Introduction
Melatonin, a neurohormone secreted predominately by
the pineal gland in animals, has been suggested to possess
anticancer properties in a number of studies (1). Physiologically, melatonin is secreted
at low nanomolar concentrations and has been demonstrated to be an
effective antioxidant, free radical scavenger (2) and regulator of antioxidant genes
(3,4). This has led to the long-term or daily
administration of melatonin as a pharmacological supplement at
concentrations almost one million-fold higher than the
physiological levels (5). The
supplementation of melatonin has been shown to alleviate the
symptoms of several degenerative diseases associated with aging
[including Alzheimer’s and Parkinson’s disease (6,7)], to
reduce the incidence of malignant tumors in vivo (8) and to increase the survival time of
patients with glioblastomas treated with radiotherapy (9). Melatonin has also been demonstrated to
suppress the growth, migration and invasion of C6 glioma cells
in vitro by modulating numerous oxidative stress pathways
(10–12), and to exert an apoptotic effect in
several types of cancer (1). These
findings, in addition to the fact that no significant side effects
have been identified with the use of melatonin (13), have formed the basis of the
hypothesis that melatonin may have a beneficial efficacy in the
prevention of cancer (5), and may
be used to further supplement adjunct therapies in the prevention
or treatment of numerous types of cancer, including glioma.
However, melatonin has also been demonstrated to
inhibit apoptotic pathways in a number of cell types (14–16),
as well as having a role in stem cell proliferation and the
epigenetic regulation of neural cell growth in vitro
(17). These findings suggest that
the modulation of melatonin may not be limited to treating cancer
or exerting an apoptotic effect, and that numerous intracellular
mechanisms may be involved in promoting cancer cell survival.
Melatonin has been shown to induce the expression of
Nestin, a type VI intermediate filament protein, in the C17.2
neural stem cell line (17). Nestin
is a marker of neural stem cells. It is transiently expressed
during the development of the nervous system and is important in
the proliferation and the non-differentiated status of neural stem
cells (18,19). Previous studies have demonstrated
the involvement of Nestin in the development of cancer, and have
suggested that several types of cancer that present with
Nestin-positive tumors have a poor prognosis (20–24).
The general differentiation status of tumor cells has been shown to
be an important factor directly correlated with the malignancy of
tumors (25,26). The finding that melatonin affects
the expression of Nestin suggests that melatonin may have an effect
on stem cell differentiation which promotes the development of
cancer.
In the present study, the effect of pharmacological
concentrations of melatonin on the proliferation, growth and
survival of C6 glioma cells was evaluated in vitro to
examine the role of melatonin in the treatment of cancer. C6 glioma
cells provided a good model to investigate the effect of melatonin
on cell growth in vitro, as these cells express two types of
extracellular melatonin receptors (MT1 and MT2) and are susceptible
to modulation by melatonin at pharmacological concentrations
(10,11). The transcription of Nestin, along
with the transcription of two other genes that are important to
nervous system development (Bmi-1 and Sox2) (27,28),
were used as markers of cell proliferation to evaluate the role of
melatonin on glioma cell differentiation and proliferation. Bmi-1
and Sox2 were selected as cell proliferation markers in this study,
as they possess similar roles in cell growth (27,29–31),
have been implicated in cancer development (32–37)
and have not been demonstrated to be affected by melatonin
(38). The effect of melatonin on
the cell morphology, viability and death of C6 glioma cells was
additionally evaluated in comparison with the levels of
transcription of these proliferation markers.
Materials and methods
Cell culture and treatment
C6 glioblastoma cells (C6 cells) were provided by
the Cell Bank of Type Culture Collection of Chinese Academy of
Sciences (Shanghai, China). C6 cells were cultured in F12K media
(Sigma, St. Louis, MO, USA) supplemented with 15% equine serum
(Hyclone, Beijing, China) and 2.5% fetal bovine serum (Hyclone).
The cells were incubated at 37.5°C and 100% humidity in 95% air and
5% CO2.
All the experiments were conducted with cells at
70–80% confluence. C6 cells were cultured for 24 h prior to the
addition of experimental treatments. The cells were treated with
melatonin (Sigma) at concentrations of 0, 1, 3, 5 and 10 mM, and
incubated for an additional 24 h. An equal volume of
phosphate-buffered saline (PBS) was used as the vehicle
treatment.
Quantitative polymerase chain reaction
(qPCR)
The mRNA transcription levels of the target genes
(Nestin, Bmi-1 and Sox2) were analyzed with qPCR. Total RNA was
extracted from C6 cells using the RNA Simple Total RNA kit
(Tiangen, Beijing, China), according to the manufacturer’s
instructions. The RNA was reverse-transcribed using the M-MLV First
Strand kit (Invitrogen Life Technologies, Beijing, China). qPCR was
performed using the iTaq™ Universal SYBR Green supermix (Bio-Rad,
Hercules, CA, USA). The primers used for qPCR were as follows:
Forward: 5′-ATGGGGTTCCTGTACTATCTG-3′ and reverse:
5′-GGTGTTGGCTCTCCTCTTTA-3′ for Nestin; forward:
5′-CCAAAGGAGGAGGTGAATGA-3′ and reverse:
5′-AGGTGTAAATGTAGGCAATGTC-3′ for Bmi-1; forward:
5′-TAGGGCTGGGAGAAAGAAGAG-3′ and reverse: 5′-ATCTGGCGGAGAATAGTTGG-3′
for Sox2; forward: 5′-GGGACCTGACAGACTACCTCA-3′ and reverse:
5′-ATTGCCGATAGTGATGACCTGA-3′ for β-actin. β-actin mRNA was used as
a loading control.
Immunostaining of cells
The C6 cells were trypsinized and mounted on glass
coverslips precoated with polyornithine and laminin (Sigma). The
cells were fixed with 4% paraformaldehyde and permeabilized with
0.4% Triton X-100 at room temperature (RT). Bovine serum albumin
(5%; Amresco, Solon, OH, USA) was used to block the cells. The
cells were then incubated with primary rabbit anti-Nestin
polyclonal antibody (Millipore, Billerica, MA, USA) at 4°C
overnight. The cells were incubated with PE-conjugated secondary
antibody (anti-rabbit IgG-PE; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) at RT, and then stained with DAPI to identify all
cell nuclei (39).
Immunofluorescence and DAPI staining were detected by laser
confocal scanning microscopy (IX81S1F-3, Olympus, Tokyo,
Japan).
MTT assay
The MTT assay (Amresco) was used to detect C6 cell
viability (40). C6 cells cultured
in 96-well plates were incubated with 10 μl MTT (5 mg/ml) in
a CO2 incubator for 4 h. The medium was then discarded
using a suction pump and 100 μl dimethylsulfoxide (DMSO) was
added to each well to dissolve the MTT formazan crystals. The
optical density at 570 nm was measured by an enzyme-linked
immunosorbent detector (Bio-Rad, Kyoto, Japan).
Flow cytometry analysis of cell survival
status
The cell survival status was measured using the
Annexin-V Apoptosis Detection kit (Becton-Dickinson, San Diego, CA,
USA), according to the manufacturer’s instructions. Fluorescence
was measured on a fluorescence activated cell sorter (the
FACSVantageSE flow cytometer; Becton-Dickinson, Heidelberg,
Germany) and analysis of the data was performed using WinMDI 2.9
software. Data are presented as dot plots of fluorescein
isothiocyanate (FITC)-conjugated Annexin-V (X axis) and propidium
iodide (PI; Y axis) staining.
Statistical analysis
The results are presented as the mean ± standard
deviation for at least three repeated individual experiments of
each group. Analysis was performed with the SPSS 13.0 statistical
software (IBM, New York City, NY, USA). Statistical differences
were determined using one-way analysis of variance (ANOVA) for
independent samples. P<0.05 was considered to indicate a
statistically significant result.
Results
qPCR
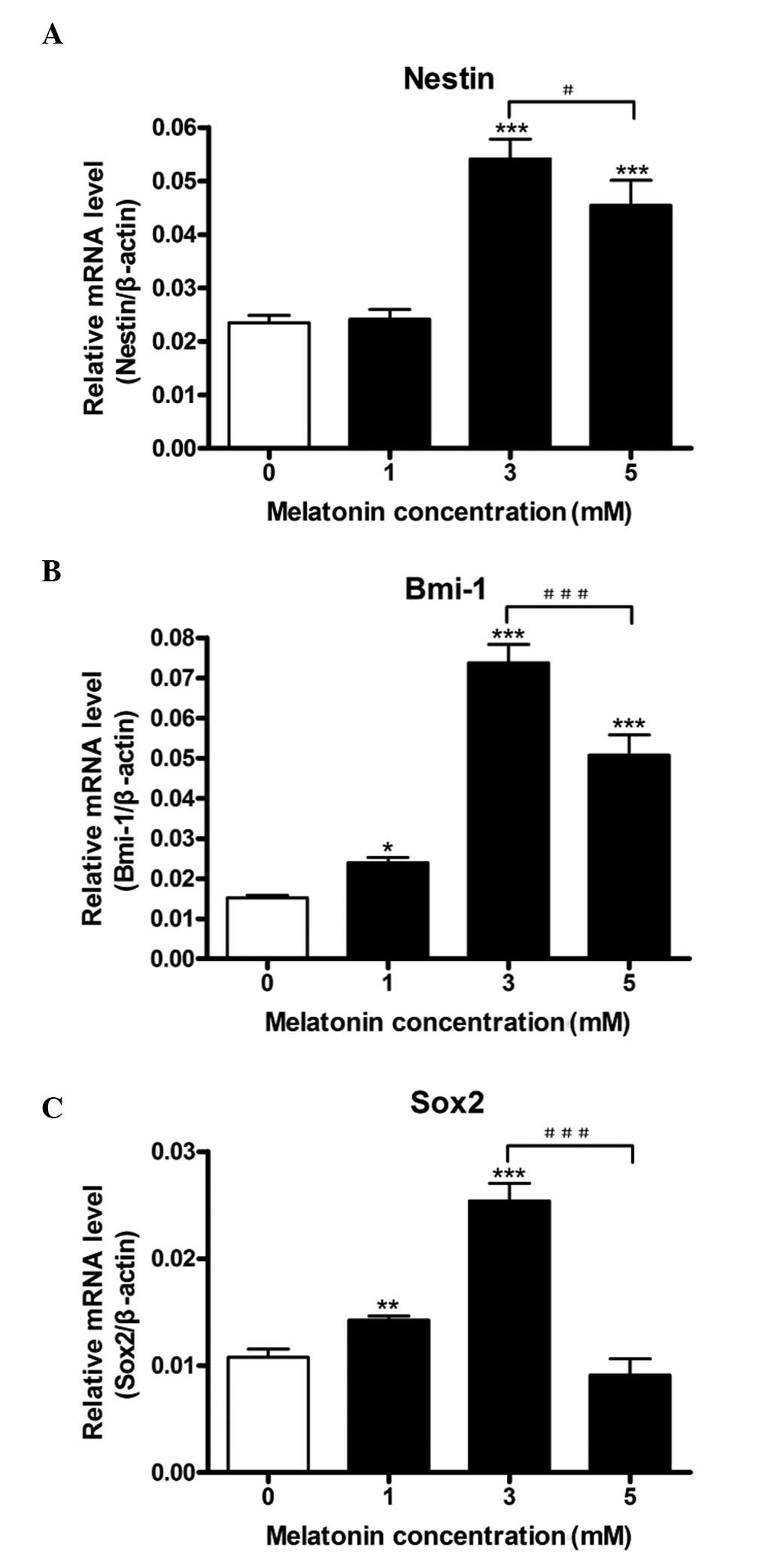
qPCR was used to study the transcription levels of
Nestin, Bmi-1 and Sox2 in C6 glioma cells under various
concentrations of melatonin. A general trend was observed for all
three cell proliferation markers (Fig.
1A–C). Melatonin was demonstrated to increase the mRNA
transcript levels in a dose-dependent manner at concentrations ≤3
mM; however, the increase in Nestin mRNA levels was not significant
at the 1 mM dose. Bmi-1 demonstrated a five- to six-fold increase
in mRNA transcript levels, whereas Nestin and Sox2 increased by
approximately three-fold at their peak transcription levels (3 mM
melatonin). At a concentration of 5 mM melatonin, all three mRNA
transcript levels were decreased in comparison with their peak
expression levels at 3 mM melatonin. At a concentration of 5 mM
melatonin, Nestin and Bmi-1 levels were significantly reduced from
their peak levels, yet remained increased from their basal levels.
By contrast, Sox2 transcription returned to basal levels.
The alterations in the mRNA transcript levels of
these cell proliferation markers with respect to the concentration
of melatonin were similar to the changes in the cell morphology of
glioma cells and the distribution pattern of Nestin expression
(Fig. 2). Untreated C6 cells
exhibited a normal morphology with Nestin filaments dispersed in
the cytoplasm and around the cell nuclei (Fig. 2A–D). Following treatment with 3 mM
melatonin, the cells became longer and thinner, and the Nestin
filaments began to condense around the cell nuclei (Fig. 2I–L). The majority of the cells
retracted to a round morphology and the Nestin filaments became
condensed around cell nuclei following treatment with 5 mM
melatonin (Fig. 2M–P).
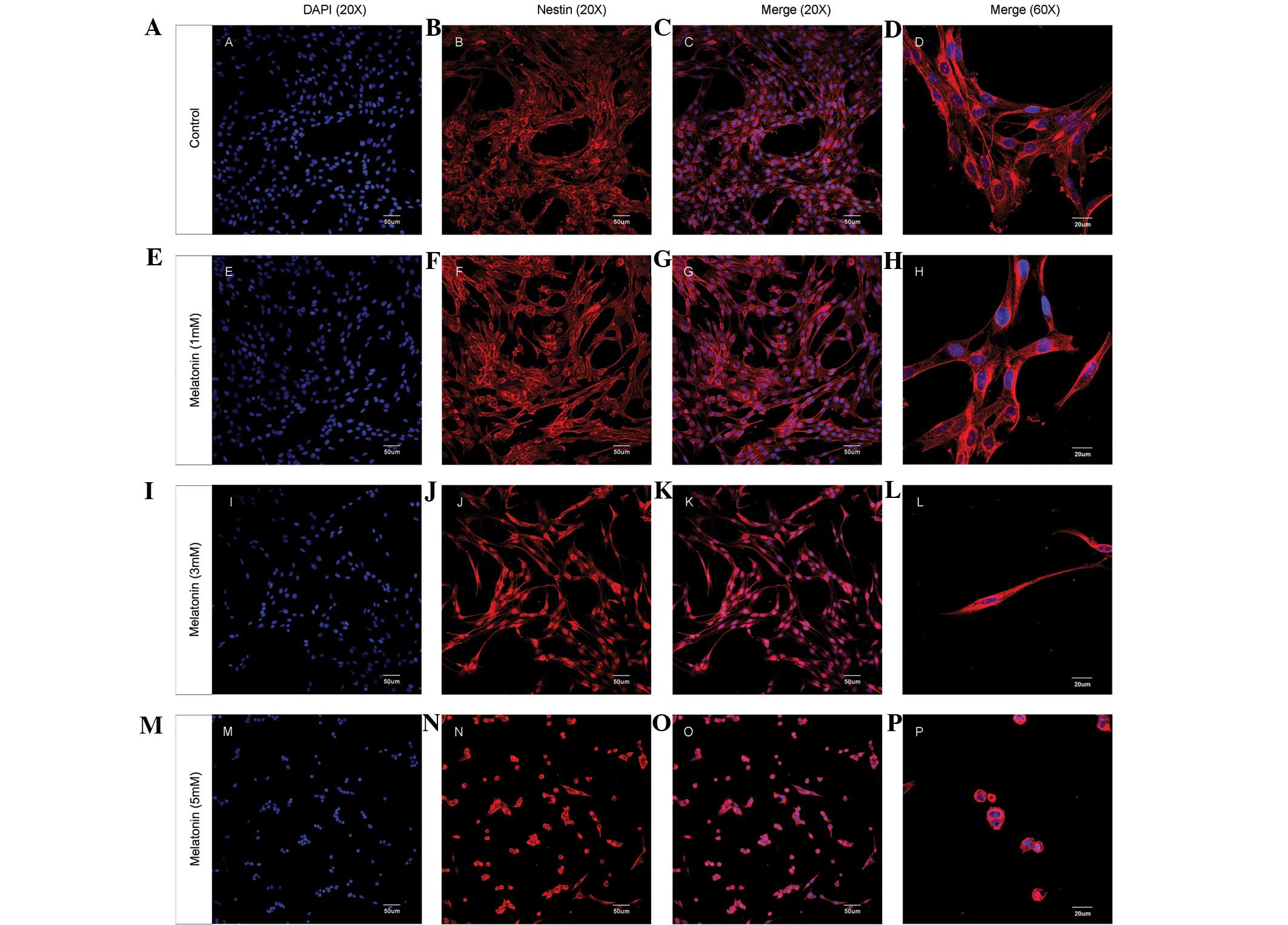 | Figure 2.Melatonin treatment affects the
protein distribution of Nestin and the morphology of C6 glioma
cells. C6 glioma cell cultures were treated with different
concentrations of melatonin (0, 1, 3 and 5 mM) for 24 h.
Immunofluorescence images of C6 glioma cells and Nestin protein
distribution are shown. (A, E, I and M) DAPI nuclear staining
(magnification, ×20); (B, F, J and N) Nestin protein
immunofluorescence (magnification, ×20); (C, G, K and O) overlay
(magnification, ×20) and (D, H, L and P) overlay (magnification,
×60). PE-conjugated secondary antibody was used as the
immunofluorescent dye. |
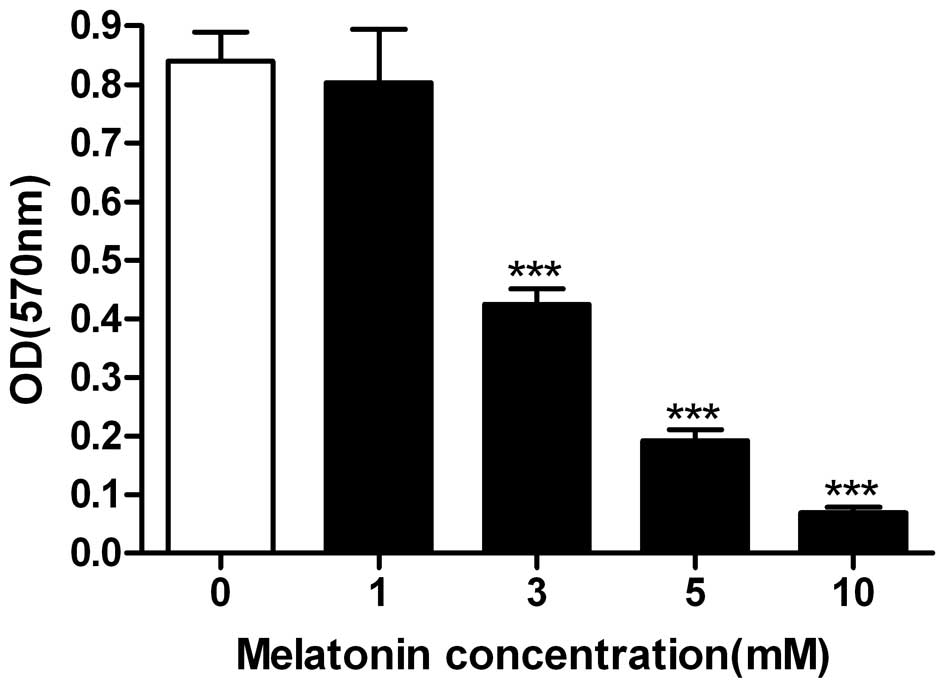
The MTT assay and flow cytometry analysis were then
performed to examine the effect of melatonin on C6 glioma cell
viability and survival. At a concentration of 1 mM, melatonin
exerted no effect on the viability of C6 cells (Fig. 3), which was consistent with the
results of previous studies (10).
However, 3 mM melatonin was demonstrated to reduce the viability of
C6 cells to ∼50%, while 10 mM melatonin almost completely
suppressed the cell viability (Fig.
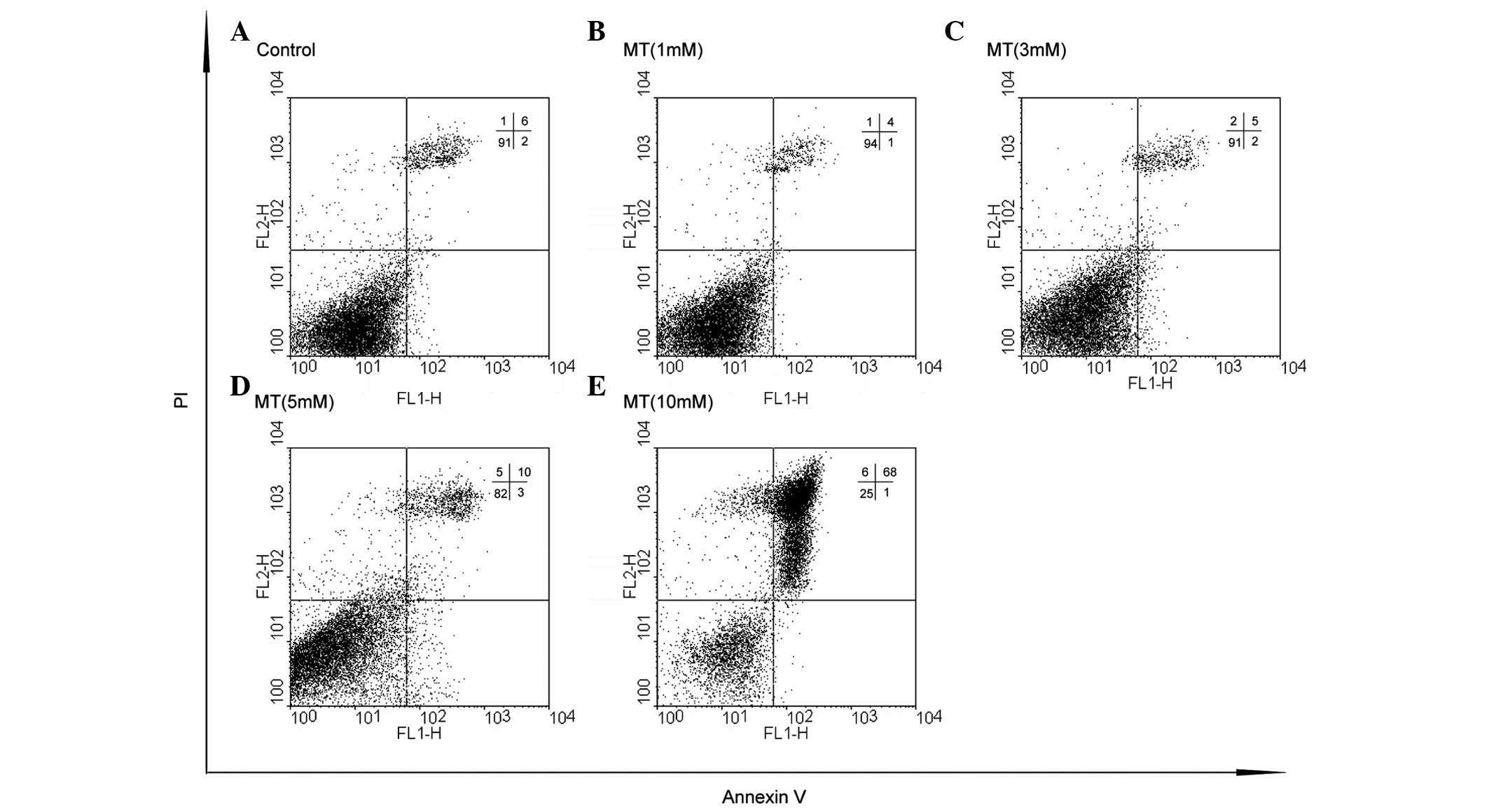
3). Similarly, a small number of dead cells were observed when
the glioma cells were treated with 0, 1 or 3 mM melatonin (Fig. 4A–C); however, these numbers
increased with higher melatonin concentrations. At a concentration
of 5 mM, melatonin was shown to induce low levels of cell death,
while 10 mM melatonin was shown to induce a significant increase in
cell death (Fig. 4D–E).
Discussion
The present study investigated the effect of
pharmacological concentrations of melatonin on C6 glioma cell
survival and viability in vitro, and evaluated the role of
melatonin on the transcriptional regulation of three genes (Nestin,
Bmi-1 and Sox2) involved in the development of the nervous system
and cancer progression. Melatonin was demonstrated to increase the
mRNA levels of Nestin, Bmi-1 and Sox2 in a similar pattern, peaking
at 3 mM melatonin. Immunofluorescence studies of Nestin-stained
cells suggested that Nestin filaments condensed and rearranged
around cell nuclei following melatonin treatment (at 3 and 5 mM).
These results corresponded with the findings that melatonin also
significantly decreased C6 cell viability at 3 mM, and induced cell
death at 5 and 10 mM. Overall, these findings suggested that
Nestin, Bmi-1 and Sox2 were strongly correlated with the survival
of C6 cells following melatonin treatment, and that high
therapeutic concentrations of melatonin (>5 mM) were required to
induce cell death.
Melatonin possesses a wide variety of physiological
functions, ranging from the regulation of the circadian rhythm
(41) to acting as a potent
antioxidant (2–4). A number of cellular membrane receptors
are regulated by melatonin, including nuclear and extracellular
membrane receptors (1), which may
partially account for the differential responses to melatonin
observed in normal and tumor cells. For example, the neural stem
cell line C17.2 has been shown to increase the transcription rate
of Nestin at physiological concentrations of melatonin (1 nM)
(17), suggesting that melatonin
has a role in stem cell proliferation in healthy tissues. The
finding that therapeutic concentrations of melatonin (3 mM) were
required to induce significant changes in the expression levels of
Nestin in C6 glioma cells suggests differences between the cell
lines; however, this result does not imply that glioma tissues are
less responsive to melatonin. It has been shown that nanomolar
concentrations of melatonin induced the transcription of glial cell
line-derived neurotrophic factor (GDNF) mRNA in C6 glioma cells
(42), and that micromolar
concentrations of melatonin protected C6 glioma cells from
amyloid-β-induced apoptosis (43).
These findings support the hypothesis that the mechanism by which
melatonin regulates cell growth is affected by the cell type and
the concentration of melatonin. In glioma tumors, these functions
may be involved in promoting cell growth, regardless of the fact
that melatonin is proposed to be a potential apoptotic agent for
cancer treatment (5).
Notably, Bmi-1 and Sox2 were upregulated at
therapeutic concentrations of melatonin in the glioma cell line.
Bmi-1 overexpression has been demonstrated to induce
epithelial-mesenchymal transition, to promote tumor metastasis
(32,33) and to cause radioresistance in cancer
therapy (44). Sox2 has also been
shown to be a marker of malignancy and is important in tumor cell
progression (34–37). Notably, a previous study
demonstrated that melatonin (100 μM) had no effect on the
transcription of Sox2 in a proliferating murine embryo stem cell
line (38), which suggested that
the induced transcription of Sox2 was the result of either a
differential response to a higher concentration of melatonin or a
specific response of the cancer-derived C6 glioma cell line. In
either case, the increase in the rate of proliferation in the
murine stem cell line and the increased levels of Sox2 expression
indicated the potential of melatonin to affect cell growth at
higher concentrations. These results imply that although differing
cells have various response mechanisms to melatonin, the induced
transcription of Nestin, Bmi-1 and Sox2 by melatonin is able to
initiate a molecular proliferative response in C6 glioma cells that
may contribute to the observed level of resistance to
melatonin.
Although the proliferation of C6 cells was not
observed, treatment with 3 mM melatonin was able to decrease cell
viability and cause marginal disturbances to the cell morphology of
glioma cells, despite increased transcript levels of Nestin, Bmi-1
and Sox2. This suggests that numerous mechanisms comprising
distinct molecular pathways in glioma cells, which either promote
cell growth or cell death, may be involved in the effects observed
following melatonin treatment. The competition between the distinct
pathways is highlighted by the correlation of increased levels of
cell death, decreased cell viability and significant changes in
cell morphology with decreased transcript levels of genes
associated with cell proliferation at higher concentrations (5 mM)
of melatonin, and the finding that 10 mM melatonin induced cell
death.
Melatonin may possess anticancer properties for the
treatment of several types of cancer, including glioma. The present
study supports the requirement for additional or adjunct therapies
in combination with melatonin treatment to fully inhibit the
progression of cancer. The potential to target mechanisms that
promote stem cell markers, including Nestin, Bmi-1 or Sox2, may
provide adequate strategies to investigate which chemotherapies are
most effective. Similar studies with regard to the activity of
these genes in other neurological tumors may provide useful data on
the cellular response to melatonin that promotes cell survival.
Although the mechanisms that promote cell proliferation remain
unclear and require further research, a number of studies have
identified differential patterns of Nestin filament distribution
and cell morphology in various types of neurogenic tumors (45–48),
which may act as a template to assess the proliferation-promoting
properties of melatonin compared with its potential apoptotic
functions in various types of neurological cancer. Bmi-1 and Sox2
may also provide a similar template to assess the regulatory
mechanisms and functions of melatonin in drug resistance. Future
studies investigating these areas will aid in the screening of
chemotherapies that function synergistically with melatonin, a
non-toxic natural product with apoptotic properties, to induce
cancer-specific cell death.
Acknowledgements
This study was supported by the
Knowledge Innovation Project of the Chinese Academy of Sciences
(grant no. KSCX2-EW-J-23).
References
|
1.
|
Sánchez-Hidalgo M, Guerrero JM, Villegas
I, Packham G and de la Lastra CA: Melatonin, a natural programmed
cell death inducer in cancer. Curr Med Chem. 19:3805–3821.
2012.PubMed/NCBI
|
|
2.
|
Tan DX, Reiter RJ, Manchester LC, et al:
Chemical and physical properties and potential mechanisms:
melatonin as a broad spectrum antioxidant and free radical
scavenger. Curr Top Med Chem. 2:181–197. 2002. View Article : Google Scholar
|
|
3.
|
Chakravarty S and Rizvi SI: Circadian
modulation of human erythrocyte plasma membrane redox system by
melatonin. Neurosci Lett. 518:32–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Reiter RJ: Oxidative damage in the central
nervous system: protection by melatonin. Prog Neurobiol.
56:359–384. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Altun A and Ugur-Altun B: Melatonin:
therapeutic and clinical utilization. Int J Clin Pract. 61:835–845.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Wu YH and Swaab DF: The human pineal gland
and melatonin in aging and Alzheimer’s disease. J Pineal Res.
38:145–152. 2005.
|
|
7.
|
Mayo JC, Sainz RM, Tan DX, Antolín I,
Rodríguez C and Reiter RJ: Melatonin and Parkinson’s disease.
Endocrine. 27:169–178. 2005.
|
|
8.
|
Tamarkin L, Cohen M, Roselle D, Reichert
C, Lippman M and Chabner B: Melatonin inhibition and pinealectomy
enhancement of 7,12-dimethylbenz(a)anthracene-induced mammary
tumors in the rat. Cancer Res. 41:4432–4436. 1981.PubMed/NCBI
|
|
9.
|
Lissoni P, Meregalli S, Nosetto L, et al:
Increased survival time in brain glioblastomas by a
radioneuroendocrine strategy with radiotherapy plus melatonin
compared to radiotherapy alone. Oncology. 53:43–46. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Wang J, Hao H, Yao L, et al: Melatonin
suppresses migration and invasion via inhibition of oxidative
stress pathway in glioma cells. J Pineal Res. 53:180–187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Martín V, Herrera F, Carrera-Gonzalez P,
et al: Intracellular signaling pathways involved in the cell growth
inhibition of glioma cells by melatonin. Cancer Res. 66:1081–1088.
2006.PubMed/NCBI
|
|
12.
|
Martín V, Herrera F, García-Santos G, et
al: Involvement of protein kinase C in melatonin’s oncostatic
effect in C6 glioma cells. J Pineal Res. 43:239–244. 2007.
|
|
13.
|
Vijayalaxmi, Reiter RJ, Tan DX, Herman TS
and Thomas CR Jr: Melatonin as a radioprotective agent: a review.
Int J Radiat Oncol Biol Phys. 59:639–653. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Andrabi SA, Sayeed I, Siemen D, Wolf G and
Horn TF: Direct inhibition of the mitochondrial permeability
transition pore: a possible mechanism responsible for
anti-apoptotic effects of melatonin. FASEB J. 18:869–871.
2004.PubMed/NCBI
|
|
15.
|
Radogna F, Paternoster L, Albertini MC, et
al: Melatonin antagonizes apoptosis via receptor interaction in
U937 monocytic cells. J Pineal Res. 43:154–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Esposito E, Iacono A, Muià C, et al:
Signal transduction pathways involved in protective effects of
melatonin in C6 glioma cells. J Pineal Res. 44:78–87.
2008.PubMed/NCBI
|
|
17.
|
Sharma R, Ottenhof T, Rzeczkowska PA and
Niles LP: Epigenetic targets for melatonin: induction of histone H3
hyperacetylation and gene expression in C17.2 neural stem cells. J
Pineal Res. 45:277–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Matsuda M, Katoh-Semba R, Kitani H and
Tomooka Y: A possible role of the nestin protein in the developing
central nervous system in rat embryos. Brain Res. 723:177–189.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Lendahl U, Zimmerman LB and McKay RD: CNS
stem cells express a new class of intermediate filament protein.
Cell. 60:585–595. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Wan F, Herold-Mende C, Campos B, et al:
Association of stem cell-related markers and survival in astrocytic
gliomas. Biomarkers. 16:136–143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Rushing EJ, Sandberg GD and
Horkayne-Szakaly I: High-grade astrocytomas show increased Nestin
and Wilms’s tumor gene (WT1) protein expression. Int J Surg Pathol.
18:255–259. 2010.PubMed/NCBI
|
|
22.
|
Zhang M, Song T, Yang L, et al: Nestin and
CD133: valuable stem cell-specific markers for determining clinical
outcome of glioma patients. J Exp Clin Cancer Res. 27:852008.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Rutka JT, Ivanchuk S, Mondal S, et al:
Co-expression of nestin and vimentin intermediate filaments in
invasive human astrocytoma cells. Int J Dev Neurosci. 17:503–515.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Flørenes VA, Holm R, Myklebost O, Lendahl
U and Fodstad O: Expression of the neuroectodermal intermediate
filament nestin in human melanomas. Cancer Res. 54:354–356.
1994.PubMed/NCBI
|
|
25.
|
Contesso G, Mouriesse H, