Introduction
Gastrointestinal stromal tumors (GISTs) are the most
common mesenchymal neoplasms of the gastrointestinal tract and are
highly resistant to conventional chemotherapy (1). C-kit (also known as CD117) expression
occurs in ∼95% of GISTs, thereby enabling differentiation from
other mesenchymal spindle-cell neoplasms (2). C-kit is a transmembrane receptor that
is activated by binding of the KIT ligand, a stem cell factor. Of
all the GISTs, ∼85–90% are associated with gain-of-function KIT
gene mutations that lead to the constitutive activation of KIT
kinase activity. A significantly smaller proportion (5%) are
associated with analogous gain-of-function mutations in PDGFRA, the
gene encoding platelet derived growth factor receptor α (PDGFRα);
<10% contain no identified receptor tyrosine kinase mutations
(3–5). Experience gained from epidemiological
studies and active GIST therapeutic trials suggests that the annual
incidence of GISTs in the United States is at least 4,000 to 6,000
new cases (roughly seven to 20 cases per million population) per
year (6).
Surgical resection remains the mainstay therapy for
GISTs, but recurrence is common. The five-year survival rates for
GISTs following complete resection range between 40 and 65%
(7–11). Imatinib mesylate selectively
inhibits certain protein tyrosine kinases; intracellular ABL
kinase, chimeric BCR-ABL fusion oncoprotein of chronic myeloid
leukemia, the transmembrane receptor KIT and platelet-derived
growth factor receptors (PDG-FR) (12–15).
Imatinib mesylate has induced a sustained objective response in
>50% of patients with advanced GISTs in Western and other
countries (16–18). However, the response to imatinib
therapy is time-limited and secondary resistance to imatinib
therapy (following initial stabilization or response) develops in
the majority of patients (19).
Sunitinib malate is an oral multi-targeted receptor
tyrosine kinase inhibitor that has shown antiangiogenic and
antitumor activities in several in vitro and in vivo
tumor models (7,20–25).
Sunitinib has shown effective activity for patients with GISTs
after imatinib failure or intolerance, and has induced a sustained
clinical benefit in advanced GISTs (7,18). A
number of imatinib-resistant mutations confer cross-resistance to
sunitinib. Therefore, various agents, including sorafenib, have
been tested as salvage therapy for patients with these resistant
GISTs (26). In a prospective
multicenter phase II study involving patients with unresectable,
KIT-positive GISTs that had progressed under imatinib and sunitinib
treatment, 55% of patients who received sorafenib had stable
disease and 13% had partial responses (27). In a retrospective analysis of 32
patients, sorafenib was shown to be significantly active in
patients with metastatic GISTs resistant to imatinib and sunitinib
(28).
Based on the limited data, guidelines have included
sorafenib as an option for patients who are no longer receiving a
clinical benefit from imatinib or sunitinib (29). Therefore, the aim of the present
study was to report the results of sorafenib treatment in Turkish
GIST patients.
Materials and methods
Patients and study design
A total of 250 patients with GISTs from ten
institutions in Turkey were retrospectively evaluated. All cases of
surgically or endoscopically resected GISTs, investigated by the
pathology departments of the participating institutions (between
January 2001 and November 2012), were reviewed. Of these, the cases
of 25 patients who received sorafenib as the third- or fourth-line
treatment from eight institutions were selected for evaluation
according to the Response Evaluation Criteria in Solid Tumors
(RECIST) (30). Follow-up data were
obtained from clinical records and histopathology reports. Written
informed consent was obtained from all patients. The GISTs were
defined as primary spindle cell, epithelioid cell and mixed
neoplasms of the tubular GI tract with an overexpression of CD117
and with or without CD34 expression, according to well-established
criteria for GIST diagnosis (31,32).
Mitoses were counted in 50 high-power fields. Tumor sizes were
recorded as the largest diameter in any dimension of the primary
tumor and were classified as <2, 2–5, 5–10 or >10 cm. The
malignant potential of the GISTs was classified according to the
risk categories proposed by Fletcher et al (32). The patient, tumor and treatment
variables were recorded. Patient data included age, gender and
presentation status. All patients with any metastatic disease were
considered to have a metastatic presentation, regardless of whether
they had received prior therapy or had also had local recurrence.
All tumors were regarded as being histologically malignant.
Statistical analysis
All data were analyzed using SPSS 17.0 software
(SPSS Inc., Chicago, IL, USA). Based on the low number of patients,
non-parametric tests were selected for the evaluation. Actuarial
survival was determined by Kaplan-Meier analysis. Tumor response
rates were evaluated as complete response (CR), partial response
(PR), stable disease (SD) and progressive disease (PD) according to
the RECIST criteria (30). CR, PR
and SD were accepted as a response to sorafenib treatment, while PD
was accepted as a non-responsive to sorafenib treatment. The
duration of imatinib usage was recorded as more or less than six
months. Progression free survival (PFS) was defined as no
progression after sorafenib use. Overall survival (OS) was defined
as survival following the administration of sorafenib and mortality
was the endpoint of the study. The associations of patient, tumor
and treatment characteristics with outcome were tested by
univariate analysis using a log-rank test. A multivariate analysis
was performed using the Cox proportional hazards model, and only
variables that were deemed statistically significant were included
in the final Cox model. Multivariate P-values were used to
characterize the independence of these factors. The 95% confidence
interval (CI) was used to quantify the association between survival
time and each independent factor. All P-values were two-sided in
the tests and P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical features
Between January 2001 and November 2012, a total of
250 patients with GISTs were evaluated and 25 who had treatment
failure with imatinib and other thyrosine-kinases inhibitors were
included in the present study. All patients were previously treated
with imatinib. Of these, 17 (68.0%) were male and eight (32.0%)
were female. The median age was 54.0 years (range, 16–82 years) and
the peak age was between 40 and 60.
