–512. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Mao ZB, Xiao MB, Huang JF, Ni HB, Ni RZ,
Wei Q and Zhang H: Expression of VEGF and its signification in
serum of gastric cancer. Shijie Huaren Xiaohua Zazhi. 10:1220–1221.
2002.
|
|
34.
|
Lou G, Gao Y, Ning XM and Zhang QF:
Expression and correlation of CD44v6, vascular endothelial growth
factor, matrix metalloproteinase-2, and matrix metalloproteinase-9
in Krukenberg tumor. World J Gastroenterol. 11:5032–5036. 2005.
|