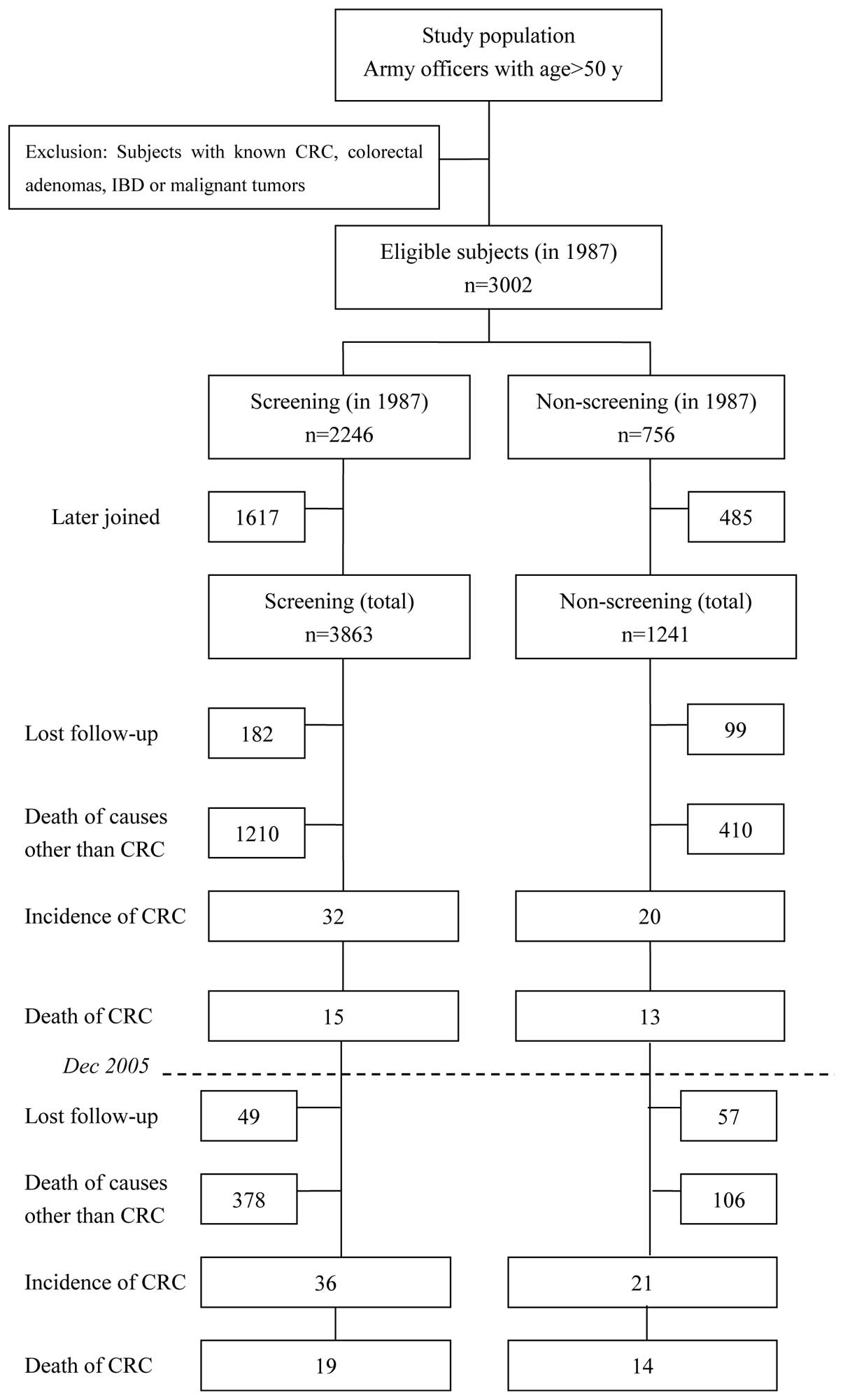
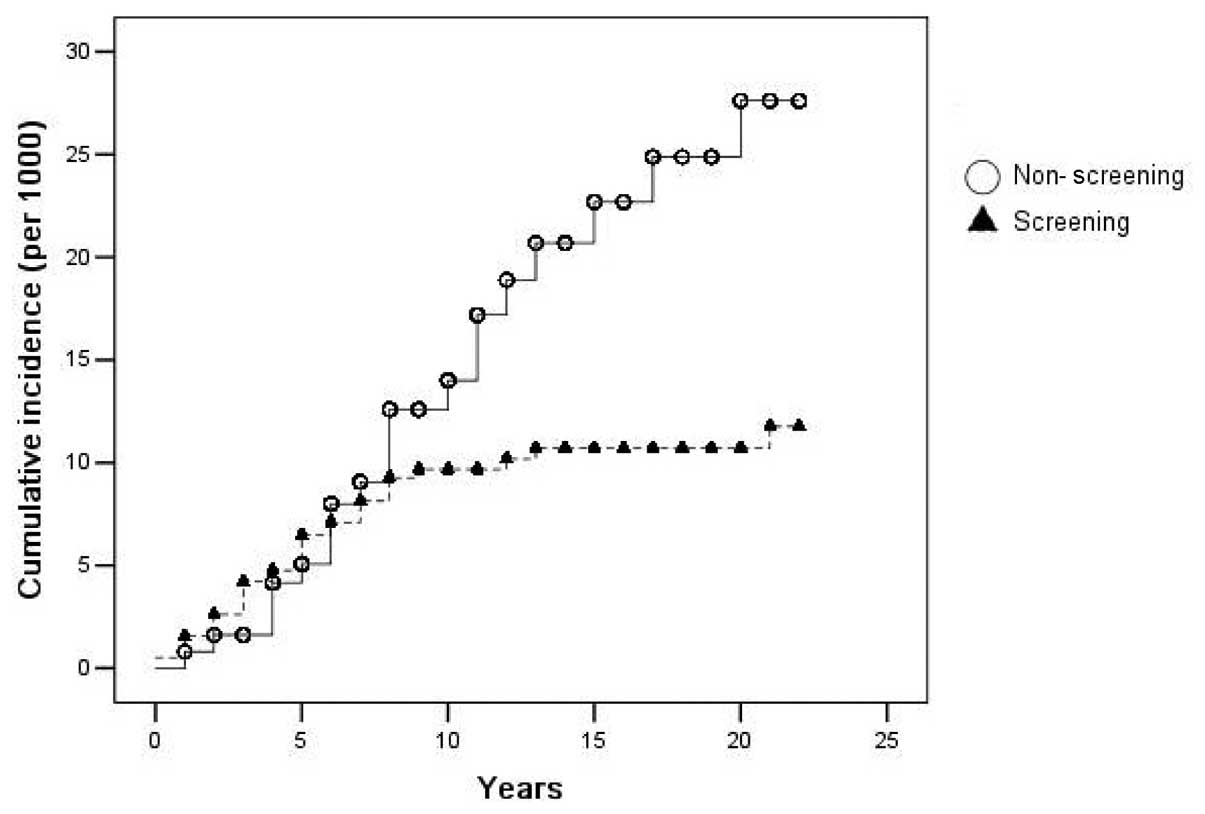
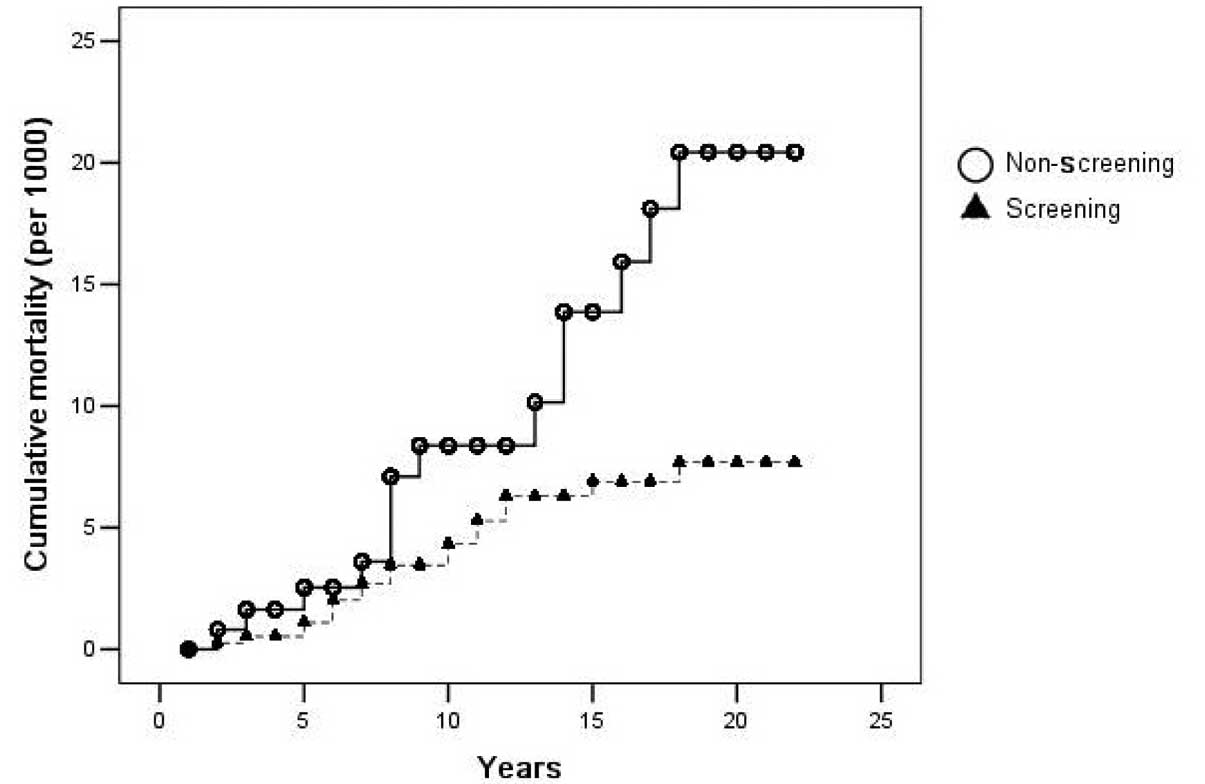
|
1.