Of the GIST patients, 77% were diagnosed as
clinically symptomatic. The most commonly presented symptoms were
abdominal pain and non-specific symptoms due to an abdominal mass
(55.5%). The tumors most commonly originated in the stomach (20.0%)
and the small intestine (64.0%). Histologically, the majority of
tumors were predominantly spindle-shaped (36.0%) or of mixed type
(36.0%). All of the tumors were CD117-positive, while 68.0% were
CD34-positive. A total of 17 patients (68.0%) presented with
metastasis at diagnosis. Among these patients, 11 of the metastasis
sites were the liver (64.7%), three were the peritoneum (17.6%),
one was the lung (5.9%) and two were other sites (11.8%). In total,
21 patients (84.0%) received imatinib for longer than six months,
while four (16.0%) received it for less than six months (Table I).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
Characteristics | Value |
|---|
| Age, years | |
| Median
(range) | 54.0 (16–82) |
| Gender, n (%) | |
| Male | 17 (68.0) |
| Female | 8 (32.0) |
| Tumor site, n
(%) | |
| Stomach | 6 (24.0) |
| Small
intestine | 16 (64.0) |
| Colon | 1 (4.0) |
| Other sites | 2 (8.0) |
| Histopathology, n
(%) | |
| Spindle cell
type | 9 (36.0) |
| Epitheloid | 7 (28.0) |
| Mixed type | 9 (36.0) |
| Cd117 positivity, n
(%) | |
|
Cd117+, Cd34+ | 17 (68.0) |
|
Cd117+, Cd34− | 8 (32.0) |
| Site of metastases,
n (%) | |
| Liver | 11 (64.7) |
| Peritoneum | 3 (17.6) |
| Lung | 1 (5.9) |
| Others | 2 (11.8) |
| Duration of
imatinib usage, n (%) | |
| <6 months | 4 (16.0) |
| >6 months | 21 (84.0) |
The majority of the tumors (72.0%) were >10 cm in
size. Based on the size of the primary tumor, the localization and
the mitotic index, 24.0% of the patients were classified as
intermediate-risk and 76.0% as high-risk according to the NIH risk
classification. There were no extremely low- or low-risk groups
(Table II).
 | Table II.Tumor characteristics of 25 patients
with gastrointestinal stromal tumors receiving sorafenib
treatment. |
Table II.
Tumor characteristics of 25 patients
with gastrointestinal stromal tumors receiving sorafenib
treatment.
| Variables | Number of patients
(%) |
|---|
| Tumor size, cm | |
| 2–5 | 3 (12.0) |
| 5–10 | 4 (16.0) |
| >10 | 18 (72.0) |
| Mitosis, HPF | |
| ≤5/50 | 8 (32.0) |
| >6–10/50 | 5 (20.0) |
| >10/50 | 12 (48.0) |
| Fletcher risk
categories | |
| Very low | 0 (0.0) |
| Low | 0 (0.0) |
| Intermediate | 6 (24.0) |
| High | 19 (76.0) |
Treatment outcomes
Sorafenib was administered to 25 patients and all
patients were followed up after the administration at regular
intervals until mortality or the time this manuscript was written.
The dosage of sorafenib was 2× 400 mg/day at the beginning of the
treatment. Treatment was continued until the patient no longer
clinically benefitted from therapy or until unacceptable toxicity
occurred. Temporary dose interruption and/or dose reduction of
sorafenib therapy was provided if an intolerance or any adverse
effects occurred.
A total of 18 (72.0%) patients received 400 mg/day
imatinib and seven patients (28.0%) with PD received 600–800 mg/day
imatinib in second-line treatment. All the patients had PD during
imatinib treatment and no patients were intolerant to imatinib.
Of the patients, 12 received sorafenib in the
third-line and 13 received it in the fourth-line treatment. Nine of
the patients who received sorafenib in the fourth-line received
sunitinib and three received nilotinib in the third-line treatment.
Overall, 10 (40%) of the patients achieved responses while
receiving sorafenib. This represents the clinical benefit of
sorafenib as determined by the sum of PR and SD. No patients
achieved CR. Six patients (24.0%) achieved PR and four (16.0%)
achieved SD during sorafenib usage. A total of 15 (60%) patients
showed PD at the time of analysis according to RECIST.
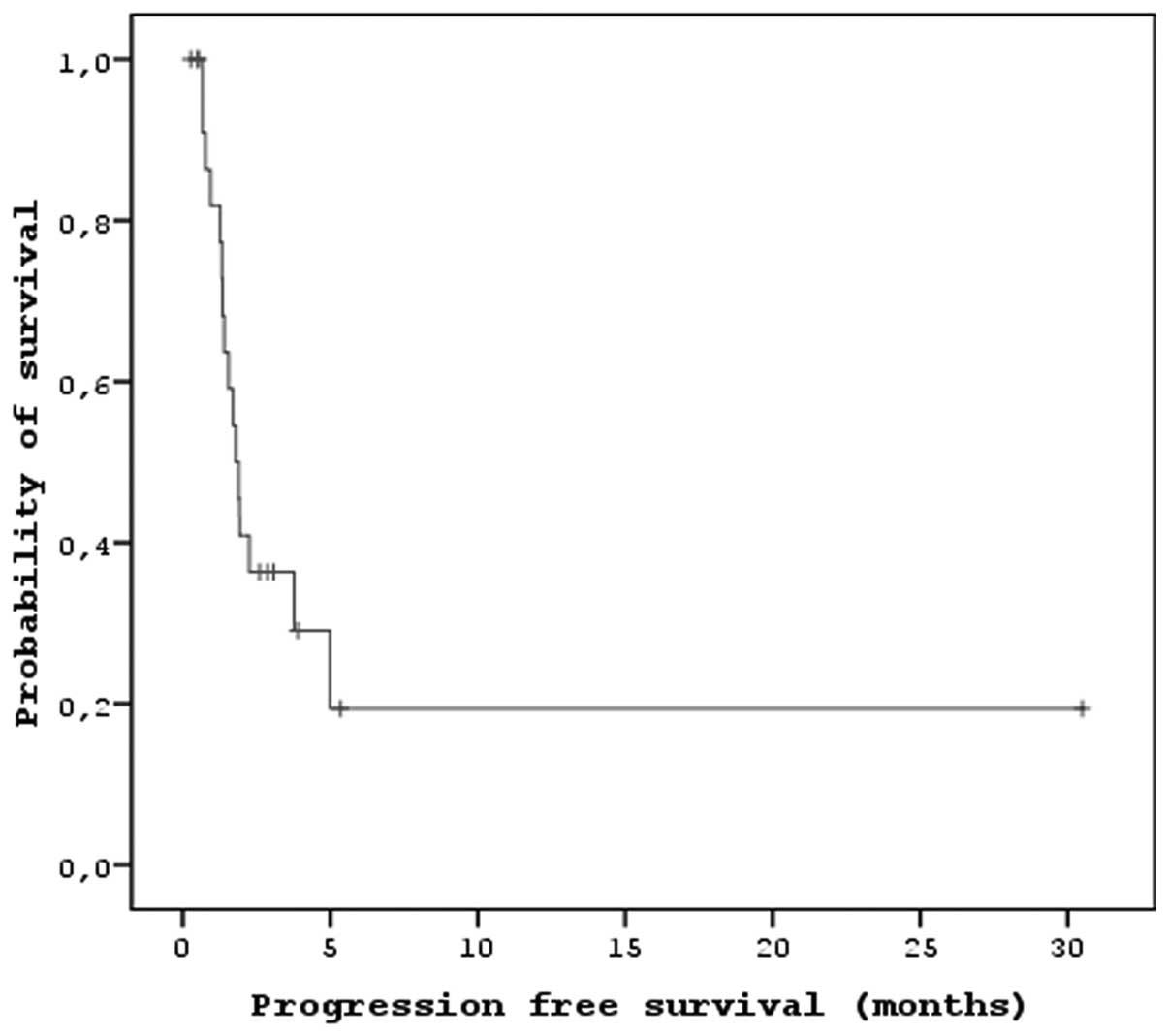
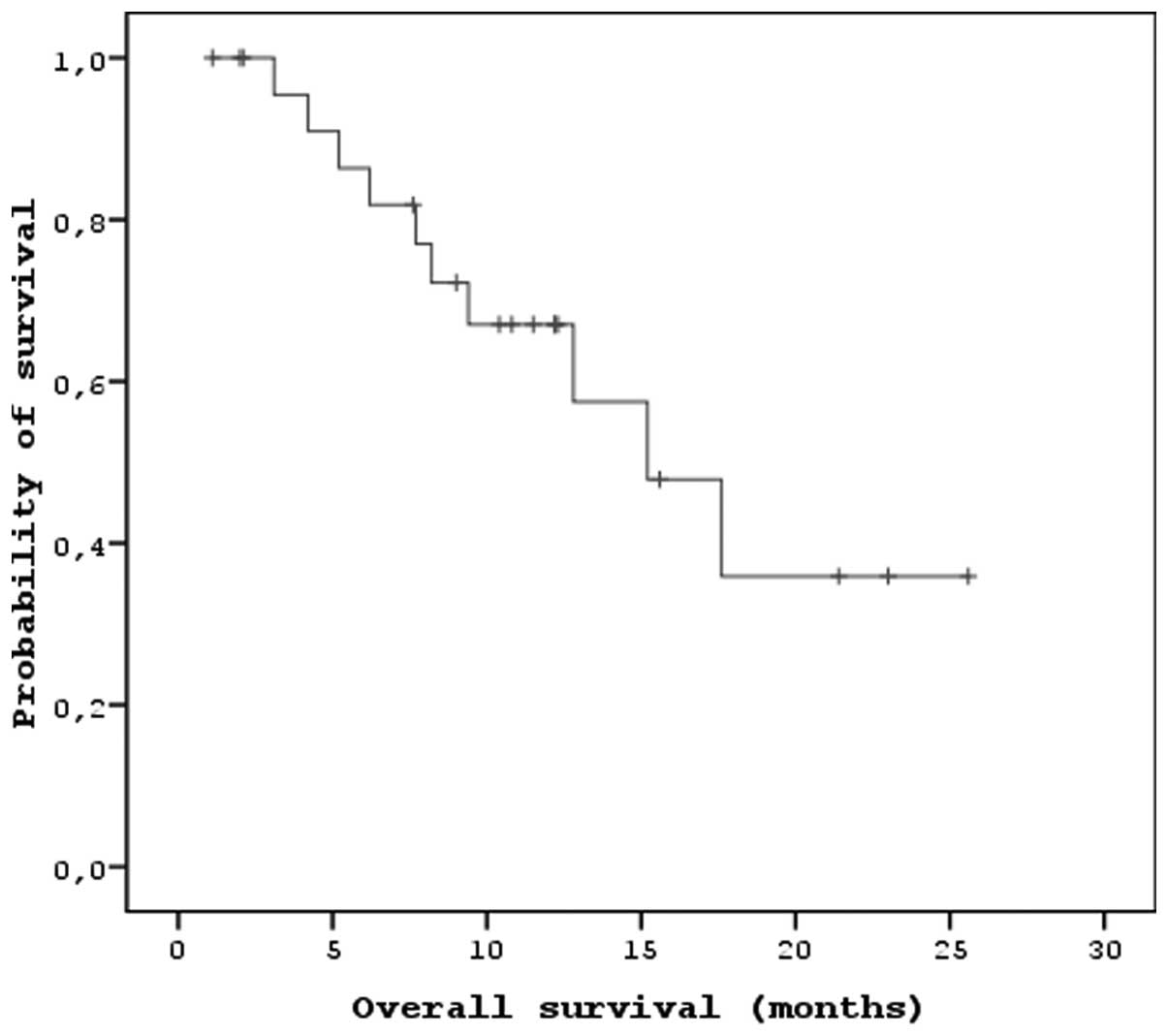
The median PFS and OS times of the patients that
received sorafenib were 7.2 months (95% CI, 5.47–8.92) and 15.2
months (95% CI, 9.26–21.13), respectively (Figs. 1 and 2). In the univariate analysis, there were
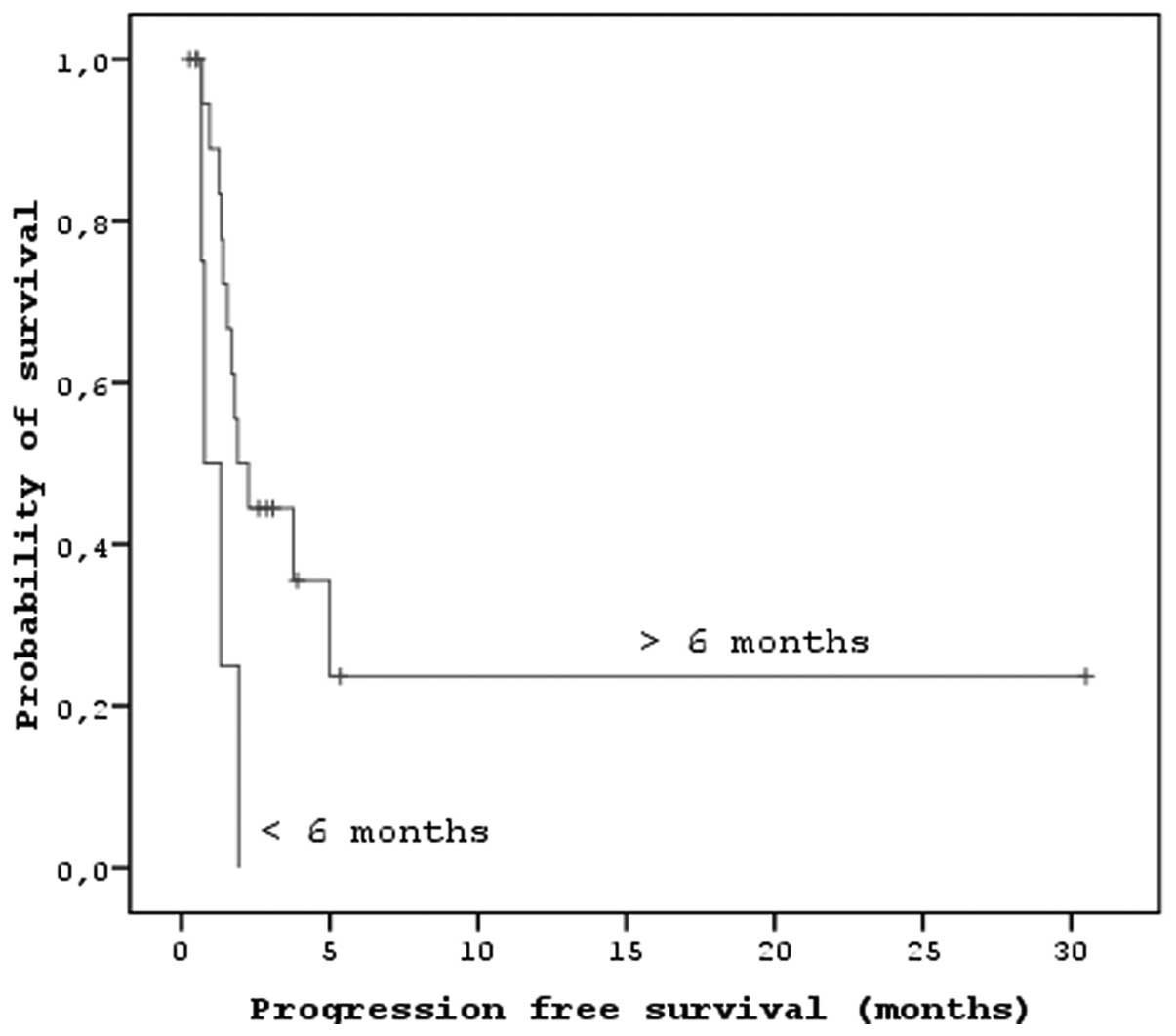
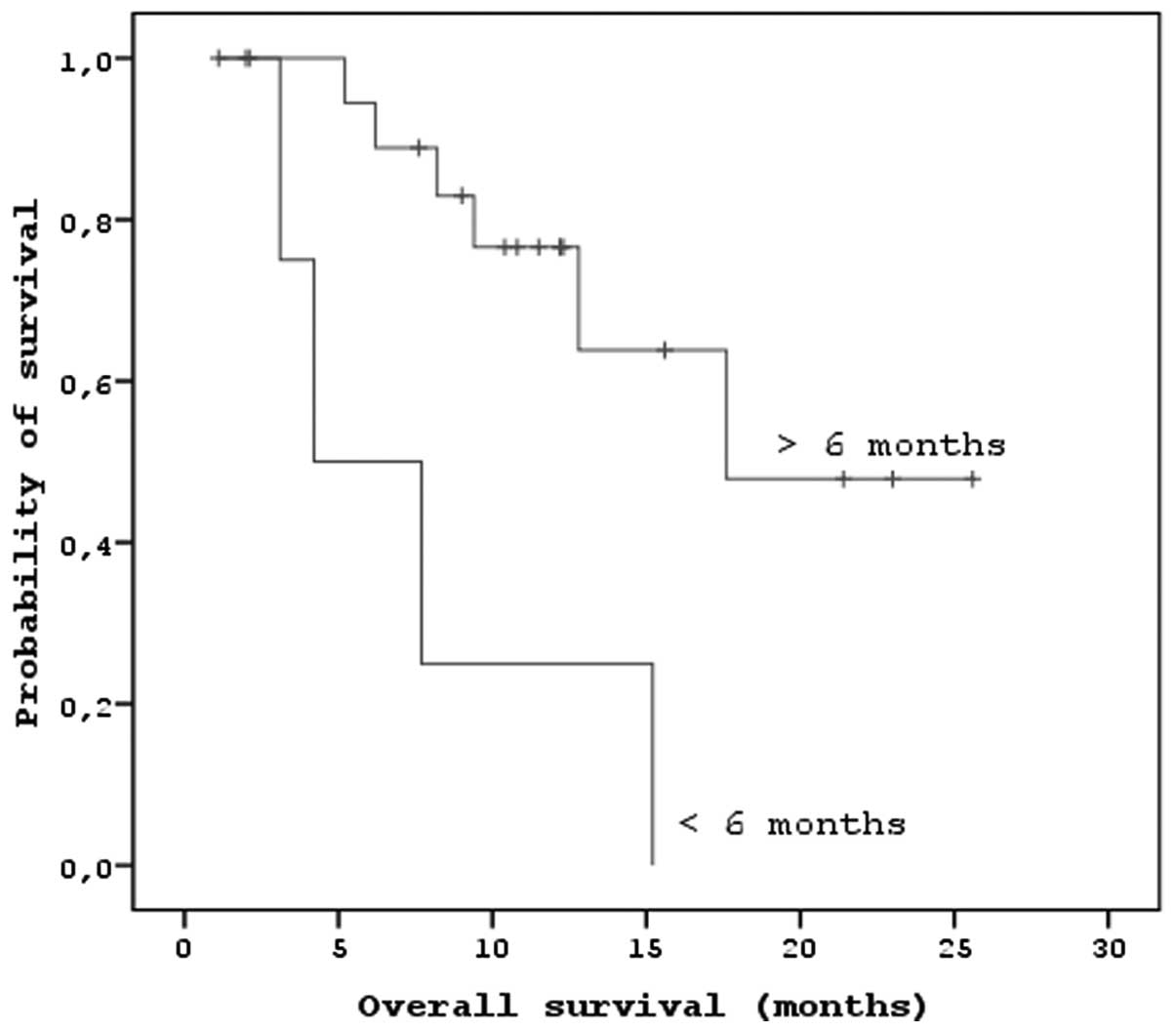
significant correlations between localization (P<0.01) and PFS.
There were also significant correlations between the duration of
imatinib usage, the response to sorafenib and the PFS and OS
(P<0.05; Figs. 3 and 4). There were no associations between age,
gender, tumor risk category, imatinib dose, duration of sunitinib
usage and PFS and OS (P>0.05). In the multivariate analysis,
there were significant associations between the duration of
imatinib usage and PFS (P<0.05) and OS (P<0.05; Tables III and IV).
 | Table III.Univariate analysis between
clinopathological characteristics of the patient group and OS and
PFS |
Table III.
