Introduction
Oesophageal carcinoma is the fourth most common type
of malignant carcinoma and has a high mortality rate in China
(1). Despite long-term studies,
understanding of the molecular changes underlying oesophageal
carcinoma is limited. Previous studies have hypothesised that
tumourigenesis represents an independent process governed by genes
carried by tumour cells (2,3). However, further studies have
demonstrated that carcinoma behaviour is also affected by the
tumour microenvironment, including the extracellular matrix, blood
vasculature, inflammatory cells and myofibroblasts (4). Notably, among these components,
cancer-associated fibroblasts (CAFs) play a predominant role in
carcinogenesis (5). The activation
of CAFs correlates with the expression of α-smooth muscle actin
(α-SMA), which is the most commonly used marker for CAFs (6,7).
Numerous families of growth factors secreted by cancer cells and
CAFs are involved in carcinoma initiation and progression.
Transforming growth factor β (TGFβ) is capable of regulating the
growth, differentiation, migration, adhesion and apoptosis of cells
by binding to the TGFβ receptors (TβR-I and -II) (8). Studies have demonstrated that the
TGFβ1-Smad signalling pathway is involved in the progression and
prognosis of several types of carcinomas, including oesophageal
(9), colorectal (10) and gastric (11) carcinoma. Hepatocyte growth factor
(HGF) is a multifunctional cytokine produced by tumour cells and
myofibroblasts in tumour stroma. HGF exerts multiple functions,
including cell proliferation, migration and metastases (12,13),
by binding to c-met, a receptor expressed in epithelial cells. The
majority of previous studies have analysed TGFβ1 (9,14) and
HGF (15,16) protein expression in oesophageal
squamous cell carcinoma (ESCC) but not in precancerous lesions. In
addition, few studies have investigated the functional and clinical
significance of TGFβ1 and HGF proteins, secreted by dysplasia
epithelial cells, cancer cells and stroma fibroblasts, in
oesophageal carcinogenesis. Therefore, the present study aimed to
examine the significance of TGFβ1 and HGF proteins in oesophageal
carcinogenesis and angiogenesis.
Materials and methods
Tissue collection and processing
A total of 136 patients, 88 males and 48 females,
treated at The Fourth Hospital of Hebei Medical University (Hebei,
China) between August, 2006 and August, 2010, were enrolled in the
current study (mean age, 62 years; range, 46–75 years). Normal
oesophageal tissue, obtained from a distance of >5 cm between
the normal oesophageal tissue and the edge of the cardiac
carcinoma, was obtained from patients undergoing cardiac carcinoma
resection. Oesophageal precancerous lesions were obtained from
patients undergoing endoscopic mucosal resection for oesophageal
precancerous lesions, and oesophageal carcinoma specimens were
obtained from patients undergoing oesophagectomy surgery. Written
informed consent was provided by all participants and the study was
approved by the ethics committee of Hebei Medical University.
Individuals had not undergone radiotherapy and chemotherapy prior
to oesophagectomy or gastrectomy, and tissues were fixed with 85%
alcohol, embedded with paraffin and serially sectioned at 5
μm. Sections were mounted onto histostick-coated slides
(Haimen Experiment Equipment Factory, Jiangsu, China) and four to
five adjacent ribbons were collected for haematoxylin and eosin and
immunohistochemical staining.
Classification of pathology
In accordance with the Classification of Tumours of
the Digestive System established by the World Health Organisation
(17), 136 specimens were divided
into 5 groups according to tissue type. These included normal, low-
and high-grade intraepithelial neoplasia (LGIEN and HGIEN,
respectively), carcinoma in situ (CIS) and squamous cell
carcinoma (SCC), and comprised 20, 26, 44, 23 and 23 cases,
respectively.
Immunohistochemistry (IHC)
Paraffin-embedded sections were deparaffinised with
xylene and rehydrated. Sections were incubated with
H2O2 (concentration, 3%) for 30 min at room
temperature. Sections were immersed in 0.01 M citrate buffer (pH
6.0) at 95°C for 10 min for antigen retrieval, and then immersed in
phosphate-buffered saline (PBS) for 15 min at room temperature.
Following blocking, the sections were incubated at 4°C overnight
with primary antibodies, including mouse anti-human α-SMA
monoclonal (1:100), mouse anti-human CD34 monoclonal (1:80; Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China), rabbit
anti-human TGFβ1 polyclonal (1:100; Bioworld Technology Inc.,
Nanjing, China) and rabbit anti-human HGF polyclonal (1:100; Santa
Cruz Biotechnology, Inc,, Santa Cruz, CA, USA) antibodies, and
subsequently washed with PBS. Sections were incubated with
biotin-conjugated goat anti-mouse or rabbit IgG at 37°C for 1 h,
and visualization was achieved with peroxidase-labelled
streptavidin-biotin and diaminobenzidine. Slides were subsequently
counterstained with Mayer’s haematoxylin, dehydrated and
mounted.
