Introduction
Colorectal cancer (CRC) is the second most common
type of cancer and the second leading cause of cancer-related
mortality in developed countries (1). In recent years, the incidence of CRC
has markedly increased in China, particularly in urban populations.
This is likely to be due to the rapid changes in social economic
factors, such as lifestyle, diet and environment. As an example, in
Beijing, the annual incidence of CRC has increased from 16 per
100,000 to 24 per 100,000 in the past decade (2).
Screening provides the ability to detect
precancerous lesions and early-stage CRC, thus saving lives, and
there are numerous different screening options presently available
(3). Among these, the fecal occult
blood test (FOBT) is the most common test in use for CRC screening.
Several large cohort studies have demonstrated the effectiveness of
FOBT-based screening in Western countries (4–6);
however, no such studies have been performed in developing
countries thus far. Furthermore, the majority of the large-scale
cohort studies have utilized the conventional chemical guaiac-based
FOBT (gFOBT) method. The gFOBT has a high false-positive rate
(range, 2–13%) (7,8) and other limitations, such as the
requirement of diet restriction. The latter is particularly
troublesome for people in Asian countries. The antibody-based
immunochemical method, the fecal immunochemical test (FIT), has
been demonstrated to have test performance characteristics with
improved sensitivity and specificity compared with those of gFOBT,
and it does not require any restrictions on diet (7). However, the higher costs associated
with FIT compared with gFOBT limit its use for screening in
countries such as China, where healthcare resources are limited.
The present study evaluates a three-tier FOBT-based program, and
presents the results of a prospective longitudinal trial for CRC
screening with this program.
Materials and methods
Subjects
The eligible subjects in the present study were army
officers (retired or non-retired) aged >50 years, who were
receiving healthcare from the Beijing Military General Hospital
(Beijing, China) and lived in the Beijing area. Subjects with known
CRC, colorectal adenomas, inflammatory bowel disease or various
types of malignant tumors were excluded from the cohort. The study
was approved by the Beijing Military General Hospital Ethics
Committee, and informed consent was obtained from each subject.
The present study was a dynamic cohort study, where
subjects who met the eligibility criteria were recruited on an
annual basis. Upon entry into the cohort study, each subject
received a complete health status check-up, which included a
physical examination, chest X-ray, ECG, abdominal ultrasound,
mammogram (for females) and serological examination, including an
analysis of glucose and lipid levels. The health check-up data,
along with the data from a baseline questionnaire (for date of
birth, gender, education, family history of malignant tumors,
lifestyle, medication use, body height/weight and previous general
health status), were entered into a database. The database was
updated yearly, based on the findings from the annual follow-up
examinations.
At the end of the initial check-up, each eligible
subject was asked by physicians for their consent to undergo CRC
screening. Those who agreed to be screened were included in the
screening group, and those who refused to be screened were included