Univariate analysis between
clinopathological characteristics of the patient group and OS and
PFS
| Variables | PFS
| OS
|
|---|
| Median
(months) | 95% CI
(months) | P-value | Median
(months) | 95% CI
(months) | P-value |
|---|
| Age, years | | | 0.052 | | | 0.482 |
| <50 | 15.08 | 6.89–29.47 | | 15.06 | 10.13–20.02 | |
| >50 | 6.94 | 2.43–8.00 | | 15.32 | 7.84–22.31 | |
| Gender | | | 0.866 | | | 0.858 |
| Men | 5.29 | 2.31–8.26 | | 16.59 | 10.37–22.87 | |
| Women | 15.08 | 8.50–17.67 | | 12.89 | 7.46–18.92 | |
| Tumor site | | | 0.008 | | | 0.223 |
| Stomach | 15.08 | 9.56–17.32 | | 17.18 | 8.83–25.52 | |
| Small
intestine | 6.29 | 5.08–6.49 | | 15.12 | 12.63–21.60 | |
| Colon | 2.73 | 2.43–2.83 | | 10.15 | 10.15–10.15 | |
| Other sites | 2.62 | NA | | 9.86 | NA | |
| Histopathology | | | 0.499 | | | 0.179 |
| Spindle cell
type | 3.02 | 2.53–6.14 | | 17.77 | NA | |
| Epitheloid | 4.76 | NA | | 11.99 | NA | |
| Mixed type | 15.08 | 8.14–30.35 | | 19.37 | 15.93–22.80 | |
| Tumor size, cm | | | 0.709 | | | 0.811 |
| 2–5 | 4.76 | NA | | 11.99 | NA | |
| 5–10 | 5.22 | 2.66–8.25 | | 10.58 | 3.94–18.81 | |
| >10 | 7.65 | 1.24–14.06 | | 15.02 | 11.33–21.12 | |
| Mitotic count,
HPF | | | 0.018 | | | 0.233 |
| ≤5/50 | 15.08 | 12.73–22.79 | | 15.00 | 13.46–22.51 | |
| >6–10/50 | 4.76 | 2.44–7.17 | | 11.99 | 7.19–14.08 | |
| >10/50 | 2.43 | 1.05–6.66 | | 6.59 | 3.44–11.16 | |
| Duration of
imatinib usage, months | | | 0.018 | | | 0.003 |
| <6 | 3.10 | 1.15–5.64 | | 4.20 | 2.78–8.56 | |
| >6 | 7.60 | 3.85–11.34 | | 17.60 | 12.78–23.68 | |
| Response to
sorafenib | | | 0.000 | | | 0.007 |
| PR-SD | 12.08 | 11.36–20.76 | | 15.87 | 11.10–34.51 | |
| PD | 5.60 | 4.12–6.83 | | 10.56 | 3.80–20.17 | |
 | Table IV.The multivariate analysis between
clinopathological characteristics of the patient group and OS and
PFS. |
Table IV.
The multivariate analysis between
clinopathological characteristics of the patient group and OS and
PFS.
| Variables | PFS
| OS
|
|---|
| Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Duration of
imatinib usage | 0.079 | 0.007–0.895 | 0.04 | 0.058 | 0.005–0.65 | 0.021 |
Treatment-related adverse events were reported in
72% of the patients. These adverse events were generally mild to
moderate in intensity and managed by dose reduction or standard
supportive medical treatments. Hypertension only occurred in one
patient and this was managed with anti-hypertensive drugs. None of
the patients discontinued sorafenib treatment due to adverse
events. The most common adverse events of any grade were skin
rashes (54%), thrombocytopenia (34%) and fatigue (38%). The most
common grade III side-effect was hand-foot syndrome (HFS; 38%); 41%
of these patients received dose reductions due to HFS.
Discussion
Approximately 50% of patients with GIST eventually
develop progression within 24 months of imatinib treatment
(33). Patients with advanced GIST
who undergo disease progression or are intolerant to first-line
imatinib therapy usually start second-line sunitinib malate
therapy. As has been observed for imatinib in a first-line setting,
the majority of patients showing an initial clinical benefit from
sunitinib develop PD (34). In a
prospective multicenter phase II study involving patients with
unresectable, KIT-positive GIST that had progressed on treatment
with imatinib and sunitinib, the median PFS and OS times were 5.2
and 11.6 months, respectively, while the one- and two-year survival
rates were 50 and 29%, respectively (27). In a retrospective analysis of 32
patients, sorafenib exhibited significant clinical activity in a
heavily pretreated group of patients with metastatic GIST resistant
to imatinib, sunitinib and nilotinib (28). In a more recent study, patients with
metastatic GISTs refractory to first-line imatinib and second-line
sunitinib were treated at the discretion of their physician. The
authors concluded that sorafenib had significant clinical activity
in imatinib-resistant and sunitinib-resistant GISTs (34). In the present study, as in these
studies, in heavily pretreated patients, the median PFS and OS
times of the patients that received sorafenib were 7.2 and 15.2
months, respectively. Thus, the present study demonstrated the
improved effect of sorafenib in patients with metastatic GIST who
experience previous treatment failure.
In the present study, the duration of imatinib usage
significantly affected PFS and OS. Demetri et al detected no
association between the duration of imatinib treatment and survival
(7). In the present study, the
majority of the patients treated with sunitinib used imatinib for
>12 months. Of these patients, those who received imatinib for
longer had improved OS and PFS times than those who received it for
a shorter time. There were no significant associations between the
dose of imatinib and OS and PFS (P>0.05).
The current risk-group stratification according to
Fletcher et al does not include the possible effect of the
tumor site (32). Another recently
suggested GIST risk-group stratification system takes the tumor
site into account, as well as tumor size and the mitotic rate,
dividing GISTs into possibly benign, uncertain or low malignancy
potential and possibly malignant (35). In the present study, the majority of
patients were classified as high-risk (76.0%) according to the NIH
risk classification. This result was significantly higher than that
reported by previous studies (36,37).
There were no associations between tumor size, mitosis, risk
category and PFS and OS. These differences may be explained by the
progressive nature of the GISTs in patients receiving sorafenib
treatment and should be evaluated with further studies.
In the present study, the clinical benefit rate of
sorafenib treatment was 40%. This clinical benefit rate was lower
than that in the phase II study reported by Kindler et al
(27). Reichardt et al
reported that 19% of patients who received sorafenib after the
failure of imatinib, sunitinib and nilotinib in fourth-line
treatment achieved partial remission, while 44% achieved disease
stabilization (28). Based on the
response rates achieved in the present study and these previous
studies, sorafenib treatment may be accepted as clinically
beneficial, although the limited experience with regard to response
rates while using sorafenib treatment should be further evaluated
with larger prospective series. Additionally, to determine the best
practice in the third-line or fourth-line treatment, randomized,
prospective comparative studies between sorafenib and other agents
such as regorafenib should be conducted.
Italiano et al observed that albumin levels
and KIT/PDGFRA mutational status were significantly associated with
PFS, whereas performance status and albumin level were associated
with OS (34). Furthermore,
Heinrich et al indicated that sorafenib was more effective
than imatinib or sunitinib for inhibiting the kinase activity of
drug-resistant KIT mutants (26).
As a limitation to the present study we were unable to determine
the kinase mutations in our patients. In Turkey, it is not a
routine practice to determine kinase mutations. Kinase mutations
may explain the differences in the longevity of the PFS and OS and
lower response rates in the present study. Therefore, an analysis
of these mutations should be performed in further studies.
Sorafenib treatment is associated with several
adverse effects. Fatigue, skin rashes and hematological toxicity
were the most common side-effects in the present study. These
side-effects are generally mild and may be managed by dose
modulation. The toxicity profile reported in the present study was
similar to that observed in previous studies, with the exception of
hypertension (27,38). No serious treatment-related
hypertension was observed with sorafenib and there was no treatment
discontinuation.
In summary, sorafenib is an active and effective
agent with a reasonable side-effect profile in the treatment of
patients with gastrointestinal stromal tumors in third- or
fourth-line treatments that are refractory to previous therapies. A
significant number of patients with advanced GIST benefitted from
sorafenib, with OS times exceeding one year. It was observed that
the duration of imatinib usage was a significant independent
prognostic factor for PFS and OS. Future prospective studies of
sorafenib in GIST should investigate these factors to clarify the
correlations of this clinical benefit.
Acknowledgements
The authors would like to thank the
patients and advocates who supported the present study and the
teams of research nurses and study coordinators at all centres who
made this work possible.
References
|
1.
|
Rubin BP, Heinrich MC and Corless CL:
Gastrointestinal stromal tumour. Lancet. 369:1731–1741. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors: review on morphology, molecular pathology,
prognosis, and differential diagnosis. Arch Pathol Lab Med.
130:1466–1478. 2006.PubMed/NCBI
|
|
3.
|
Hirota S, Isozaki K, Moriyama Y, et al:
Gain-of-function mutations of c-kit in human gastrointestinal
stromal tumors. Science. 279:577–580. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Heinrich MC, Corless CL, Demetri GD, et
al: Kinase mutations and imatinib response in patients with
metastatic gastrointestinal stromal tumor. J Clin Oncol.
21:4342–4349. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Heinrich MC, Corless CL, Duensing A, et
al: PDGFRA activating mutations in gastrointestinal stromal tumors.
Science. 299:708–710. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Tran T, Davila JA and El-Serag HB: The
epidemiology of malignant gastrointestinal stromal tumors: an
analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol.
100:162–168. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Demetri GD, von Mehren M, Blanke CD, et
al: Efficacy and safety of imatinib mesylate in advanced
gastrointestinal stromal tumors. N Engl J Med. 347:472–480. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Akwari OE, Dozois RR, Weiland LH and
Beahrs OH: Leiomyosarcoma of the small and large bowel. Cancer.
42:1375–1384. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Shiu MH, Farr GH, Papachristou DN and
Hajdu SI: Myosarcomas of the stomach: natural history, prognostic
factors and management. Cancer. 49:177–187. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
McGrath PC, Neifeld JP, Lawrence W Jr, Kay
S, Horsley JS 3rd and Parker GA: Gastrointestinal sarcomas.
Analysis of prognostic factors. Ann Surg. 206:706–710. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ng EH, Pollock RE, Munsell MF, Atkinson EN
and Romsdahl MM: Prognostic factors influencing survival in
gastrointestinal leiomyosarcomas. Implications for surgical
management and staging. Ann Surg. 215:68–77. 1992. View Article : Google Scholar
|
|
12.
|
Druker BJ, Tamura S, Buchdunger E, et al:
Effects of a selective inhibitor of the Abl tyrosine kinase on the
growth of Bcr-Abl positive cells. Nat Med. 2:561–566. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Buchdunger E, Cioffi CL, Law N, et al: Abl
protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal
transduction mediated by c-kit and platelet-derived growth factor
receptors. J Pharmacol Exp Ther. 295:139–145. 2000.
|
|
14.
|
Heinrich MC, Griffith DJ, Druker BJ, Wait
CL, Ott KA and Zigler AJ: Inhibition of c-kit receptor tyrosine
kinase activity by STI 571, a selective tyrosine kinase inhibitor.
Blood. 96:925–932. 2000.PubMed/NCBI
|
|
15.
|
Wang WL, Healy ME, Sattler M, et al:
Growth inhibition and modulation of kinase pathways of small cell
lung cancer cell lines by the novel tyrosine kinase inhibitor STI
571. Oncogene. 19:3521–3528. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Yeh CN, Chen TW, Wu TJ, Hsueh S and Jan
YY: Treatment of patients with advanced gastrointestinal stromal
tumor of small bowel: implications of imatinib mesylate. World J
Gastroenterol. 12:3760–3765. 2006.PubMed/NCBI
|
|
17.
|
Yeh CN, Chen TW, Lee HL, et al: Kinase
mutations and imatinib mesylate response for 64 Taiwanese with
advanced GIST: preliminary experience from Chang Gung Memorial
Hospital. Ann Surg Oncol. 14:1123–1128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Chen YY, Yeh CN, Cheng CT, et al:
Sunitinib for Taiwanese patients with gastrointestinal stromal
tumor after imatinib treatment failure or intolerance. World J
Gastroenterol. 17:2113–2119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Rutkowski P, Debiec-Rychter M and Ruka W:
Gastrointestinal stromal tumors: key to diagnosis and choice of
therapy. Mol Diagn Ther. 12:131–143. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Osusky KL, Hallahan DE, Fu A, et al: The
receptor tyrosine kinase inhibitor SU11248 impedes endothelial cell
migration, tubule formation, and blood vessel formation in vivo,
but has little effect on existing tumor vessels. Angiogenesis.
7:225–233. 2004. View Article : Google Scholar
|
|
21.
|
Abrams TJ, Lee LB, Murray LJ, Pryer NK and
Cherrington JM: SU11248 inhibits KIT and platelet-derived growth
factor receptor beta in preclinical models of human small cell lung
cancer. Mol Cancer Ther. 2:471–478. 2003.PubMed/NCBI
|
|
22.
|
Mendel DB, Laird AD, Xin X, et al: In vivo
antitumor activity of SU11248, a novel tyrosine kinase inhibitor
targeting vascular endothelial growth factor and platelet-derived
growth factor receptors: determination of a
pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res.
9:327–337. 2003.
|
|
23.
|
Murray LJ, Abrams TJ, Long KR, et al:
SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an
experimental breast cancer bone metastasis model. Clin Exp
Metastasis. 20:757–766. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
O’Farrell AM, Abrams TJ, Yuen HA, et al:
SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent
activity in vitro and in vivo. Blood. 101:3597–3605.
2003.PubMed/NCBI
|
|
25.
|
Schueneman AJ, Himmelfarb E, Geng L, et
al: SU11248 maintenance therapy prevents tumor regrowth after
fractionated irradiation of murine tumor models. Cancer Res.
63:4009–4016. 2003.PubMed/NCBI
|
|
26.
|
Heinrich MC, Marino-Enriquez A, Presnell
A, et al: Sorafenib inhibits many kinase mutations associated with
drug-resistant gastrointestinal stromal tumors. Mol Cancer Ther.
11:1770–1780. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Kindler HL, Campbell NP, Wroblewski K, et
al: Sorafenib (SOR) in patients (pts) with imatinib (IM) and
sunitinib (SU)-resistant (RES) gastrointestinal stromal tumors
(GIST): Final results of a University of Chicago Phase II
Consortium trial. J Clin Oncol (ASCO Meeting Proceedings).
29:100092011.
|
|
28.
|
Reichardt P, Montemurro M, Gelderblom H,
et al: Sorafenib fourth-line treatment in imatinib-, sunitinib-,
and nilotinib-resistant metastatic GIST: A retrospective analysis.
J Clin Oncol (2009 ASCO Annual Meeting). 27(15 Suppl): Abstract
10564,. 2009.
|
|
29.
|
National Comprehensive Cancer Network:
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines):
Soft Tissue Sarcoma. Version 2. 2012.www.NCCN.orghttps://www.NCCN.org. Accessed June 17,
2012.
|
|
30.
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
31.
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors - definition, clinical, histological,
immunohistochemical, and molecular genetic features and
differential diagnosis. Virchows Arch. 438:1–12. 2001. View Article : Google Scholar
|
|
32.
|
Fletcher CD, Berman JJ, Corless C, et al:
Diagnosis of gastrointestinal stromal tumors: A consensus approach.
Hum Pathol. 33:459–465. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Verweij J, Casali PG, Zalcberg J, et al:
Progression-free survival in gastrointestinal stromal tumours with
high-dose imatinib: randomised trial. Lancet. 364:1127–1134. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Italiano A, Cioffi A, Coco P, et al:
Patterns of care, prognosis, and survival in patients with
metastatic gastrointestinal stromal tumors (GIST) refractory to
first-line imatinib and second-line sunitinib. Ann Surg Oncol.
19:1551–1559. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Miettinen M, El-Rifai W, Sobin LH and
Lasota J: Evaluation of malignancy and prognosis of
gastrointestinal stromal tumors: a review. Hum Pathol. 33:478–483.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Nilsson B, Bümming P, Meis-Kindblom JM, et
al: Gastrointestinal stromal tumors: the incidence, prevalence,
clinical course, and prognostication in the preimatinib mesylate
era - a population-based study in western Sweden. Cancer.
103:821–829. 2005. View Article : Google Scholar
|
|
37.
|
Cao H, Zhang Y, Wang M, et al: Prognostic
analysis of patients with gastrointestinal stromal tumors: a single
unit experience with surgical treatment of primary disease. Chin
Med J (Engl). 123:131–136. 2010.PubMed/NCBI
|
|
38.
|
Keohan M, D’Adamo D, Qin L, et al:
Analysis of toxicity in a phase II study of sorafenib in soft
tissue sarcoma (STS). J Clin Oncol 2007 ASCO Annual Meeting
Proceedings (Post-Meeting Edition). 25(18 Suppl): 100612007.
|