Introduction
MPE is a rare benign variant of ependymoma that
occurs most commonly in the cauda equina and filum terminale of the
spinal cord (1,2). The majority of patients with MPEs are
young adults and only a limited number of MPEs have been reported
in elderly patients. MPEs are considered to represent grade I
tumors characterized by slow growth. The tumor is often intradural,
although local invasion and distant metastases are occasionally
observed (3,4). The typical histopathological features
of MPEs have been well described, whereas reports of hyaline
changes and low cellular variants are rarely published. The current
study presents an unusual case of MPE with marked hyaline
degeneration in an elderly male. The clinicopathological
observations of the case are described and analyzed. Written
informed consent was obtained from the patient.
Case report
Clinical presentation and diagnosis
A 72-year-old male presented with a 10-month history
of lower back pain and dysesthesia at the lower abdominal level.
The pain was of sudden onset with radiation to the legs. Weakness
in the legs was also reported and the clinical signs were worsened
by sitting. There was no history of trauma. The patient showed
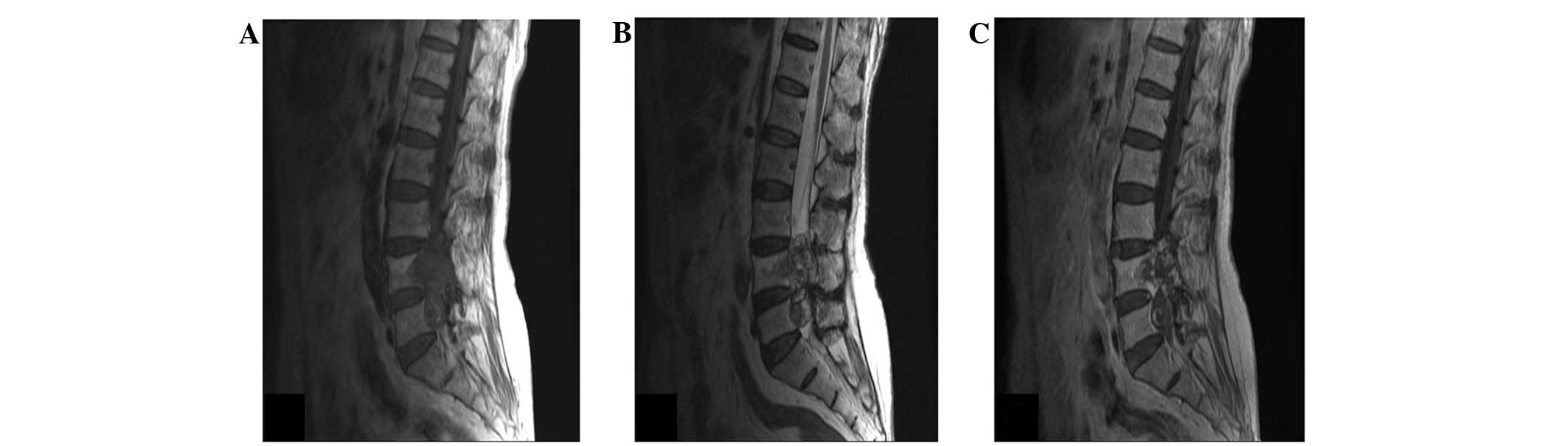
intermittent claudication after walking 200 m. Magnetic resonance
imaging revealed a mass involving the L4 and L5 vertebrae with
local bone destruction. The mass was hypointense on T1-weighted
images, hyperintense on T2-weighted images and enhanced
heterogeneously on post-contrast T1-weighted images (Fig. 1A–C). A total resection was performed
and the patient’s post-operative course was uneventful. No
subsequent adjuvant therapy was deemed necessary.
Pathological analysis
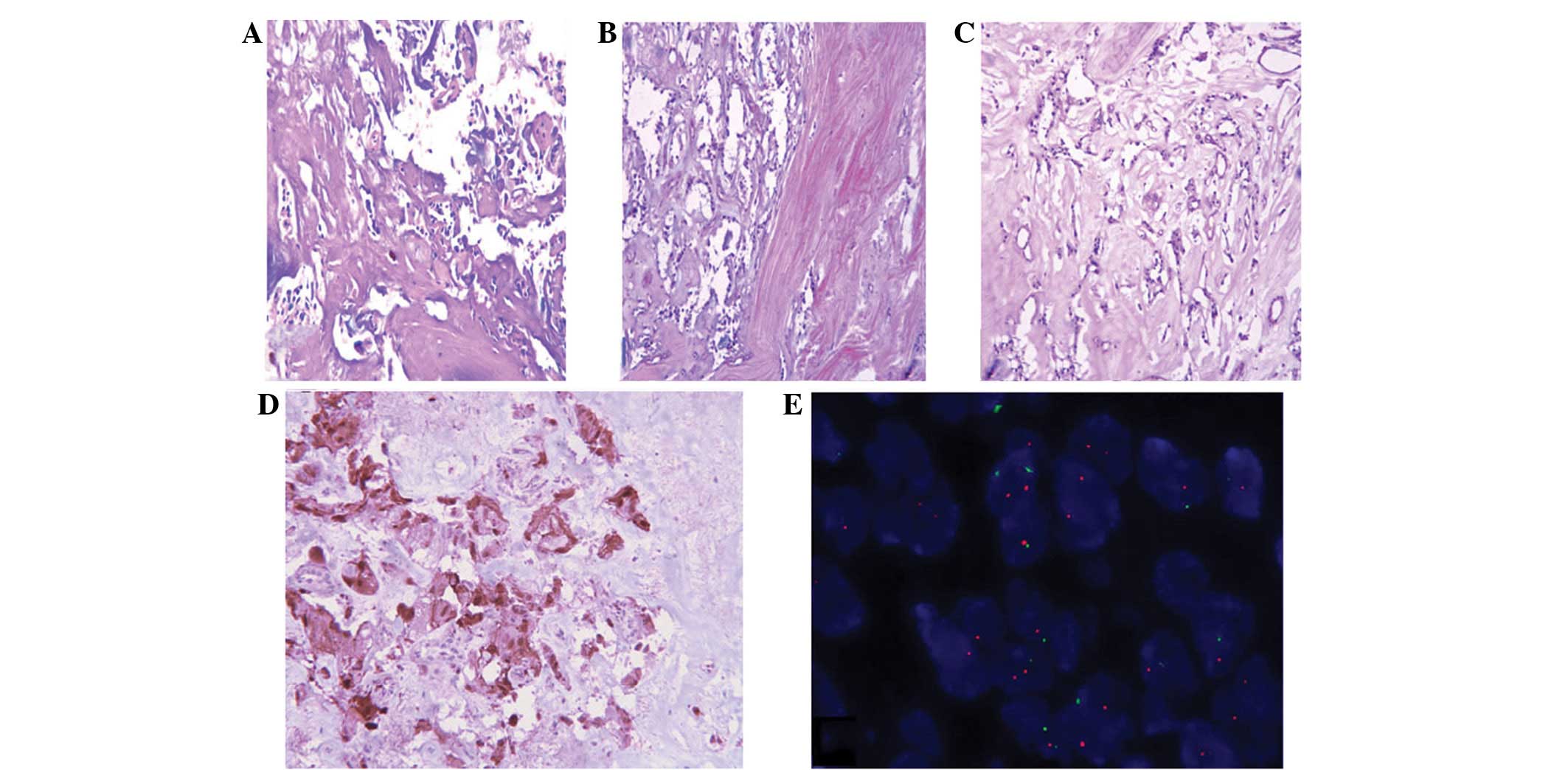
Microscopically, the tumor largely consisted of
areas with low cellularity. In less cellular areas, marked hyaline
changes were observed in the blood vascular walls and stroma
(Fig. 2A). In other areas, the
tumor showed pseudopapillary or reticular patterning formed by
cuboidal cells on a hyaline background (Fig. 2B and C). Mitotic figures and
necrosis were absent. The tumor cells showed marked positivity for
glial fibrillary acidic protein (GFAP; Fig. 2D) and S-100 proteins, whereas the
cells were negative for epithelial membrane antigen (EMA),
cytokeratins (CK) and epidermal growth factor receptor (EGFR). The
Ki-67 labeling index was ∼3%. Since EGFR may be a predictor of
relapse in MPE (5), the EGFR gene
was analyzed by fluorescence in situ hybridization. No
amplification of the EGFR gene was observed (Fig. 2E). The results of pathological and
immunohistochemical studies were consistent with the ependymal
nature of neoplastic cells.