in a non-screening group. Other than the FOBT that was performed in
the screening group on an annual basis, the subjects in the
screening and non-screening groups were investigated in the same
manner, with an annual examination for other chronic conditions, as
detailed above.
The annual FOBT-based CRC screening began in May
1987 and ended in December 2005. Following this, the two groups
were followed up until December 2008. In 1987, there were 3,002
eligible subjects, including 2,260 males and 742 females, with a
mean age of 61.8 years (2,809 subjects were aged ≤74 years). The
number of subjects in the screening group was 2,246, and the number
of subjects in the non-screening group was 756. For the subsequent
years (1988–2005), an additional 2,102 eligible subjects were
entered into the cohort. In total, there were 3,863 subjects in the
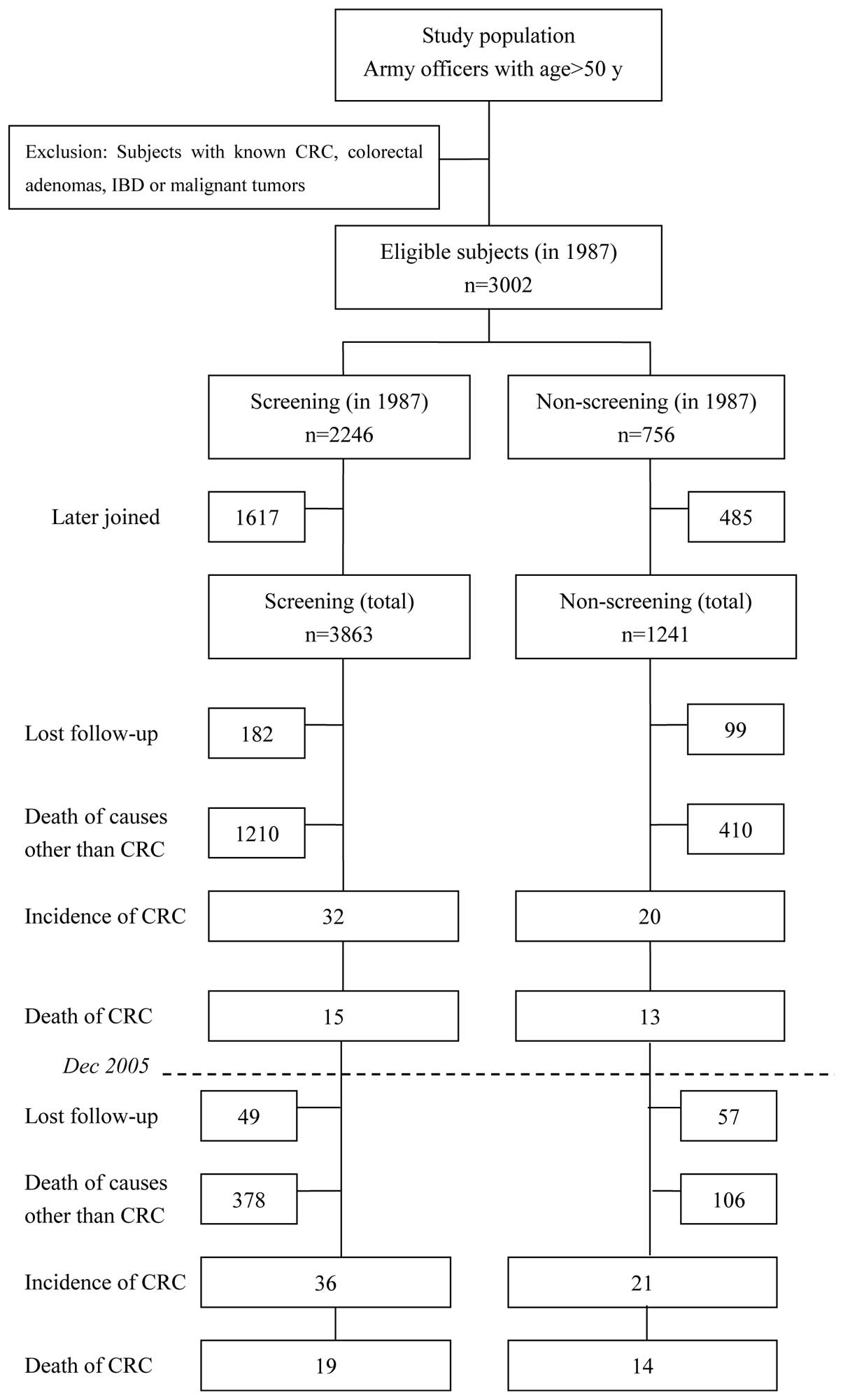
screening group and 1,241 in the non-screening group (Fig. 1).
Screening methods
For the screening, each subject provided one fecal
sample every year for the FOBT, which was performed in the Clinical
Laboratory of Beijing Military General Hospital. No restrictions,
with regard to diet, drugs or other food items, were imposed on the
subjects prior to stool collection. Between 1987 and 2005, a
three-tier screening program was implemented: gFOBT (rehydrated;
produced by Baso Diagnostics Inc., Zhuhai, China) was performed,
followed by FIT (gold gel stripe; provided by WanhuaPuman
Biological Engineering Co., Ltd., Tech Lt. Comp., Beijing, China)
for gFOBT-positive fecal samples, and then colonoscopy for those
who had positive FIT results. According to the manufacturer, the
positive threshold of FIT was 0.2 μg/ml. For patients who
refused colonoscopy, a double-contrast barium enema (DCBE) was
performed. For patients with negative (no cancer detected)
colonoscopy or DCBE results, the aforementioned screening steps
were repeated in the following year.
On identification of a lesion (polyp, tumor or
otherwise) during colonoscopy or DCBE, a biopsy (or resection for a
polyp or tumor) was performed. If a malignant tumor was identified,
appropriate management was provided according to the final
pathology, and the subject was regarded as an incidence case (the
secondary endpoint) and continued to be followed up until they
succumbed to the disease (the primary endpoint). Patients with
adenoma were to be followed annually, as were the other non-cancer
subjects, following complete resection.
Data and statistical analyses
The primary endpoint of the follow up was
CRC-related mortality, and the secondary endpoint was the incidence
of CRC. Overall, 82% of the mortalities occurred in the Beijing
Military General Hospital, and the cause was determined based on a
review of the medical records (International Classification of
Diseases, ICD-9 or -10). For the subjects who succumbed elsewhere,
a telephone interview with the next of kin was performed and the
cause of death was determined from the death certificate.
A positive predictive value (PPV) was estimated for
each screening round, while only positive tests followed by
colonoscopy or DCBE were used in the computation. The general
sensitivity was evaluated by the number of true positives relative
to the number of individuals with carcinomas, while a positive test
result was considered to be a true positive if a carcinoma was
detected during the entire follow-up period.
To evaluate potential selection bias, the
χ2 test or the independent samples t-test for the
baseline characteristics [age at baseline, gender, education,
family history of malignant tumors, body mass index (BMI), smoking
status (never, former or current), alcohol intake (none, occasional
or regular), physical exercise (at least once per week or less),
meat intake (at least three times per week or less) and aspirin use
(regular or never)] and status at time of mortality from all of
these causes were utilized. A χ2 test was used to
examine the CRC incidence and mortality in the screening and
non-screening groups, and the relative risk (RR) and 95% confidence
intervals (CIs) were calculated. The Kaplan-Meier survival analysis
was used to evaluate the effect of screening on CRC incidence and
mortality, and the cumulative incidence and cumulative mortality
curves were determined. The Cox proportional hazards regression
model was used to control potential confounding factors (age at
baseline, gender, education, family history of malignant tumors,
BMI, smoking status, alcohol intake, physical exercise, meat intake
and aspirin use) for CRC incidence and mortality. SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA) was used for the
statistical analyses. All statistical tests that were performed
were two-sided, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Subjects and screening results
Fig. 1 shows a
diagram depicting the screened vs. non-screened population and the
outcome of the study. For the entire 22-year study period, there
were a total of 5,104 eligible subjects. Of these, 3,863
participated in the screening for CRC (screening group) and 1,241
did not (non-screening group). Over the course of the study, 231
subjects in the screening group and 156 in the non-screening group
were lost to follow-up due to a change in residence (6.0 and 12.6%,
respectively; P=0.000). In addition, 1,588 subjects in the
screening group and 516 in the non-screening group had succumbed
due to causes other than CRC (41.1 and 41.6%, respectively;
P=0.769). Up to December, 2008, the person-years of observation in
the screening and non-screening groups were 49,566 and 15,826,
respectively. The baseline characteristics of the subjects were not
significantly different in the screening group compared with those
in the non-screening group (Table
I).
 | Table I.Baseline characteristics of subjects
in the screening and non-screening groups. |
Table I.
Baseline characteristics of subjects
in the screening and non-screening groups.
| Characteristics | Screening
(n=3863) | Non-screening
(n=1241) | P-value |
|---|
| Gender, n (%) | | | |
| Male | 2913 (75.4) | 934 (75.3) | 0.917 |
| Female | 950 (24.6) | 307 (24.7) | |
| Age (years) | | | |
| Mean age ± SD | 62.1±6.9 | 62.0±7.0 | 0.613 |
| Education, n (%) | | | |
| Up to high
school | 3549 (91.9) | 1133 (91.3) | 0.523 |
| College or
higher | 314 (8.1) | 108 (8.7) | |
| Family history of
malignant tumors, n (%) | | | |
| With | 118 (3.1) | 31 (2.5) | 0.311 |
| Without | 3745 (96.9) | 1210 (97.5) | |
| Body mass index | | | |
| Mean BMI ± SD | 23.1±1.6 | 23.2±1.6 | 0.736 |
| Smoking status, n
(%) | | | |
| Never | 1339 (34.7) | 406 (32.7) | 0.082 |
| Former | 1120 (29.0) | 401 (32.3) | |
| Current | 1404 (36.3) | 434 (35.0) | |
| Alcohol intake, n
(%) | | | |
| None | 747 (19.3) | 244 (19.7) | 0.630 |
| Occasional | 2810 (72.7) | 909 (73.2) | |
| Regular | 306 (7.9) | 88 (7.1) | |
| Physical exercise, n
(%) | | | |
| <Once per
week | 1853 (48.0) | 593 (47.8) | 0.910 |
| ≥Once per week | 2010 (52.0) | 648 (52.2) | |
| Meat intake, n
(%) | | | |
| <3 times per
week | 1038 (26.9) | 331 (26.7) | 0.891 |
| ≥3 times per
week | 2825 (73.1) | 910 (73.3) | |
| Aspirin, n (%) | | | |
| No use | 2957 (76.5) | 958 (77.2) | 0.638 |
| Regular use | 906 (23.5) | 283 (22.8) | |
Table II shows the
results of each screening round. The positive result rates of gFOBT
ranged from 4.6 to 16.2%, while FIT ranged from 1.1 to 2.6%.
Between 1987 and 2005, 778 colonoscopies and 33 DCBEs were
performed in the screening group, with a total follow-up rate of
87.0% (811/932) in patients with positive FIT results. A total of
36 cases of CRC occurred in the screening group. Among the 36 CRC
cases, 25 were detected by the screening program, while four cases
with positive FIT results refused to undergo follow-up colonoscopy
or DCBE, and were later diagnosed by clinical means. Seven cases
were missed due to a false-negative FOBT result. The general
sensitivity of this three-tier FOBT was 80.6% (29/36; 95% CI,
65.3–91.1). There were 153 (4.0%) subjects who were identified to
have adenomas of ≥1 cm in size in the screening group.
 | Table II.Results of each annual screening round
and the test performance of FOBT. |
Table II.
Results of each annual screening round
and the test performance of FOBT.
| Year | No. of subjects | Screened subjects
| gFOBT+
| FIT+
| Colonoscopy or DCBE
| Cancer
| Cancer and adenomas
≥1 cm
|
|---|
| n | % | n | % | n | % | n | %a | n | PPVb | n | PPVb |
|---|
| 1987 | 2246 | 2246 | 100.0 | 202 | 9.0 | 51 | 2.3 | 45 | 88.2 | 1 | 2.2 | 10 | 22.2 |
| 1988 | 2239 | 2233 | 99.7 | 175 | 7.8 | 48 | 2.1 | 39 | 81.3 | 1 | 2.6 | 8 | 20.5 |
| 1989 | 2240 | 2228 | 99.5 | 237 | 10.6 | 52 | 2.3 | 47 | 90.4 | 2 | 4.3 | 8 | 17.0 |
| 1990 | 2211 | 2205 | 99.7 | 156 | 7.1 | 47 | 2.1 | 35 | 74.5 | 0 | 0.0 | 7 | 20.0 |
| 1991 | 2184 | 2164 | 99.1 | 233 | 10.8 | 52 | 2.4 | 45 | 86.5 | 1 | 2.2 | 12 | 26.7 |
| 1992 | 2286 | 2253 | 98.6 | 254 | 11.3 | 55 | 2.4 | 49 | 89.1 | 2 | 4.1 | 8 | 16.3 |
| 1993 | 2293 | 2285 | 99.7 | 216 | 9.5 | 54 | 2.4 | 46 | 85.2 | 1 | 2.2 | 11 | 23.9 |
| 1994 | 2264 | 2251 | 99.4 | 365 | 16.2 | 58 | 2.6 | 53 | 91.4 | 2 | 3.8 | 10 | 18.9 |
| 1995 | 2308 | 2302 | 99.7 | 269 | 11.7 | 61 | 2.6 | 57 | 93.4 | 3 | 5.3 | 15 | 26.3 |
| 1996 | 2280 | 2247 | 98.6 | 271 | 12.1 | 54 | 2.4 | 51 | 94.4 | 2 | 3.9 | 11 | 21.6 |
| 1997 | 2220 | 2214 | 99.7 | 204 | 9.2 | 52 | 2.3 | 46 | 88.5 | 1 | 2.2 | 7 | 15.2 |
| 1998 | 2217 | 2201 | 99.3 | 316 | 14.4 | 50 | 2.3 | 44 | 88.0 | 1 | 2.3 | 12 | 27.3 |
| 1999 | 2255 | 2249 | 99.7 | 198 | 8.8 | 53 | 2.4 | 39 | 73.6 | 1 | 2.6 | 9 | 23.1 |
| 2000 | 2253 | 2236 | 99.2 | 215 | 9.6 | 52 | 2.3 | 50 | 96.2 | 1 | 2.0 | 14 | 28.0 |
| 2001 | 2232 | 2218 | 99.4 | 192 | 8.7 | 48 | 2.2 | 43 | 89.6 | 1 | 2.3 | 5 | 11.6 |
| 2002 | 2289 | 2273 | 99.3 | 183 | 8.1 | 45 | 2.0 | 41 | 91.1 | 1 | 2.4 | 11 | 26.8 |
| 2003 | 2457 | 2415 | 98.3 | 112 | 4.6 | 26 | 1.1 | 21 | 80.8 | 1 | 4.8 | 6 | 28.6 |
| 2004 | 2457 | 2445 | 99.5 | 227 | 9.3 | 48 | 2.0 | 36 | 75.0 | 2 | 5.6 | 6 | 16.7 |
| 2005 | 2456 | 2431 | 99.0 | 145 | 6.0 | 26 | 1.1 | 24 | 92.3 | 1 | 4.2 | 8 | 33.3 |
Incidence and mortality rates of CRC in
screening and non-screening groups
During the 19-year screening period (1987–2005), 32
CRC cases occurred in the screening group and 20 occurred in the
non-screening group. In the last 3 years of follow-up (2006–2008),
an additional four cases occurred in the screening group and one
occurred in the non-screening group (Fig. 1 and Table III). Until December 2005, the CRC
incidence in the screening group was 0.75/1,000 person-years, which
was significantly lower than that of the non-screening group
(1.43/1,000 person-years; P=0.027). The mortality was 0.35/1,000
person-years in the screening group, which was also significantly
lower than that of the non-screening group (0.93/1,000, P= 0.013).
The relative risks of incidence and mortality adjusted for the
baseline characteristics of the subjects were 0.49 (95% CI,
0.28–0.85) and 0.31 (95% CI, 0.14–0.65), respectively (Both
P<0.05; Table III). Three years
subsequent to the termination of the screening program, the
incidence and mortality in the screening group was significantly
lower than that of the non-screening group (P=0.034 and P=0.022,
respectively), with adjusted relative risks of 0.51 (95% CI,
0.30–0.87) and 0.36 (95% CI, 0.18–0.71), respectively (both
P<0.05; Table III). Therefore,
there was a 49% decrease in incidence and a 64% decrease in
mortality in the screening group compared with the non-screening
group during the entire 22-year study period. Mortality rates from
all causes did not differ between the two groups (P=0.516 for
between 1987 and 2008).
 | Table III.Incidence and mortality rates of CRC
between 1987 and 2008 (per 1000 person-years). |
Table III.
Incidence and mortality rates of CRC
between 1987 and 2008 (per 1000 person-years).
| Characteristic | May 1987-December
2005
| May 1987-December
2008
|
|---|
| Screening | Non-screening | Screening | Non-screening |
|---|
| Person-years of
observation | 42881 | 13974 | 49566 | 15826 |
| Colorectal
cancer | | | | |
| No. of
patients | 32 | 20 | 36 | 21 |
| Incidence
rate | 0.75 | 1.43 | 0.73 | 1.33 |
| RR (95% CI) | 0.52
(0.30–0.93) | 0.55
(0.32–0.95) |
| Adjusted RR (95%
CI)a | 0.50
(0.29–0.88) | 0.52
(0.30–0.89) |
| Adjusted RR (95%
CI)b | 0.49
(0.28–0.85) | 0.51
(0.30–0.87) |
| Mortality from
CRC | | | | |
| No. of
deaths | 15 | 13 | 19 | 14 |
| Mortality
rate | 0.35 | 0.93 | 0.38 | 0.88 |
| RR (95% CI) | 0.38
(0.18–0.81) | 0.43
(0.22–0.88) |
| Adjusted RR (95%
CI)a | 0.31
(0.15–0.67) | 0.36
(0.18–0.72) |
| Adjusted RR (95%
CI)b | 0.31
(0.14–0.65) | 0.36
(0.18–0.71) |
| Mortality from all
causes | | | | |
| No. of
deaths | 1225 | 423 | 1607 | 530 |
| Mortality
rate | 28.57 | 30.27 | 32.42 | 33.49 |
| RR (95% CI) | 0.94
(0.85–1.05) | 0.97
(0.88–1.07) |
In total, the study was comprised of 4,777 subjects
aged between 50 and 74 years, and 327 subjects aged >75 years on
recruitment. A stratified analysis revealed that a significant
decrease in the incidence and mortality of CRC was only evident in
the subgroup who were aged between 50 and 74 years (P=0.028 and
P=0.024, respectively; Table
IV).
 | Table IV.Incidence and mortality of CRC
stratified by age (between May, 1987 and December, 2008). |
Table IV.
Incidence and mortality of CRC
stratified by age (between May, 1987 and December, 2008).
| 50–74 years
| ≥75 years
|
|---|
| Screening | Non-screening | Screening | Non-screening |
|---|
| No. of
subjects | 3614 | 1163 | 249 | 78 |
| No. of CRC
cases | 29 (0.8%) | 18 (1.5%) | 7 (2.8%) | 3 (3.8%) |
| RR (95% CI) | 0.52
(0.29–0.93) | 0.71
(0.18–2.74) |
| Adjusted RR (95%
CI)a | 0.50
(0.28–0.90) | 0.69
(0.18–2.67) |
| Mortality from
CRC | 14 (0.4%) | 11 (0.9%) | 5 (2.0%) | 3 (3.8%) |
| RR (95% CI) | 0.40
(0.18–0.89) | 0.43
(0.10–1.83) |
| Adjusted RR (95%
CI)a | 0.36
(0.16–0.80) | 0.41
(0.10–1.74) |
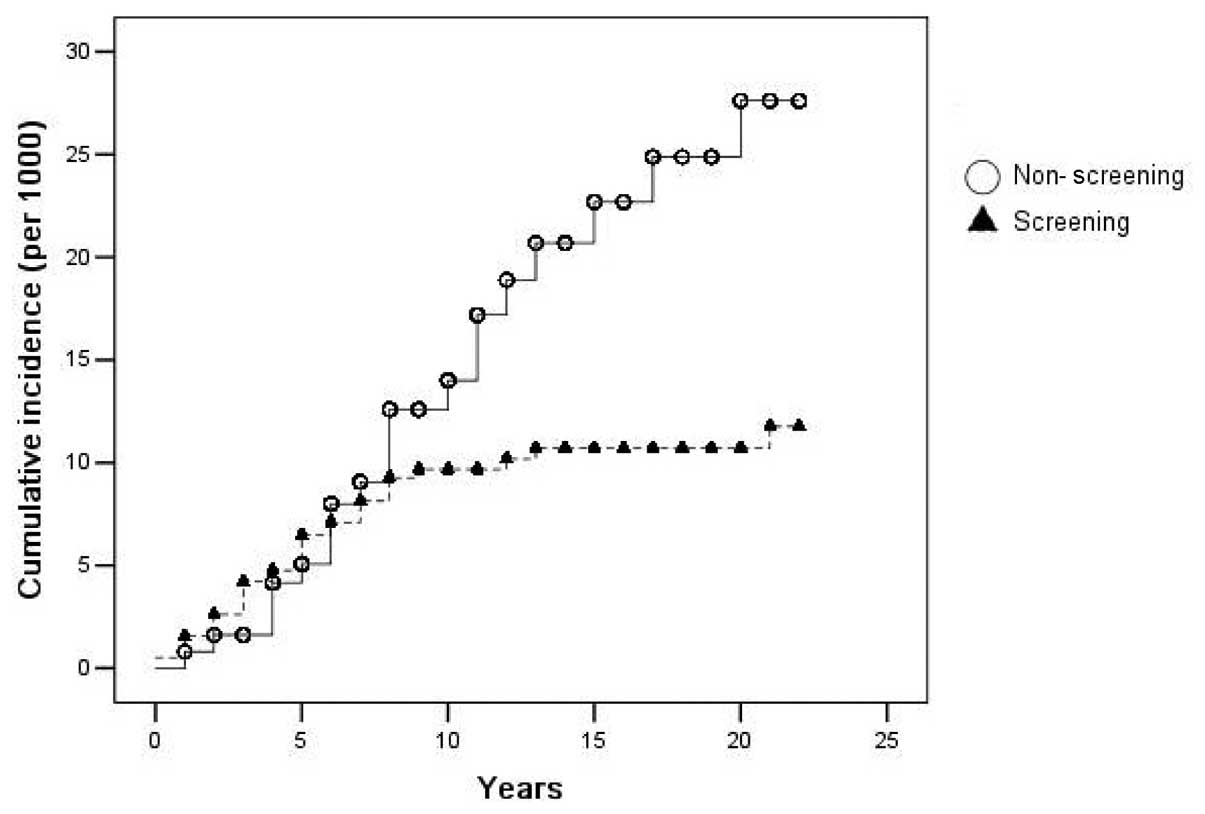
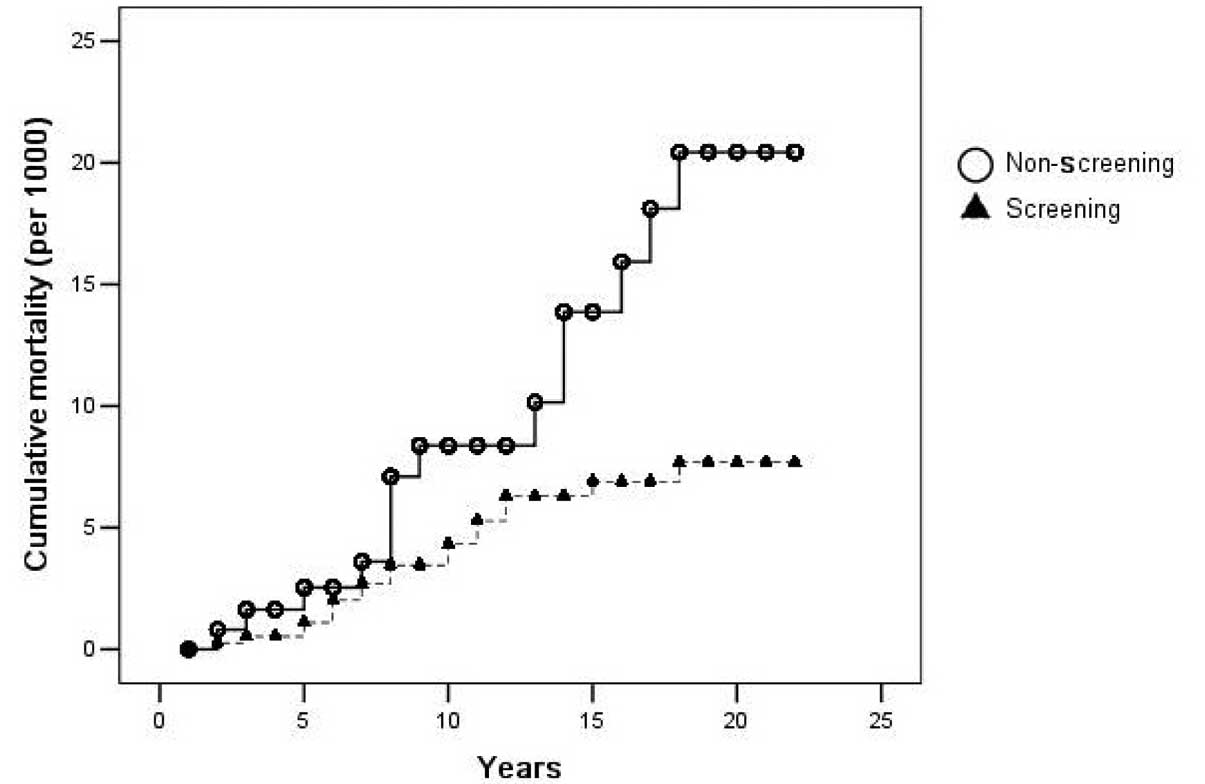
Figs. 2 and 3 show the cumulative incidence and
mortality of CRC, as analyzed by the Kaplan-Meier method. The
differences in the cumulative incidence and mortality between the
two groups was evident after the seventh and eighth years of
screening, and more so thereafter.
Discussion
The current study presented a three-tier FOBT-based
screening program, which has been described previously (9,10).
Certain cross-sectional studies for this type of combined gFOBT
with FIT have demonstrated compatible sensitivity to gFOBT, but
notable increased specificity (7,10). The
difficulty of dietary restriction led to a poor specificity of
guaiac-based tests in the Chinese population (11). Moreover, the advantage of using a
combined test is that it reduces the cost of the FIT assay, as the
FIT is only developed when the gFOBT result is positive. The
observed sensitivity for CRC detection in the present study was
substantially greater than that identified by Allison et al
(8) (65% for CRC) in a
cross-sectional study with a similar three-tier method. This is
likely to be due to the longitudinal nature of the present study,
which meant that the cases missed in one screening round were
identified in subsequent repeat tests.
In this 22-year longitudinal, controlled trial
involving a dynamic cohort, it was demonstrated that annual
FOBT-based screening resulted in a 49% decrease in colon cancer
incidence and a 64% decrease in CRC-related mortality, even 3 years
after the termination of the screening program. The differences in
cumulative incidence and mortality were evident between the two
groups after only 7 to 8 years of the study, which may indicate
that the lead time of screening is ∼7–8 years. To the best of our
knowledge, this study is the longest follow-up of a colon cancer
screening trial in a developing country. The study included a
highly stable cohort with relatively few subjects lost to follow-up
(<10% in the 22-year follow-up), but a high censoring rate due
to mortality from other causes. The compliance rate was also high
(almost 100% for the first level test and 83% for colonoscopy in
patients with a positive FIT result). This may be ascribed partly
to bringing the screening program to a military healthcare
system.
However, the study also has several limitations. The
population of the study consisted only of military officials and
may not consequently be representative of the general Chinese
population. Additionally, the study was not randomized; the
screening group consisted of subjects who chose to be screened.
This may have produced a selection bias, although the screened and
non-screened populations shared similar demographic features.
Furthermore, there is no definite final age for the screening.
Routine screening for CRC is recommended against in adults >75
years (12). The present data also
revealed that the effect of screening on the incidence and
mortality of CRC was less significant among subjects aged >75
years. Moreover, the sample size was relatively small compared with
previous trials involving gFOBT.
Taking into account the aforementioned limitations,
the present study demonstrated that a three-tier FOBT-based annual
screening, even with one fecal sample, resulted in the detection of
>80% of CRCs. The study also revealed a marked decrease in
CRC-related mortality (64% after controlling potential confounding
factors) compared with that which has been identified previously
with gFOBT, which was in the range of 15 to 30% (4–6). The
present findings were similar to the longitudinal non-controlled
observational study in the Japanese population by Lee et al
(13), which utilized FIT for
screening and demonstrated a 70% decrease in mortality over a
13-year study period. Moreover, there are a limited number of
studies demonstrating an effect of FOBT on incidence (14), while the present data revealed a 49%
decrease in CRC incidence in the screening group compared with that
in the non-screening group. This dramatic decrease in the incidence
and mortality of CRC may be ascribed partly to the higher
compliance and adenoma detection rates with subsequent resection in
the present study. In the study 4% of the screening subjects were
identified to have adenomas of ≥1 cm in size, while 0.8–1.7% were
identified in a previous study (15). However, since the present study was
not randomized, the effect of selection bias on the differences in
incidence and mortality observed may not be excluded, although the
two groups (screening and non-screening) exhibited similar baseline
demographic characteristics. Furthermore, the grouping in the
present study was according to the preference of the subjects, i.e.
whether to undergo CRC screening or not. It is supposed that
individuals who refuse screening have higher CRC incidence and
mortality rates than those who accept testing (16). The rates of loss to follow-up were
significantly higher in the non-screening group, which may indicate
less benefit from the military healthcare system in the
non-screening group compared with that in the screening group. All
of the aforementioned factors may have contributed to the decreases
in the incidence and mortality of CRC, along with the efficacy of
the screening process.
In conclusion, when accepted, annual FOBT-based
screening using a three-tier system in an average-risk Chinese
population aged >50 years demonstrated >80% sensitivity in
detecting CRC. The screening significantly reduced the incidence
and mortality rates of CRC. These findings suggested that annual
FOBT examination coupled with a complete colonoscopy follow-up for
cases with positive FOBT results is an effective approach for CRC
control in China.
Acknowledgements
This study was supported by the
Chinese Zonghou Medical Foundation (grant no. BJZ07).
References
|
1.
|
Stewart BW and Kleihues P: World cancer
report. Lyon: IARC Press; pp. 163–166. 2003
|
|
2.
|
Zhang SW, Chen WQ, Kong LZ, Li LD, Lu FZ,
Li GL, Meng J and Zhao P: An analysis of cancer incidence and
mortality from 30 cancer registries in China, 1998∼2002. Zhongguo
Zhongliu. 15:430–448. 2006.
|
|
3.
|
Levin B, Lieberman DA, McFarland B,
Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S,
Johnson D, Johnson CD, et al American Cancer Society Colorectal
Cancer Advisory Group; US Multi-Society Task Force; American
College of Radiology Colon Cancer Committee: Screening and
surveillance for the early detection of colorectal cancer and
adenomatous polyps, 2008: a joint guideline from the American
Cancer Society, the US Multi-Society Task Force on Colorectal
Cancer, and the American College of Radiology. Gastroenterology.
134:1570–1595. 2008. View Article : Google Scholar
|
|
4.
|
Mandel JS, Bond JH, Church TR, Snover DC,
Bradley GM, Schuman LM and Ederer F: Reducing mortality from
colorectal cancer by screening for fecal occult blood. Minnesota
Colon Cancer Control Study. N Engl J Med. 328:1365–1371. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kronborg O, Fenger C, Olsen J, Jorgensen
OD and Søndergaard O: Randomised study of screening for colorectal
cancer with faecal-occult-blood test. Lancet. 348:1467–1471. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hardcastle JD, Chamberlain JO, Robinson
MH, Moss SM, Amar SS, Balfour TW, James PD and Mangham CM:
Randomised controlled trial of faecal-occult-blood screening for
colorectal cancer. Lancet. 348:1472–1477. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Allison JE, Sakoda LC, Levin TR, Tucker
JP, Tekawa IS, Cuff T, Pauly MP, Shlager L, Palitz AM, Zhao WK,
Schwartz JS, Ransohoff DF and Selby JV: Screening for colorectal
neoplasms with new fecal occult blood tests: update on performance
characteristics. J Natl Cancer Inst. 99:1462–1470. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Allison JE, Tekawa IS, Ransom LJ and
Adrain AL: A comparison of fecal occult-blood tests for
colorectal-cancer screening. N Engl J Med. 334:155–159. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Li S, Nie Z, Li N, Li J, Zhang P, Yang Z,
Mu S, Du Y, Hu J, Yuan S, Qu H, et al: Colorectal cancer screening
for the natural population of Beijing with sequential fecal occult
blood test: a multicenter study. Chin Med J (Engl). 116:200–202.
2003.PubMed/NCBI
|
|
10.
|
Li S, Wang H, Hu J, Li N, Liu Y, Wu Z,
Zheng Y, Wang H, Wu K, Ye H and Rao J: New immunochemical fecal
occult blood test with two-consecutive stool sample testing is a
cost-effective approach for colon cancer screening: Results of a
prospective multicenter study in Chinese patients. Int J Cancer.
118:3078–3083. 2006. View Article : Google Scholar
|
|
11.
|
Wong BC, Wong WM, Cheung KL, Tong TS,
Rozen P, Young GP, Chu KW, Ho J, Law WL, Tung HM, Lai KC, Hu WH,
Chan CK and Lam SK: A sensitive guaiac faecal occult blood test is
less useful than an immunochemical test for colorectal cancer
screening in a Chinese population. Aliment Pharmacol Ther.
18:941–946. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Calogne N, Petitti DB, DeWitt TG, Dietrich
AJ, Gregory KD, Harris R, Isham G, LeFevre ML, Leipzig RM,
Loveland-Cherry C, Marion LN, et al U.S. Preventive Services Task
Force: Screening for colorectal cancer: U.S. Preventie Services
Task Force recommendation statement. Ann Intern Med. 149:627–637.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lee KJ, Inoue M, Otani T, Iwasaki M,
Sasazuki S and Tsugane S; Japan Public Health Center-based
Prospective Study: Colorectal cancer screening using fecal occult
blood test and subsequent risk of colorectal cancer: a prospective
cohort study in Japan. Cancer Detect Prev. 31:3–11. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Mandel JS, Church TR, Bond JH, Ederer F,
Geisser MS, Mongin SJ, Snover DC and Schuman LM: The effect of
fecal occult-blood screening on the incidence of colorectal cancer.
N Engl J Med. 343:1603–1607. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
van Rossum LG, van Rijn AF, Laheij RJ, van
Oijen MG, Fockens P, van Krieken HH, Verbeek AL, Jansen JB and
Dekker E: Random comparison of guaiac and immunochemical fecal
occult blood tests for colorectal cancer in a screening population.
Gastroenterology. 135:82–90. 2008.
|
|
16.
|
Niv Y, Lev-El M, Fraser G, Abuksis G and
Tamir A: Protective effect of faecal occult blood test screening
for colorectal cancer: worse prognosis for screening refusers. Gut.
50:33–37. 2002. View Article : Google Scholar : PubMed/NCBI
|