|
Stewart BW and Kleihues P: World cancer
report. Lyon: IARC Press; pp. 163–166. 2003
|
|
2.
|
Zhang SW, Chen WQ, Kong LZ, Li LD, Lu FZ,
Li GL, Meng J and Zhao P: An analysis of cancer incidence and
mortality from 30 cancer registries in China, 1998∼2002. Zhongguo
Zhongliu. 15:430–448. 2006.
|
|
3.
|
Levin B, Lieberman DA, McFarland B,
Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S,
Johnson D, Johnson CD, et al American Cancer Society Colorectal
Cancer Advisory Group; US Multi-Society Task Force; American
College of Radiology Colon Cancer Committee: Screening and
surveillance for the early detection of colorectal cancer and
adenomatous polyps, 2008: a joint guideline from the American
Cancer Society, the US Multi-Society Task Force on Colorectal
Cancer, and the American College of Radiology. Gastroenterology.
134:1570–1595. 2008. View Article : Google Scholar
|
|
4.
|
Mandel JS, Bond JH, Church TR, Snover DC,
Bradley GM, Schuman LM and Ederer F: Reducing mortality from
colorectal cancer by screening for fecal occult blood. Minnesota
Colon Cancer Control Study. N Engl J Med. 328:1365–1371. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kronborg O, Fenger C, Olsen J, Jorgensen
OD and Søndergaard O: Randomised study of screening for colorectal
cancer with faecal-occult-blood test. Lancet. 348:1467–1471. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hardcastle JD, Chamberlain JO, Robinson
MH, Moss SM, Amar SS, Balfour TW, James PD and Mangham CM:
Randomised controlled trial of faecal-occult-blood screening for
colorectal cancer. Lancet. 348:1472–1477. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Allison JE, Sakoda LC, Levin TR, Tucker
JP, Tekawa IS, Cuff T, Pauly MP, Shlager L, Palitz AM, Zhao WK,
Schwartz JS, Ransohoff DF and Selby JV: Screening for colorectal
neoplasms with new fecal occult blood tests: update on performance
characteristics. J Natl Cancer Inst. 99:1462–1470. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Allison JE, Tekawa IS, Ransom LJ and
Adrain AL: A comparison of fecal occult-blood tests for
colorectal-cancer screening. N Engl J Med. 334:155–159. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Li S, Nie Z, Li N, Li J, Zhang P, Yang Z,
Mu S, Du Y, Hu J, Yuan S, Qu H, et al: Colorectal cancer screening
for the natural population of Beijing with sequential fecal occult
blood test: a multicenter study. Chin Med J (Engl). 116:200–202.
2003.PubMed/NCBI
|
|
10.
|
Li S, Wang H, Hu J, Li N, Liu Y, Wu Z,
Zheng Y, Wang H, Wu K, Ye H and Rao J: New immunochemical fecal
occult blood test with two-consecutive stool sample testing is a
cost-effective approach for colon cancer screening: Results of a
prospective multicenter study in Chinese patients. Int J Cancer.
118:3078–3083. 2006. View Article : Google Scholar
|
|
11.
|
Wong BC, Wong WM, Cheung KL, Tong TS,
Rozen P, Young GP, Chu KW, Ho J, Law WL, Tung HM, Lai KC, Hu WH,
Chan CK and Lam SK: A sensitive guaiac faecal occult blood test is
less useful than an immunochemical test for colorectal cancer
screening in a Chinese population. Aliment Pharmacol Ther.
18:941–946. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Calogne N, Petitti DB, DeWitt TG, Dietrich
AJ, Gregory KD, Harris R, Isham G, LeFevre ML, Leipzig RM,
Loveland-Cherry C, Marion LN, et al U.S. Preventive Services Task
Force: Screening for colorectal cancer: U.S. Preventie Services
Task Force recommendation statement. Ann Intern Med. 149:627–637.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lee KJ, Inoue M, Otani T, Iwasaki M,
Sasazuki S and Tsugane S; Japan Public Health Center-based
Prospective Study: Colorectal cancer screening using fecal occult
blood test and subsequent risk of colorectal cancer: a prospective
cohort study in Japan. Cancer Detect Prev. 31:3–11. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Mandel JS, Church TR, Bond JH, Ederer F,
Geisser MS, Mongin SJ, Snover DC and Schuman LM: The effect of
fecal occult-blood screening on the incidence of colorectal cancer.
N Engl J Med. 343:1603–1607. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
van Rossum LG, van Rijn AF, Laheij RJ, van
Oijen MG, Fockens P, van Krieken HH, Verbeek AL, Jansen JB and
Dekker E: Random comparison of guaiac and immunochemical fecal
occult blood tests for colorectal cancer in a screening population.
Gastroenterology. 135:82–90. 2008.
|
|
16.
|
Niv Y, Lev-El M, Fraser G, Abuksis G and
Tamir A: Protective effect of faecal occult blood test screening
for colorectal cancer: worse prognosis for screening refusers. Gut.
50:33–37. 2002. View Article : Google Scholar : PubMed/NCBI
|