Interpretation of IHC
Cytoplasmic staining of HGF and TGFβ1 was scored by
the percentage of positive cells (0, <10%; 1, 10–25%; 2, 26–50%
and 3, >51%), where 0 was classified as negative expression (−)
and 1–3 was classified as positive expression (+). A specimen of
invasive breast and hepatic carcinoma served as a positive control
for TGFβ1 and HGF, respectively. Absence of α-SMA immunostaining in
the myofibroblasts was classified as negative (−) and
immunostaining patterns, including focal or diffuse, weak or
strong, were classified as positive (+).
Anti-CD34 antibody was used to stain endothelial
cells and detect microvessel density (MVD), as described previously
(18). A single endothelial cell or
cluster of endothelial cells, with or without a lumen, was
hypothesised to represent individual vessels. However, vessels with
thick muscular walls or of a caliber of >8 red blood cells were
excluded. Highly vascular areas were identified by scanning
sections at low power magnification (×100) to determine three hot
spots. The MVD is presented as the mean of the highest three counts
at high power magnification (×200), and the slides were interpreted
by two independent observers (Xiaoling Wang and Zhiming Dong).
Statistical analysis
Results were analysed using SPSS software, version
13.0 (SPSS, Inc., Chicago, IL, USA). Comparison of the expression
of α-SMA, TGFβ1 and HGF among various clinical and histological
parameters was performed using the Pearson’s χ2 test and
the Fisher’s exact test. Correlations among the various factors
were analysed using the Spearman’s rank correlation and values of
MVD were analysed with analysis of variance and Dunnett’s tests.
Data are presented as the mean ± standard deviation. A two-sided
P<0.05 was considered to indicate a statistically significant
difference.
Results
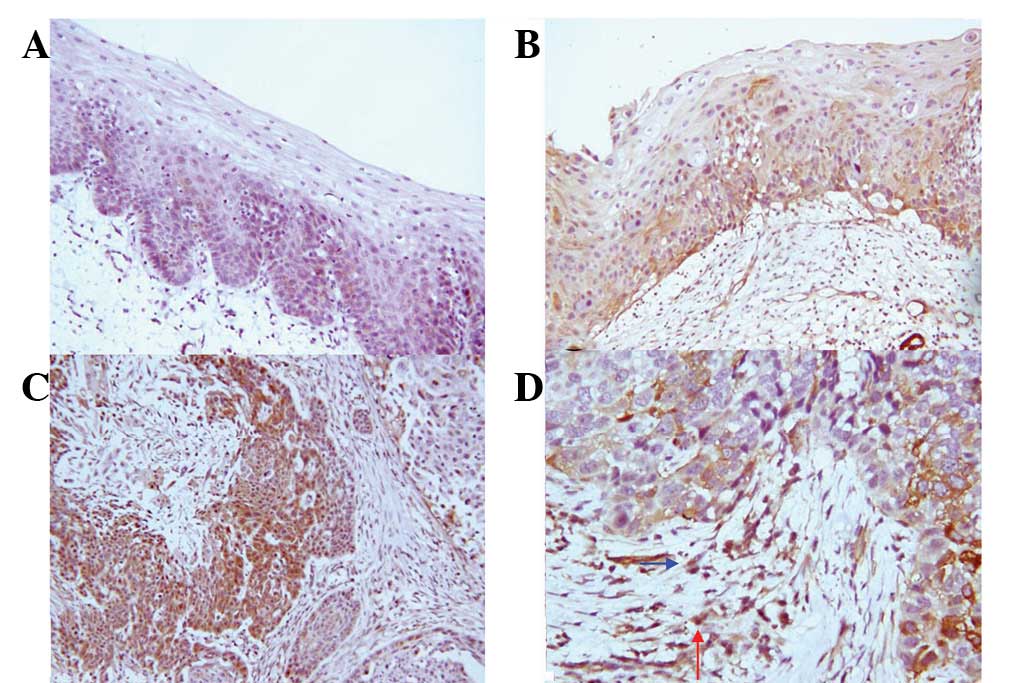
Stromal fibroblast expression of α-SMA in
oesophageal carcinogenesis
The IHC results showed ascending rates of stromal
fibroblast expression of α-SMA in oesophageal carcinogenesis. The
majority of α-SMA-positive fibroblasts were distributed in the
oesophageal stroma surrounding cancer nests or adjacent to
dysplasia cells (Fig. 1). No
significant differences were identified between the positive rates
of α-SMA expression with respect to gender and age. The positive
rates of α-SMA expression in the HGIEN, CIS and SCC groups were
statistically significant when compared with that of the normal
group; however, no significant difference in α-SMA expression rates
was identified between the LGIEN and normal groups.
