Introduction
Osteosarcoma is the most common type of
non-hematopoietic primary malignant bone tumor. Following the
initial diagnosis, patients are usually treated with multi-agent
preoperative chemotherapy and surgical resection, followed by
postoperative chemotherapy. Despite significant progress in
chemotherapy, patients who have metastases at diagnosis continue to
have poor prognoses (1). Therefore,
it is essential to identify additional biomarkers for use in
diagnosis and novel therapeutic strategies.
T cell Ig- and mucin-domain-containing molecules
(TIMs) are a recently described family. Three members of which,
TIM-1, TIM-3 and TIM-4, have been identified in humans (2). TIM-1 was demonstrated to be
preferentially expressed on Th2 cells and to support T-cell
activation, promoting the pathogenesis of asthma and allergies
(3–5). TIM-4 was observed on
antigen-presenting cells (APCs) and mediates the phagocytosis of
apoptotic cells (6). By contrast,
TIM-3 was originally identified as a surface molecule expressed on
CD4+ Th1 cells. The interaction of TIM-3 with its
potential ligand, galectin-9, induces Th1 cells to undergo
apoptosis and inhibits their production of IFN-γ (7–9).
In addition to activated T cells, ectopic expression
of TIMs has been observed on tumor cells, where they were also
described as being actively involved in the pathogenesis of tumor
development. For example, non-small cell lung cancer patients whose
tumor tissues were positive for TIM-3 had significantly shorter
survival times compared with those with TIM-3- tumor tissues
(10). In patients with hepatitis B
virus-associated hepatocellular carcinoma, the number of
TIM-3+ infiltrating tumor cells was negatively
associated with patient survival (11). Moreover, TIMs were also shown to
function as specific markers for the diagnosis of Langerhans cell
sarcoma, head and neck cancer and follicular B cell non-Hodgkin
lymphoma (12,13), while the combined blockade of TIM-3
and TIM-4 augments the efficacy of cancer vaccines against
established melanomas (14).
Epithelial-mesenchymal transition (EMT) is an
essential process for normal development and is also crucial in
cancer dissemination, endowing cells with metastatic and cancer
stem cell properties (15). EMT is
characterized by the down-regulation of epithelial markers (such as
E-cadherin) and the upregulation of vimentin, as well as other
mesenchymal markers, resulting in numerous phenotypic changes,
including the loss of cell-cell adhesion and cell polarity and the
acquisition of migratory and invasive properties (16). TWIST1, SNAIL and SLUG are
transcription factors that govern EMT and are regulated by TGF-β
and microRNA (17,18). The increased expression of EMT
biomarkers has been associated with poor prognostic
clinicopathological features of various types of cancer, including
osteosarcoma (19–21).
In the present study, the expression of TIMs in
osteosarcoma samples was analyzed by immunohistochemistry, and the
associations between TIMs and EMT biomarkers were further analyzed
by dual immunofluorescence staining.
Materials and methods
Patients
Samples from nine cases of osteosarcoma were
collected at the Department of Orthopedics, Affiliated Hospital of
Chifeng University (Chifeng, China). The histopathology and
pathological characteristics of the patients were analyzed by CT
examination and H&E staining, with the results demonstrating
that all patients had osteosarcoma (Fig. 1). All osteosarcoma samples were
obtained from the legs, and the tissues were fixed in 10% neutral
buffered formalin, and then embedded in paraffin. This study
protocol was approved by the review board of the ethics committee
of Chifeng University. Written informed consent was obtained from
all patients.
Immunohistochemistry
The immunohistochemistry was performed as published
previously, but with slight modifications (22). Briefly, paraffin-embedded tissue
blocks were cut into 2–3-μm sections and mounted on
poly-L-lysine-charged glass slides (Sigma, St. Louis, MO, USA).
After the sections were dewaxed and rehydrated, antigen retrieval
was performed by microwaving in 10 mM citrate buffer (pH 6.0). The
sections were cooled to room temperature (RT) and endogenous
peroxidase activity was blocked by incubation with a solution of
0.5% hydrogen peroxide in 50% methanol for 1 h. The sections were
then incubated in 3% BSA (Sigma) with 0.1% Nonidet P-40 (Beyotime,
Haimen, China) in PBS (Wuhan Boster Biological Technology Ltd.,
Wuhan, China) for 1 h at RT to block nonspecific binding.
Subsequently, the sections were incubated overnight at 4°C with
primary antibodies (Table I),
including anti-TIM-1, anti-TIM-3 or anti-TIM-4, diluted in 1% BSA.
After washing, the sections were incubated with the corresponding
secondary antibodies for 1 h at RT. The Vectastain ABC kit (Vector
Laboratories, San Diego, CA, USA) was used for the avidin-biotin
complex method according the manufacturer’s instructions. Sections
incubated with isotype-matched, concentration-matched Ig without
primary antibodies were used as isotype controls. Peroxidase
activity was visualized with the DAB Elite kit (K3465; Dako,
Copenhagen, Denmark) and brown coloration of tissues represented
positive staining. The sections were lightly counterstained with
hematoxylin, dehydrated through an ethanol series, cleared in
xylene and mounted. Subsequently, the sample sections were viewed
using a light microscope (Axioplan 2; Zeiss, Berlin, Germany).
 | Table I.Immunohistochemical study: Antibodies,
source and dilution. |
Table I.
Immunohistochemical study: Antibodies,
source and dilution.
| Ab | Dilution | Clone | Source |
|---|
| TIM-1 | 1:100 | Polyclonal Goat
IgG | R&D System |
| TIM-3 | 1:100 | Polyclonal Goat
IgG | R&D System |
| TIM-4 | 1:100 | Polyclonal Goat
IgG | R&D System |
| CD3 | 1:50 | Monoclonal mouse IgG
(F7.2.38) | Dako |
| CK-18 | 1:400 | Monoclonal mouse IgG
(C-04) | Santa Cruz |
| CD68 | 1:50 | Monoclonal mouse IgG
(3F103) | Santa Cruz |
| CD31 | 1:50 | Monoclonal mouse IgG
(10G9) | Santa Cruz |
| CD1a | 1:200 | Monoclonal mouse IgG
(7A7) | Abcam |
| PCNA | 1:200 | Monoclonal mouse IgG
(F-2) | Santa Cruz |
| Bcl-2 | 1:100 | Monoclonal mouse IgG
(C-2) | Santa Cruz |
| Slug | 1:100 | Monoclonal mouse
IgG | Abcam |
| Snail | 1:100 | Polyclonal Goat
IgG | Abcam |
| Smad2 | 1:100 | Polyclonal Goat
IgG | Santa Cruz |
| Smad3 | 1:100 | Polyclonal Rabbit
IgG | Santa Cruz |
| Vimentin | 1:50 | Monoclonal mouse IgG
(RV202) | Santa Cruz |
| E-cad | 1:200 | Polyclonal Rabbit
IgG | Santa Cruz |
Dual immunofluorescence staining
For dual immunofluorescence staining, the sections
were incubated with primary anti-TIM-3 antibodies at 4°C overnight.
After washing with PBS (three washes, 5 min per wash), the sections
were incubated with Alexa Fluor® 555-conjugated goat
anti-mouse/rabbit IgG antibodies (Invitrogen, Carlsbad, CA, USA)
for 1 h. The sections were further incubated with anti-CD68,
anti-CD31, anti-PCNA, anti-Bcl-2, anti-Snail, anti-Slug, anti-Smad,
anti-pSmad2/3 or anti-CK-18 antibodies at 4°C overnight, and
incubated with Alexa Fluor® 488-conjugated goat
anti-mouse/rabbit IgG1 antibodies (Invitrogen) for an additional
hour. Subsequently, the sections were incubated with 1 μg/ml
DAPI (Sigma) for 10 min to stain the nuclei. Sections incubated
with the appropriate isotype control primary antibodies and
fluorescently labeled secondary antibodies were used as negative
controls. The results were analyzed by fluorescence microscopy
(Axioplan 2; Zeiss).
Results
Expression and anatomical distribution of
TIMs in sections from osteosarcoma
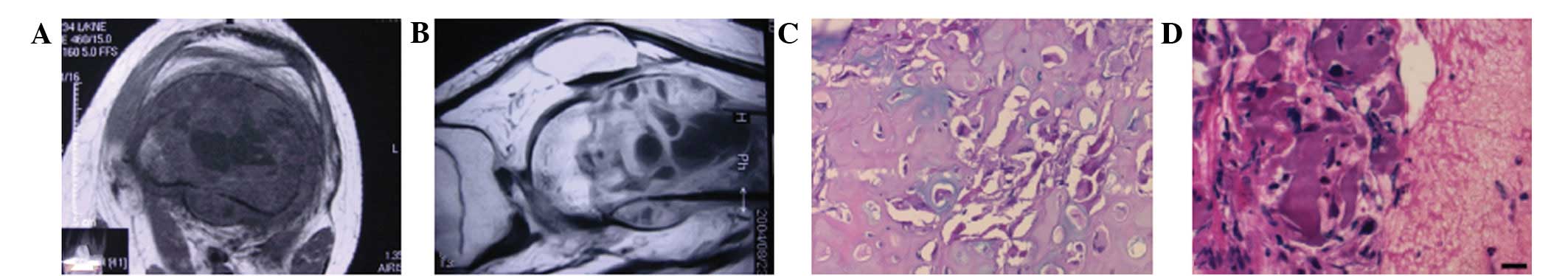
Axial and sagittal CT images demonstrated that tumor
development originated from the bone (Fig. 1A and B). The specimens from all nine
cases of osteosarcoma were highly cellular tumors consisting of
enlarged round cells. The neoplastic cells showed the presence of
cytological atypia, with multiple hyperchromatic and prominent
nucleoli (Fig. 1C and D).
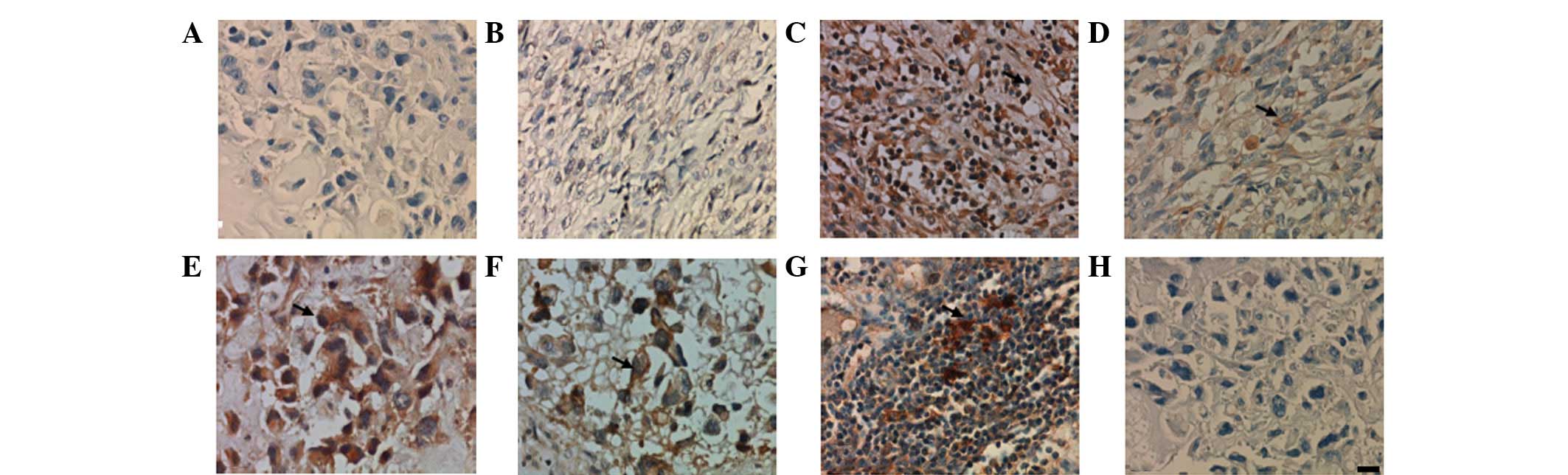
Immunohistochemistry showed that TIM-3+ and
TIM-4+, but not TIM-1+, cells were observed
in all cases. These molecules were located on cell membranes and in
the cytoplasm. However, the distributions of TIM-3 and TIM-4 were
noticeably different. TIM-3 was identified on macrophages (Fig. 2A), infiltrated inflammatory cells
(Fig. 2B) and tumor cells (Fig. 2C and D), and TIM-3+ cells
were distributed throughout the tissue sections. TIM-4, however,
was expressed on macrophage-like cells (Fig. 2E) and was absent from tumor cells
(Fig. 2F). No positive results were
observed in sections incubated with only secondary antibodies (goat
IgG1), which were used as the negative controls (data not
shown).
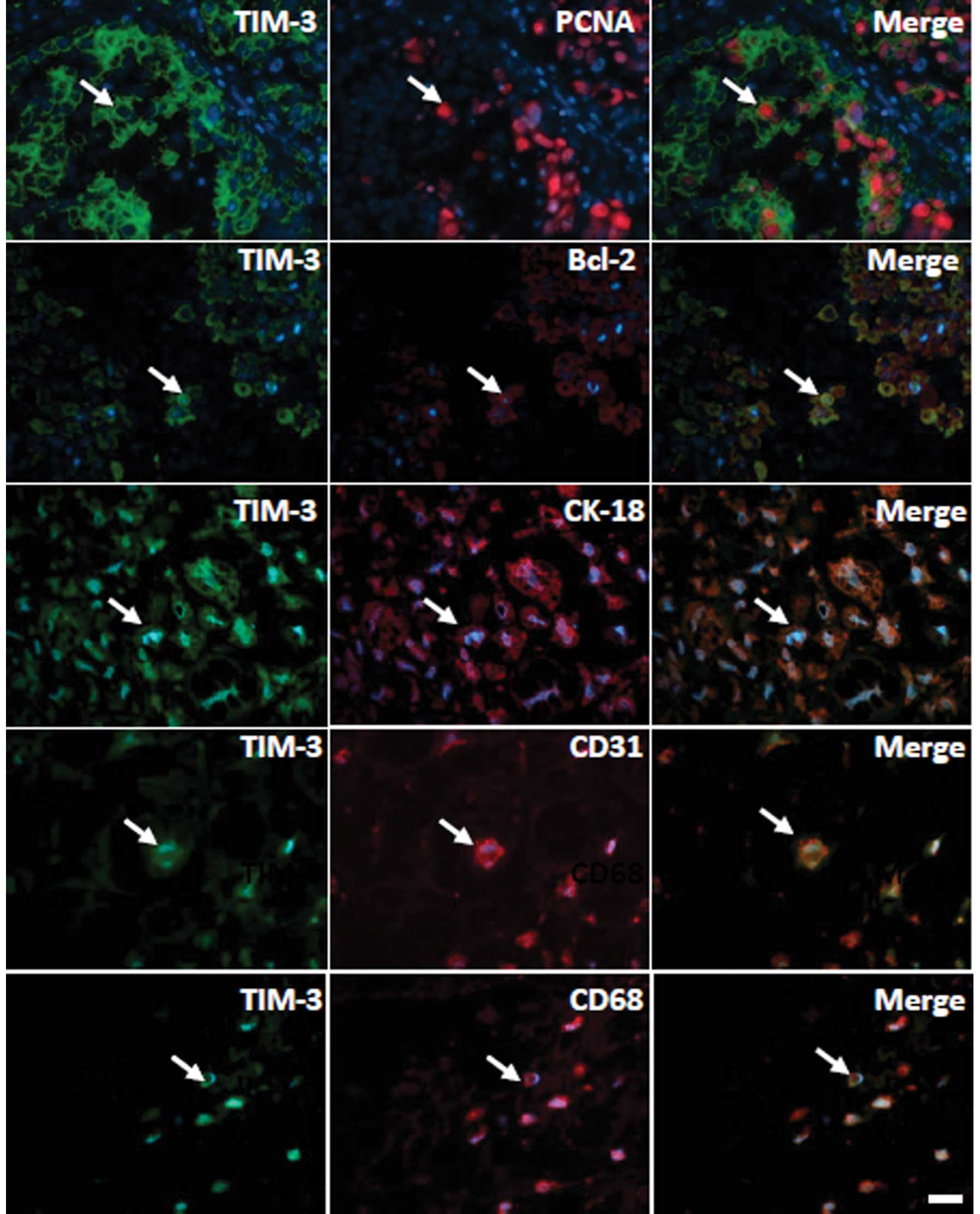
Phenotypes of TIM-3 in sections from
osteosarcoma
As TIM-3 was observed in tumor cells from
osteosarcomas, the phenotypes of the TIM-3+ cells were
further examined by dual immunofluorescence staining. As shown in
Fig. 3, TIM-3 was expressed on
CD31+ endothelial cells, CK-18+ epithelial
cells and CD68+ macrophages. In addition, TIM-3 was
co-expressed with Bcl-2 and PCNA, indicating that TIM-3 may
regulate tumor cell apoptosis and proliferation (Fig. 3).
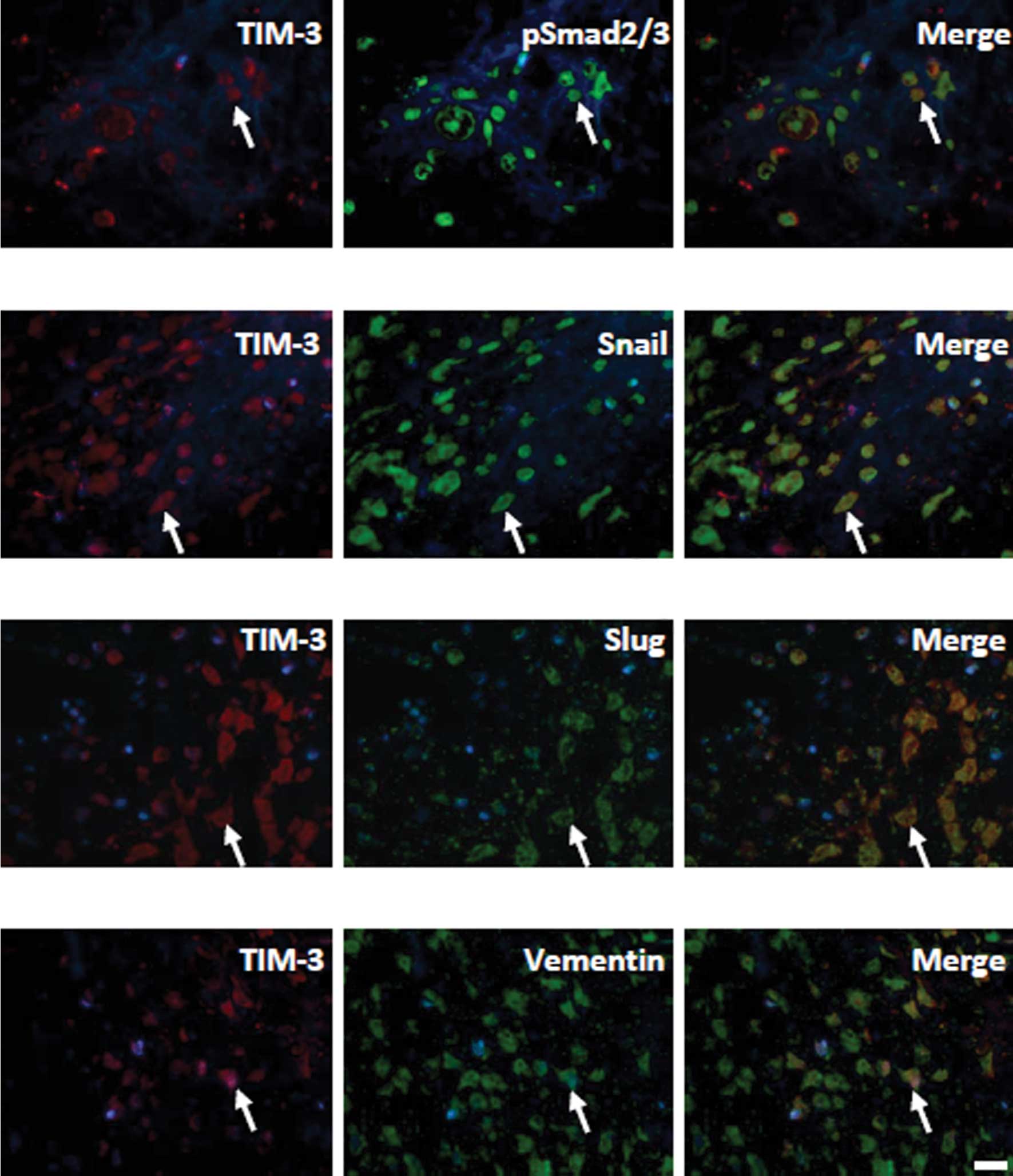
Associations between TIM-3 and EMT
biomarkers in sections from osteosarcoma
Previous studies demonstrated that the expression of
EMT biomarkers, including Slug, Snail and Smad, could be detected
in samples from patients with osteosarcoma, suggesting that EMT may
be involved in the pathogenesis of osteosarcoma (23,24).
In the present study, the correlations between TIM-3 and these EMT
biomarkers were also investigated. The results showed that TIM-3
was co-expressed with Slug, Snail and Smad in the same sarcoma
cells (Fig. 4).
Discussion
Osteosarcoma, derived from primitive mesenchymal
cells and originating from bone, is the most common type of primary
bone tumor in children and adolescents. Characteristically, this
sarcoma occurs frequently in the metaphyseal regions of long bones
and metastasizes preferentially to the lung (1,25).
Although it is a relatively uncommon type of cancer, the incidence
is increasing (26,27). With advances in multimodal
treatments consisting of adjuvant chemotherapy and surgical
resection, the prognosis and quality of life of patients with
non-metastatic osteosarcoma of the extremities are greatly
improved. Nevertheless, the five-year progression-free survival of
high-grade osteosarcoma is only ∼50% due to the failure of rescue
chemotherapy (28). Therefore, it
is necessary to establish new therapeutic strategies to improve the
overall rate of survival.
TIMs are proteins that are actively involved in
tumor development, in addition to the pathogenesis of rheumatoid
arthritis, asthma, systemic lupus erythematosus, multiple sclerosis
and diabetes (2). TIMs have been
reported to be aberrantly expressed in carcinoma tissues, and the
presence of TIM-3 modulates tumor development and carcinoma traits
(3). In the present study, the
expression of TIMs was analyzed in nine cases of osteosarcoma and
the results demonstrated that only TIM-3, rather than TIM-1 or
TIM-4, was detected on the tumor cells of osteosarcoma patients.
Notably, the morphological analysis demonstrated that TIM-3 was
also present on CD68+ macrophages, CD31+
endothelial cells, CK-18+ epithelial cells, as well as
on Bcl-2+ and PCNA+ tumor cells. These
results suggested that TIM-3 is involved in the progression of
osteosarcoma via the promotion of tumor cell proliferation, as well
as the inhibition of apoptosis.
In patients with advanced cancer, widespread
manifestation of distant metastases is a major cause of
cancer-associated mortality. Despite this important clinical
problem, little is known about the mediators that promote tumor
outgrowth in the metastatic organ. At present, the
transdifferentiation of polarized epithelial cells to mesenchymal
cells (EMT), which occurs during tumor invasion and metastasis, is
recognized as a key developmental process (29). The acquisition of invasiveness by
cancer cells through the invasion and destruction of the basement
membrane is considered to represent the onset of a multistep
process that eventually leads to metastatic dissemination with
life-threatening consequences. In addition to promoting tumor cell
invasion and metastasis, EMT generates cancer cells with stem
cell-like characteristics, including increased self-renewal and
tumor-initiating capabilities, as well as increased resistance to
apoptosis and chemotherapy (30).
Previous studies have examined the expression of EMT biomarkers,
such as Slug, Snail and Smad, in osteosarcoma sections, suggesting
that EMT may be involved in the pathogenesis of osteosarcoma
(23,24). The present study also detected
correlations between TIM-3 expression and the expression of these
EMT biomarkers. The results showed that TIM-3 was co-expressed with
Slug, Snail and Smad in the same sarcoma cells, suggesting that
TIM-3 may trigger the process of EMT to promote tumor development.
However, the exact mechanism of this requires further
investigation.
In summary, the present study investigated the
expression of TIMs in sections from osteosarcoma patients and an
understanding of the functional roles of TIM-3 may aid in the
development of novel strategies for disease diagnosis or
immunotherapy.
Acknowledgements
The present study was supported by
grants from the National Natural Science Foundation of China (grant
no. 81171585).
References
|
1.
|
Szuhai K, Cleton-Jansen AM, Hogendoorn PC
and Bovée JV: Molecular pathology and its diagnostic use in bone
tumors. Cancer Genet. 205:193–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Kuchroo VK, Dardalhon V, Xiao S and
Anderson AC: New roles for TIM family members in immune regulation.
Nat Rev Immunol. 8:577–580. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kuchroo VK, Umetsu DT, DeKruyff RH and
Freeman GJ: The TIM gene family: emerging roles in immunity and
disease. Nat Rev Immunol. 3:454–462. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Sonar SS, Hsu YM, Conrad ML, et al:
Antagonism of TIM-1 blocks the development of disease in a
humanized mouse model of allergic asthma. J Clin Invest.
120:2767–2781. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
de Souza AJ, Oriss TB, O’malley KJ, Ray A
and Kane LP: T cell Ig and mucin 1 (TIM-1) is expressed on in
vivo-activated T cells and provides a costimulatory signal for T
cell activation. Proc Natl Acad Sci USA. 102:17113–17118.
2005.PubMed/NCBI
|
|
6.
|
Rodriguez-Manzanet R, Sanjuan MA, Wu HY,
et al: T and B cell hyperactivity and autoimmunity associated with
niche-specific defects in apoptotic body clearance in
TIM-4-deficient mice. Proc Natl Acad Sci USA. 107:8706–8711. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Monney L, Sabatos CA, Gaglia JL, et al:
Th1-specific cell surface protein Tim-3 regulates macrophage
activation and severity of an autoimmune disease. Nature.
415:536–541. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Sakuishi K, Apetoh L, Sullivan JM, Blazar
BR, Kuchroo VK and Anderson AC: Targeting Tim-3 and PD-1 pathways
to reverse T cell exhaustion and restore anti-tumor immunity. J Exp
Med. 207:2187–2194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Freeman GJ, Casasnovas JM, Umetsu DT and
DeKruyff RH: TIM genes: a family of cell surface phosphatidylserine
receptors that regulate innate and adaptive immunity. Immunol Rev.
235:172–189. 2010.PubMed/NCBI
|
|
10.
|
Zhuang X, Zhang X, Xia X, et al: Ectopic
expression of TIM-3 in lung cancers: a potential independent
prognostic factor for patients with NSCLC. Am J Clin Pathol.
137:978–985. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Li H, Wu K, Tao K, et al: Tim-3/galectin-9
signaling pathway mediates T-cell dysfunction and predicts poor
prognosis in patients with hepatitis B virus-associated
hepatocellular carcinoma. Hepatology. 56:1342–1351. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Dorfman DM, Hornick JL, Shahsafaei A and
Freeman GJ: The phosphatidylserine receptors, T cell immunoglobulin
mucin proteins 3 and 4, are markers of histiocytic sarcoma and
other histiocytic and dendritic cell neoplasms. Hum Pathol.
41:1486–1494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Yang ZZ, Grote DM, Ziesmer SC, et al:
IL-12 upregulates TIM-3 expression and induces T cell exhaustion in
patients with follicular B cell non-Hodgkin lymphoma. J Clin
Invest. 122:1271–1282. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Baghdadi M, Nagao H, Yoshiyama H, Akiba H,
Yagita H, Dosaka-Akita H and Jinushi M: Combined blockade of TIM-3
and TIM-4 augments cancer vaccine efficacy against established
melanomas. Cancer Immunol Immunother. 62:629–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Lim J and Thiery JP:
Epithelial-mesenchymal transitions: insights from development.
Development. 139:3471–3486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Wu CY, Tsai YP, Wu MZ, Teng SC and Wu KJ:
Epigenetic reprogramming and post-transcriptional regulation during
the epithelial-mesenchymal transition. Trends Genet. 28:454–463.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Fernandez IE and Eickelberg O: The impact
of TGF-β on lung fibrosis: from targeting to biomarkers. Proc Am
Thorac Soc. 9:111–116. 2012.
|
|
18.
|
Hermeking H: MicroRNAs in the p53 network:
micromanagement of tumour suppression. Nat Rev Cancer. 12:613–626.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Gao D, Vahdat LT, Wong S, Chang JC and
Mittal V: Microenvironmental regulation of epithelial-mesenchymal
transitions in cancer. Cancer Res. 72:4883–4889. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Rangel MC, Karasawa H, Castro NP, Nagaoka
T, Salomon DS and Bianco C: Role of Cripto-1 during
epithelial-to-mesenchymal transition in development and cancer. Am
J Pathol. 180:2188–2200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Hamilton DH, Litzinger MT, Fernando RI,
Huang B and Palena C: Cancer vaccines targeting the
epithelial-mesenchymal transition: tissue distribution of brachyury
and other drivers of the mesenchymal-like phenotype of carcinomas.
Semin Oncol. 39:358–366. 2012. View Article : Google Scholar
|
|
22.
|
Li H, Wang C, Guo G, Gao C, Wu Y and Chen
Y: The characteristic expression of B7-associated proteins in
Langerhans cell sarcoma. Acta Histochem. 114:733–743. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Guo Y, Zi X, Koontz Z, Kim A, Xie J,
Gorlick R, Holcombe RF and Hoang BH: Blocking Wnt/LRP5 signaling by
a soluble receptor modulates the epithelial to mesenchymal
transition and suppresses met and metalloproteinases in
osteosarcoma Saos-2 cells. J Orthop Res. 25:964–971. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Niinaka Y, Harada K, Fujimuro M, et al:
Silencing of autocrine motility factor induces
mesenchymal-to-epithelial transition and suppression of
osteosarcoma pulmonary metastasis. Cancer Res. 70:9483–9493. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Sarsilmaz A, Argin M, Sezak M, Altay C and
Erdogan N: Primary osteosarcoma arising from subcutaneous tissue:
5-year follow-up. Clin Imaging. 36:402–405. 2012.PubMed/NCBI
|
|
26.
|
Eyre R, Feltbower RG, James PW, Blakey K,
Mubwandarikwa E, Forman D, McKinney PA, Pearce MS and McNally RJ:
The epidemiology of bone cancer in 0–39 year olds in northern
England, 1981–2002. BMC Cancer. 10:3572010.
|
|
27.
|
Andrews EB, Gilsenan AW, Midkiff K,
Sherrill B, Wu Y, Mann BH and Masica D: The US postmarketing
surveillance study of adult osteosarcoma and teriparatide: Study
design and findings from the first 7 years. J Bone Miner Res.
27:2429–2437. 2012.
|
|
28.
|
Mavrogenis AF, Rossi G, Palmerini E, et
al: Palliative treatments for advanced osteosarcoma. J BUON.
17:436–445. 2012.
|
|
29.
|
McConkey DJ, Choi W, Marquis L, et al:
Role of epithelial-to-mesenchymal transition (EMT) in drug
sensitivity and metastasis in bladder cancer. Cancer Metastasis
Rev. 28:335–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Dunning NL, Laversin SA, Miles AK and Rees
RC: Immunotherapy of prostate cancer: should we be targeting stem
cells and EMT? Cancer Immunol Immunother. 60:1181–1193. 2011.
View Article : Google Scholar : PubMed/NCBI
|