Discussion
MPE is a benign variant of ependymoma that has a
peak incidence between the third and fifth decades of life. It
generally occurs in the filum terminale, but has also been
identified in extra-spinal locations, including subcutaneous tissue
and the brain (6,7). Clinical symptoms are directly
associated with the mass location of the tumor. Histologically, it
is typically characterized by papillae formed by the arrangement of
cuboidal to columnar cells surrounding a central core, which
contains blood vessels and myxoid change. The genesis of the
stromal myxoid changes are indicative of an inundation of the
plasma proteins generally located within blood vessels (8). In the current case, the pronounced
deposition of hyaline materials was distributed among the tumor
cells and within the blood vessel walls. Lim et al (9) previously hypothesized that the
‘proteinaceous’ deposits and hyaline vascular change were a result
of increased vascular permeability that led to inundation of plasma
proteins surrounding extravascular spaces. MPEs are generally
slow-growing, therefore, the chronic long-standing anoxic
conditions may lead to various regressive changes and necrosis. An
additional explanation for the stromal change is the complicated
structure of the conus medullaris and filum terminale, as this
region is composed of an admixture of connective tissue, nerve
fibers and neuroglia. It may be possible that these unique
structural features result from the anatomical relationships of the
MPE (10). Certain studies have
indicated that, in specific circumstances, MPE cells produce basal
lamina material, particularly in regions where ependymal cells are
opposed to connective tissue (11,12).
In the present case, tumor cells showed marked cytoplasmic staining
for GFAP and S-100 protein, whereas immunoreactivity for CK, EMA,
chromogranin, EGFR and synaptophysin were all negative. In
addition, marked hyaline deposits were identified around the tumor
cells, and pseudopapillary patterns or ependymal rosettes were
rare. Therefore, in cases such as these, it is important to rule
out the possibility of schwannomas, chordomas and metastatic
mucinous adenocarcinomas. Spinal schwannomas appear on the myelin
sheath of the spine and represent between one-quarter to one-third
of all spinal tumors. Diagnostic features include a fibrous capsule
and Antoni A and Antoni B areas. Similar to in MPEs, hyaline
vessels and stroma are common in schwannomas and S100 is markedly
expressed. However, GFAP is negative. It is extremely important to
differentiate between a schwannoma and MPE prior to surgery, as an
MPE has the potential to disseminate through the cerebrospinal
fluid throughout the neuraxis and must be removed completely. The
most common differential diagnosis at this location is that of a
chordoma. The tumor cells are large with characteristic
physaliferous cytoplasm, and the immunocytochemistry of CK is
positive (13). Metastatic mucinous
adenocarcinomas show epithelial cords and groups with an acinar
arrangement. The cancer cells are more pleomorphic and are CK- and
EMA-positive. An analysis using electron microscopy is extremely
important for forming a differential diagnosis. Specific
ultrastructural features, including microvilli, cilia, desmosomal
attachments and cytoplasmic filaments, are indicative of a
diagnosis of MPE (14,15).
In conclusion, specific regressive changes are
observed in MPE and marked hyaline degeneration may lead to an
acellular growth pattern, therefore, it is important to rule out
the possibility of other tumors. The best curative treatment lies
in a complete surgical resection, and long-term follow-up of the
whole neuraxis must be performed.
Acknowledgements
The authors would like to thank Dr
Wanchun Li (Department of Pathology, Nanjing Jinling Hospital) for
gathering clinical information from medical records and Dr Ozgur
Sahin (Department of Molecular and Cellular Oncology, The
University of Texas MD Anderson Cancer Center) for linguistic
assistance.
References
|
1.
|
Bagley CA, Kothbauer KF, Wilson S,
Bookland MJ, Epstein FJ and Jallo GI: Resection of myxopapillary
ependymomas in children. J Neurosurg. 106(4 Suppl): 261–267.
2007.PubMed/NCBI
|
|
2.
|
Nakamura M, Ishii K, Watanabe K, et al:
Long-term surgical outcomes for myxopapillary ependymomas of the
cauda equina. Spine. (Phila Pa 1976). 34:E756–E760. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kulesza P, Tihan T and Ali SZ:
Myxopapillary ependymoma: cytomorphologic characteristics and
differential diagnosis. Diagn Cytopathol. 26:247–250. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Zacharoulis S, Ji L, Pollack IF, et al:
Metastatic ependymoma: a multi-institutional retrospective analysis
of prognostic factors. Pediatr Blood Cancer. 50:231–235. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Verma A, Zhou H, Chin S, Bruggers C,
Kestle J and Khatua S: EGFR as a predictor of relapse in
myxopapillary ependymoma. Pediatr Blood Cancer. 59:746–748. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Tseng YC, Hsu HL, Jung SM and Chen CJ:
Primary intracranial myxopapillary ependymomas: report of two cases
and review of the literature. Acta Radiol. 45:344–347. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lee KJ, Min BW, Seo HJ and Cho CH:
Subcutaneous sacrococcygeal myxopapillary ependymoma in asian
female: a case report. J Clin Med Res. 4:61–63. 2012.PubMed/NCBI
|
|
8.
|
Sato H, Ohmura K, Mizushima M, Ito J and
Kuyama H: Myxopapillary ependymoma of the lateral ventricle. A
study on the mechanism of its stromal myxoid change. Acta Pathol
Jpn. 33:1017–1025. 1983.PubMed/NCBI
|
|
9.
|
Lim SC and Jang SJ: Myxopapillary
ependymoma of the fourth ventricle. Clin Neurol Neurosurg.
108:211–214. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Rawlinson DG, Herman MM and Rubinstein LJ:
The fine structure of a myxopapillary ependymoma of the filum
terminale. Acta Neuropathol. 25:1–13. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Miller C: The ultrastructure of the conus
medullaris and filum terminale. J Comp Neurol. 132:547–566. 1968.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Matyja E, Naganska E, Zabek M and Koziara
H: Myxopapillary ependymoma of the lateral ventricle with local
recurrences: histopathological and ultrastructural analysis of a
case. Folia Neuropathol. 41:51–57. 2003.
|
|
13.
|
Estrozi B, Queiroga E, Bacchi CE, et al:
Myxopapillary ependymoma of the posterior mediastinum. Ann Diagn
Pathol. 10:283–287. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Stern JB and Helwig EB: Ultrastructure of
subcutaneous sacrococcygeal myxopapillary ependymoma. Arch Pathol
Lab Med. 105:524–526. 1981.PubMed/NCBI
|
|
15.
|
Kindblom LG, Lodding P, Hagmar B and
Stenman G: Metastasizing myxopapillary ependymoma of the
sacrococcygeal region. A clinico-pathologic, light- and
electronmicroscopic, immunohistochemical, tissue culture, and
cytogenetic analysis of a case. Acta Pathol Microbiol Immunol Scand
A. 94:79–90. 1986.
|