 | Figure 1.Expression of α-SMA in stromal
fibroblasts in esophageal carcinogenesis. (A and B) α-SMA-negative
NM and LGIEN tissues, respectively (SP staining; magnification,
×200). (C–E) α-SMA-positive expression in HGIEN, CIS and SCC
tissues, respectively (SP staining; magnification, ×200). (F)
Magnification of Fig. 1C (SP
staining; magnification, ×400). Red arrows indicate myofibroblast
staining. α-SMA, α-smooth muscle actin; NM, normal; LGIEN,
low-grade intraepithelial neoplasia; HGIEN, high-grade
intraepithelial neoplasia; CIS, carcinoma in situ; SCC,
squamous cell carcinoma; SP, streptavidin-peroxidase. |
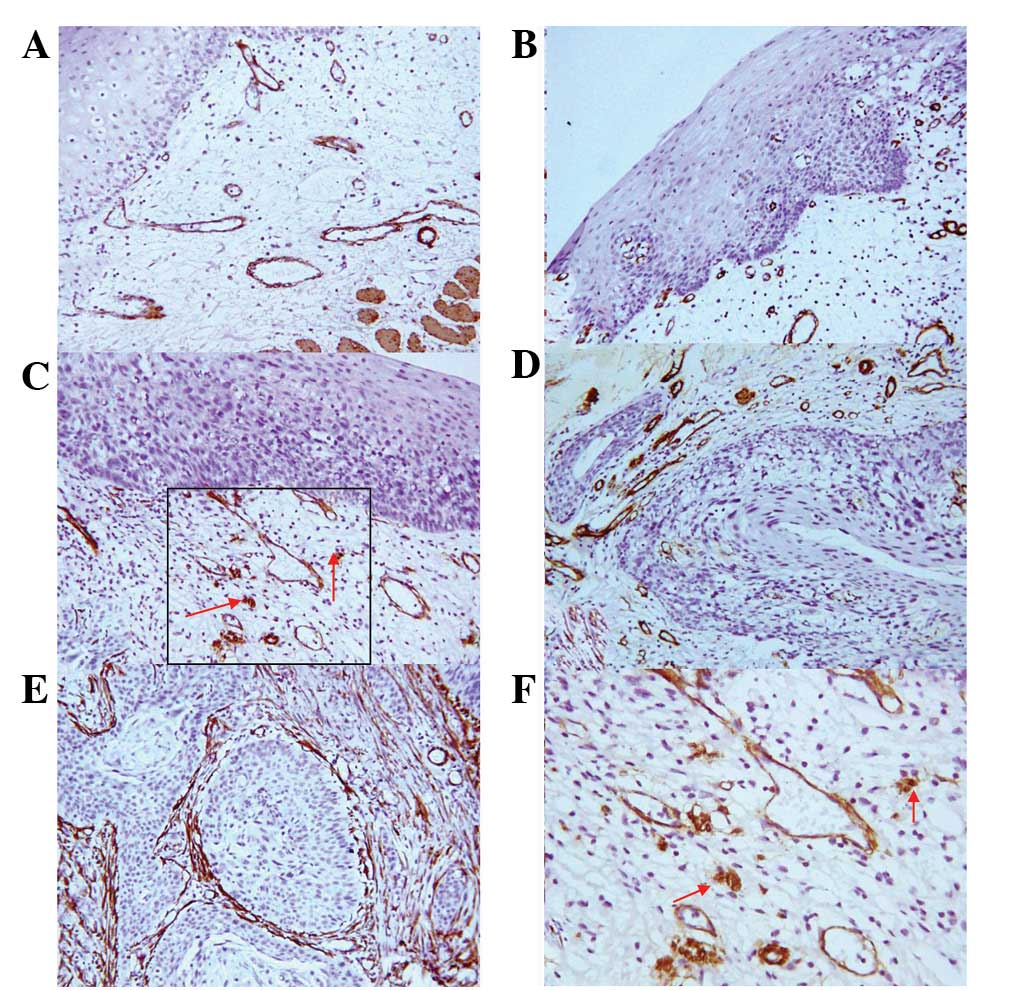
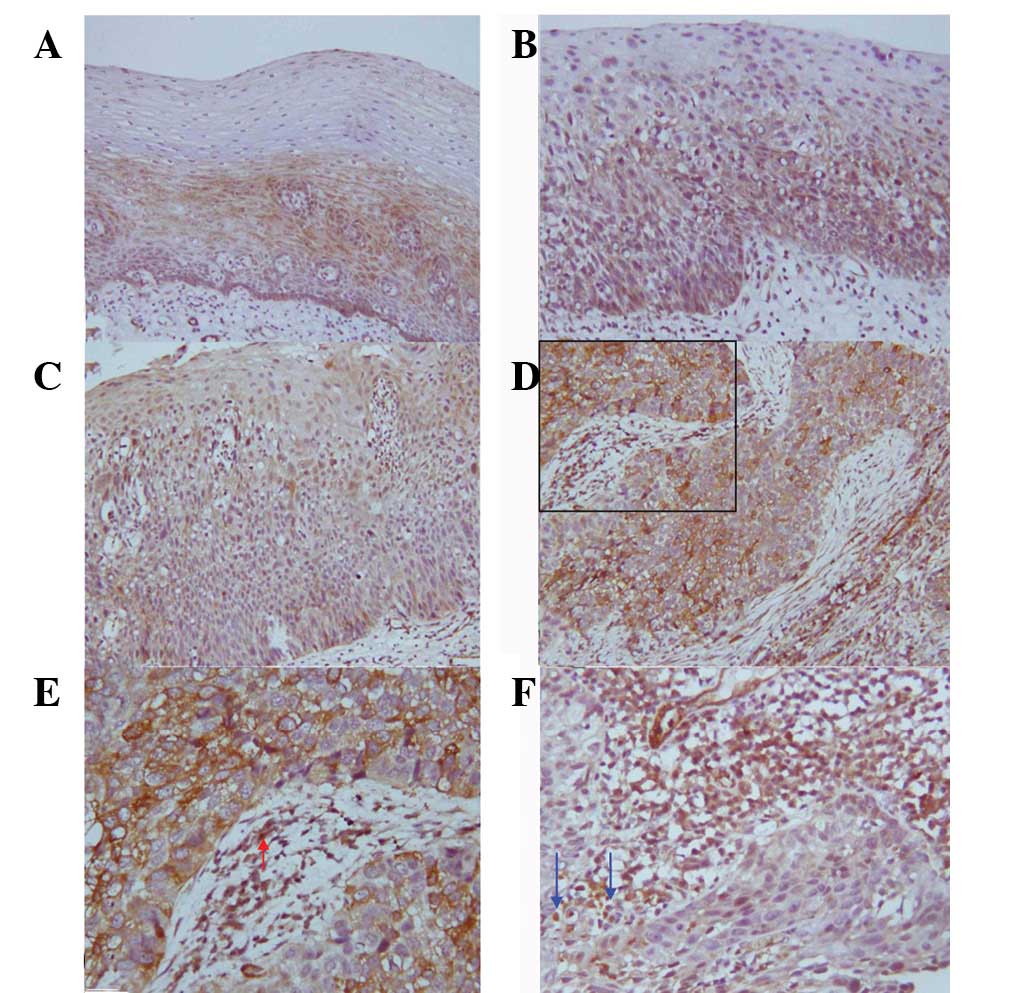
Expression of TGFβ1 and HGF in
oesophageal carcinogenesis
The majority of TGFβ1 and HGF expression was
localised in the cytoplasm of tumour and dysplasia cells, and
positive cells were distributed in the proliferative basal cell
zone. In the ESCC tissues, positive staining of TGFβ1 and HGF was
observed in stromal fibroblasts and inflammatory cells adjacent to
tumour cells, particularly at the invasive edges of tumours
(Figs. 2 and 3). Ascending positive expression levels of
TGFβ1 and HGF were observed in oesophageal carcinogenesis. The
positive immunostaining rate for TGFβ1 and HGF was low in the
normal group but increased progressively from LGIEN to HGIEN, CIS
and SCC groups, successively (Table
I). A significant difference in TGFβ1 and HGF expression was
observed between the normal epithelia and the epithelia of LGIEN,
HGIEN, CIS and SCC (with the exception of LGIEN for HGF). TGFβ1 and
HGF expression exhibited a linear correlation with the progression
of the various lesions (P<0.05). The values of TGFβ1 and HGF
linear correlations with various lesions were −0.356 and −0.437,
respectively.
 | Figure 2.Expression of TGFβ1 in esophageal
carcinogenesis. (A–D) TGFβ1-positive LGIEN, HGIEN, CIS and SCC
tissues, respectively (SP staining; magnification, ×200). (E and F)
TGFβ1-positive expression in myofibroblasts (red arrows) and
inflammatory cells (blue arrows) (SP staining; magnification,
×400). TGFβ1, transforming growth factor β1; LGIEN, low-grade
intraepithelial neoplasia; HGIEN, high-grade intraepithelial
neoplasia; CIS, carcinoma in situ; SCC, squamous cell
carcinoma; SP, streptavidin-peroxidase |
 | Table I.Expression of α-SMA, TGFβ1 and HGF at
various clinical and histological parameters. |
Table I.
Expression of α-SMA, TGFβ1 and HGF at
various clinical and histological parameters.
| Characteristic | n | α-SMA | TGFβ1 | HGF |
|---|
|
|
|
|---|
| n (%) | P-value | n (%) | P-value | n (%) | P-value |
|---|
| Gender | | | | | | | |
| Male | 88 | 40 (45.5) | | 46 (52.3) | | 28 (31.8) | |
| Female | 48 | 19 (39.6) | 0.509 | 32 (66.7) | 0.105 | 8 (16.7) | 0.056 |
| Age, years | | | | | | | |
| <60 | 61 | 26 (42.6) | | 36 (59.0) | | 16 (26.2) | |
| ≥60 | 75 | 33 (42.7) | 0.872 | 42 (56.0) | 0.724 | 20 (26.7) | 0.954 |
| Histological
type | | | | | | | |
| NM | 20 | 0 (0.0) | | 1 (5.0) | | 0 (0.0) | |
| LGIEN | 26 | 3 (11.5) | 0.246a | 15 (57.7) | 0.000a | 1 (3.8) | 0.375a |
| HGIEN | 44 | 18 (40.9) | 0.001a | 29 (65.9) | 0.000a | 13 (29.5) | 0.006a |
| CIS | 23 | 15 (65.2) | 0.000a | 16 (69.6) | 0.000a | 9 (39.1) | 0.002a |
| SCC | 23 | 23 (100.0) | 0.000a | 17 (70.0) | 0.000a | 13 (56.5) | 0.000a |
No significant differences were identified between
TGFβ1 and HGF expression with respect to gender and age. However, a
significant difference was identified in TGFβ1 and HGF expression
in ESCC and each stage of oesophageal precancerous lesions when
compared with that in the normal group.
Correlation between α-SMA and TGFβ1
expression in oesophageal carcinogenesis
The frequency of TGFβ1 overexpression was higher in
α-SMA-positive groups when compared with that of the α-SMA-negative
groups. The correlation between α-SMA and TGFβ1 was positive and
statistically significant [correlation coefficient (r), 0.365;
P=0.000; Table II].
 | Table II.Correlation between α-SMA and TGFβ1
protein expression. |
Table II.
Correlation between α-SMA and TGFβ1
protein expression.
| α-SMA | TGFβ1 | r | P-value |
|---|
|
|---|
| − | + |
|---|
| − | 45 | 32 | | |
| + | 13 | 46 | 0.365 | 0.000a |
MVD among various clinical and
histological parameters
No significant differences in the MVD were
identified with regard to gender and age. The MVD was 12.3±1.6,
15.7±1.9, 20.9±2.2, 21.4±1.9 and 22.0±2.3 in the normal, LGIEN,
HGIEN, CIS and SCC groups, respectively. The higher values of MVD
in the HGIEN, CIS and SCC groups were statistically significant
when compared with that of the normal and LGIEN groups. α-SMA-,
HGF- and TGFβ1-positive groups exhibited significantly higher MVDs
when compared with that of their negative counterparts (Table III; Fig.
4).
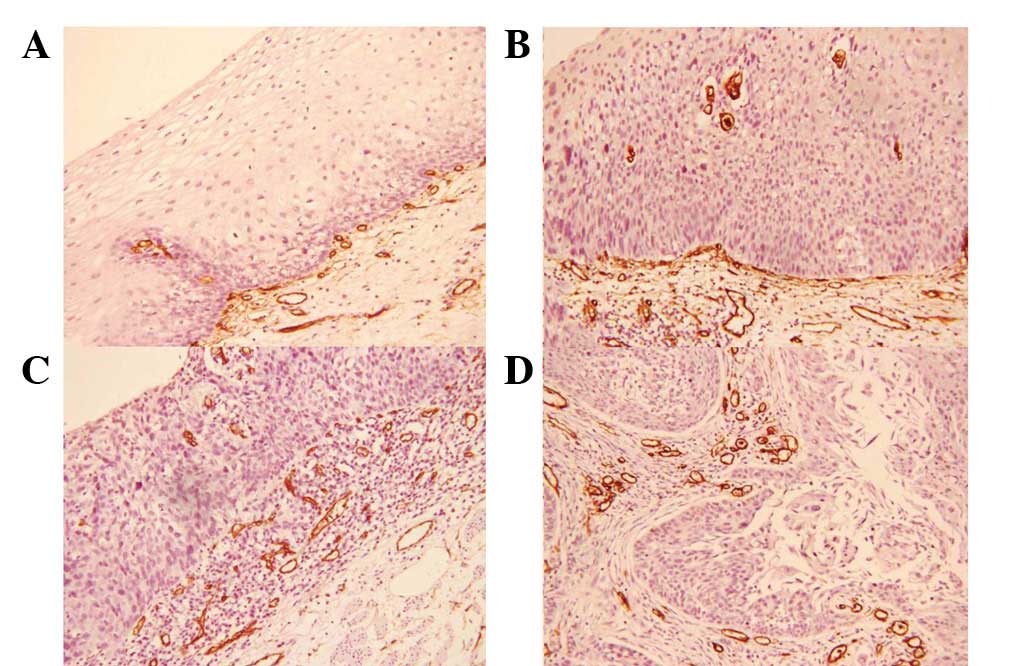 | Figure 4.MVD in various esophageal lesions (SP
staining; magnification, ×200). (A) NM, (B) HGIEN, (C) CIS and (D)
invasive SCC. MVD, microvessel density; NM, normal; LGIEN,
low-grade intraepithelial neoplasia; HGIEN, high-grade
intraepithelial neoplasia; CIS, carcinoma in situ; SCC,
squamous cell carcinoma; SP, streptavidin-peroxidase. |
 | Table III.MVD at various clinical and
histological parameters. |
Table III.
MVD at various clinical and
histological parameters.
| Characteristic | n | MVD | P-value |
|---|
| Gender | | | |
| Male | 88 | 21.2±3.5 | |
| Female | 48 | 20.8±3.8 | 0.755 |
| Age, years | | | |
| ≥60 | 75 | 21.2±3.4 | |
| <60 | 61 | 22.0±3.1 | 0.886 |
| Pathological
grade | | | |
| Normal | 20 | 12.3±1.6 | |
| LGIEN | 26 | 15.7±1.9a | 0.041a |
| HGIEN | 44 |
20.9±2.2a,b | 0.012a |
| CIS | 23 |
21.4±1.9a,b,c | 0.009a |
| SCC | 23 |
22.0±2.3a,b,c | 0.005a |
| α-SMA | | | |
| − | 77 | 15.3±7.3 | |
| + | 59 | 22.8±5.6 | 0.044 |
| TGFβ1 | | | |
| − | 58 | 15.6±4.9 | |
| + | 78 | 20.9±4.6 | 0.047 |
| HGF | | | |
| − | 100 | 15.2±3.3 | |
| + | 36 | 28.3±5.8 | 0.008 |
Discussion
Activated fibroblasts in tumour stroma are known as
CAFs and are commonly identified by the expression of α-SMA
(19,20). Studies have demonstrated that CAFs
are significant promoters of tumour growth and progression via
growth factors, including TGFβ1 and HGF, which are secreted by the
CAFs themselves and/or by carcinoma cells (21). In the present study, positive α-SMA
expression rates increased from LGIEN to HGIEN, CIS and SCC groups,
successively. The majority of α-SMA was localised to atypical
fibroblasts (AFs) and CAFs surrounding cancer nests. The expression
pattern of α-SMA from LGIEN to HGIEN and CIS groups was weak and
focal. By contrast, marked and diffuse staining of α-SMA was
observed in the ESCC tissues, particularly in invasive carcinomas.
Of note, overexpression of TGFβ1 positively correlated with the
number of α-SMA-positive fibroblasts. Numerous studies (22–25)
have demonstrated that TGFβ1 is capable of inducing α-SMA-negative
fibroblasts into α-SMA-positive fibroblasts. The specific mechanism
has been hypothesised to involve TGFβ1 activation of RhoA, which
induces α-SMA expression via activation of the endothelial growth
factor receptor (26).
Previous studies have demonstrated that
overexpression of TGFβ1 (27) and
HGF (28) is associated with
advanced stage oesophageal (Barrett’s) adenocarcinoma. However, few
studies have analysed the overexpression of TGFβ1 and HGF in
oesophageal squamous cell carcinogenesis. In the present study,
carcinoma cells and CAFs surrounding cancer nests markedly
expressed TGFβ1 and HGF in ESCC when compared with that of
precancerous lesions, particularly at the invasive edges of the
carcinoma. Expression of TGFβ1 and HGF in inflammatory cells was
also shown in regions adjacent to cancer nests. In addition, the
positive staining rates of TGFβ1 and HGF increased significantly in
the LGIEN, HGIEN, CIS and SCC groups, when compared with that in
the normal group. Thus, the immunoreactivity of TGFβ1 and HGF
occurred during the early stages of oesophageal carcinogenesis. No
significant differences were identified between TGFβ1 and HGF
expression in the HGIEN, CIS and SCC groups. However, TGFβ1 and HGF
expression levels showed linear correlations with oesophageal
pathological grade. It has been hypothesised that the
overexpression of TGFβ1 and HGF may be involved in
hyperproliferation of oesophageal epithelial cells.
Angiogenesis is an essential step for the transition
of a small cluster of harmless cells into a large tumour. ESCC is a
highly angiogenic tumour and biochemical studies (29) have shown that the TGFβ-vascular
endothelial growth factor (VEGF) pathway may induce vascular
network formation during fibroblast activation in the ESCC stroma.
TGFβ1 may be associated with gastric tumour progression by
indirectly stimulating angiogenesis through the upregulation of
VEGF expression (30). In addition,
HGF is a significant angiogenic growth factor involved in the
progression of ESCC (31,32). In the current study, the MVD
increased rapidly through the normal, LGIEN, HGIEN, CIS and SCC
groups, successively. However, no significant differences were
identified in the MVD between the HGIEN, CIS and SCC groups.
Coexpression of TGFβ1 and HGF was examined, and the correlations
with MVD were evaluated in the 136 specimens. The values of MVD in
the LGIEN, HGIEN, CIS and SCC groups with positive TGFβ1 and HGF
expression were higher compared with that in the groups that were
negative for TGFβ1 and HGF (data not shown). The results of the
present study have indicated that TGFβ1 and HGF contribute to
oesophageal angiogenesis at the stage of initiation via their
respective pathways.
From the results of the present study and previous
studies performed in mouse models (33,34),
we hypothesise that the suitable microenvironment created by AFs
and CAFs in the stroma not only contributes to cancer progression
but also to angiogenesis in oesophageal precancerous lesions and
carcinoma. In addition, TGFβ1 and HGF may be important for
oesophageal carcinogenesis and angiogenesis.
Acknowledgements
The authors would like to thank Z.
Dong Wang and Y. Jun Wang for their pathological examination and
analysis.
References
|
1.
|
Wei WQ, Qiao YL, Wang GQ, et al: Progress
in prevention and control of esophageal carcinoma in high-risk
population. J Pract Oncol. 6:371–373. 2001.(In Chinese).
|
|
2.
|
Martin de Civetta MT and Civetta JD:
Carcinogenesis. Salud Publica Mex. 53:405–414. 2011.(In
Spanish).
|
|
3.
|
Weiss RA: Multistage carcinogenesis. Br J
Cancer. 12:1981–1982. 2004. View Article : Google Scholar
|
|
4.
|
Xing F, Saidou J and Watabe K: Cancer
associated fibroblasts (CAFs) in tumor microenvironment. Front
Biosci. 15:166–179. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Bhowmick NA, Neilson EG and Moses HL:
Stromal fibroblasts in cancer initiation and progression. Nature.
432:332–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Sugimoto H, Mundel TM, Kieran MW and
Kalluri R: Identification of fibroblast heterogeneity in the tumour
microenvironment. Cancer Biol Ther. 5:1640–1646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Erez N, Ttuitt M, Olson P, et al:
Cancer-associated fibroblasts are activated in incipient neoplasia
to orchestrate tumour-promoting inflammation in an
NF-kappaB-dependent manner. Cancer Cell. 17:135–147. 2010.
View Article : Google Scholar
|
|
8.
|
Zhang B, Halder SK, Zhang S and Datta PK:
Targeting transforming growth factor-beta signaling in liver
metastasis of colon cancer. Cancer Lett. 277:114–120. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Gholamin M, Moaven O, Memar B, et al:
Overexpression and interactions of interleukin-10, transforming
growth factor beta, and vascular endothelial growth factor in
esophageal squamous cell carcinoma. World J Surg. 33:1439–1445.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Langenskiöld M, Holmdahl L, Falk P, et al:
Increased TGF-beta 1 protein expression in patients with advanced
colorectal cancer. J Surg Oncol. 97:409–415. 2008.PubMed/NCBI
|
|
11.
|
Hawinkels LJ, Verspaget HW, van Duijn W,
et al: Tissue level, activation and cellular localisation of
TGF-beta1 and association with survival in gastric cancer patients.
Br J Cancer. 97:398–404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Matsumoto K, Date K, Ohmichi H, et al:
Hepatocyte growth factor in lung morphogenesis and tumour invasion:
role as a mediator in epithelium-mesenchyme and tumour-stroma
interactions. Cancer Chemother Pharmacol. 38(Suppl): S42–S47. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Uchida D, Kawamata H, Omotehara F, et al:
Role of HGF/c-met system in invasion and metastasis of oral
squamous cell carcinoma cells in vitro and its clinical
significance. Int J Cancer. 93:489–496. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Zhou Q, Dong Wang L, Du F, et al: Changes
of TGFbeta1 and TGFbetaRII expression in esophageal precancerous
and cancerous lesions: a study of a high-risk population in Henan,
northern China. Dis Esophagus. 15:74–79. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Ren Y, Cao B, Law S, et al: Hepatocyte
growth factor promotes cancer cell migration and angiogenic factors
expression: a prognostic marker of human esophageal squamous cell
carcinomas. Clin Cancer Res. 11:6190–6197. 2005. View Article : Google Scholar
|
|
16.
|
Takada N, Yano Y, Matsuda T, et al:
Expression of immunore-active human hepatocyte growth factor in
human esophageal squamous cell carcinomas. Cancer Lett. 97:145–148.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND; World Health Organization. Classification of Tumours of
the Digestive System. IARC Press; Lyon: 2010
|
|
18.
|
El-Shahat M, Lotfy M, Fahmy L, et al:
Prognostic value of microvessel density, matrix metalloproteinase-9
and p53 protein expression in esophageal cancer. J Egypt Natl Canc
Inst. 16:224–230. 2004.PubMed/NCBI
|
|
19.
|
Rønnov-Jessen L, Petersen OW and Bissell
MJ: Cellular changes involved in conversion of normal to malignant
breast: importance of the stromal reaction. Physiol Rev. 76:69–125.
1996.PubMed/NCBI
|
|
20.
|
Gabbiani G: The myofibroblast in wound
healing and fibrocontractive diseases. J Pathol. 200:500–503. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Mueller MM and Fusenig NE: Friends or
foes-bipolar effects of the tumour stroma in cancer. Nat Rev
Cancer. 4:839–849. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Fuyuhiro Y, Yashiro M, Noda S, et al:
Upregulation of cancer-associated myofibroblasts by TGF-β from
scirrhous gastric carcinoma cells. Br J Cancer. 105:996–1001.
2011.
|
|
23.
|
Abe R, Donnelly SC, Peng T, et al:
Peripheral blood fibrocytes: differentiation pathway and migration
to wound sites. J Immunol. 166:7556–7562. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Tuxhorn JA, McAlhany SJ, Yang F, et al:
Inhibition of transforming growth factor-beta activity decreases
angiogenesis in a human prostate cancer-reactive stroma xenograft
model. Cancer Res. 62:6021–6025. 2002.
|
|
25.
|
Brenmoehl J, Miller SN, Hofmann C, et al:
Transforming growth factor-beta 1 induces intestinal myofibroblast
differentiation and modulates their migration. World J
Gastroenterol. 15:1431–1442. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Masszi A, Di Ciano C, Sirokmány G, et al:
Central role for Rho in TGF-beta1-induced alpha-smooth muscle actin
expression during epithelial-mesenchymal transition. Am J Physiol
Renal Physiol. 284:F911–F924. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Von Rahden BH, Stein HJ, Feith M, et al:
Overexpression of TGF-beta1 in esophageal (Barrett’s)
adenocarcinoma is associated with advanced stage of disease and
poor prognosis. Mol Carcinog. 45:786–794. 2006.PubMed/NCBI
|
|
28.
|
Herrera LJ, El-Hefnawy T, Queiroz de
Oliveira PE, et al: The HGF receptor c-Met is overexpressed in
esophageal adenocarcinoma. Neoplasia. 7:75–84. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Noma K, Smalley KS, Lioni M, et al: The
essential role of fibroblasts in esophageal squamous cell
carcinoma-induced angiogenesis. Gastroenterology. 134:1981–1993.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Saito H, Tsujitani S, Oka S, et al: The
expression of transforming growth factor-beta1 is significantly
correlated with the expression of vascular endothelial growth
factor and poor prognosis of patients with advanced gastric
carcinoma. Cancer. 86:1455–1462. 1999. View Article : Google Scholar
|
|
31.
|
Grugan KD, Miller CG, Yao Y, et al:
Fibroblast-secreted hepatocyte growth factor plays a functional
role in esophageal squamous cell carcinoma invasion. Proc Natl Acad
Sci USA. 107:11026–11031. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Oshima Y, Yajima S, Yamazaki K, et al:
Angiogenesis-related factors are molecular targets for diagnosis
and treatment of patients with esophageal carcinoma. Ann Thorac
Cardiovasc Surg. 16:389–393. 2010.PubMed/NCBI
|
|
33.
|
Kuperwasser C, Chavarria T, Wu M, et al:
Reconstruction of functionally normal and malignant human breast
tissues in mice. Proc Natl Acad Sci USA. 101:4966–4971. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Bhowmick NA, Chytilt A, Plieth D, et al:
TGF-beta signaling in fibroblasts modulates the oncogenic potential
of adjacent epithelia. Science. 303:848–851. 2004. View Article : Google Scholar : PubMed/NCBI
|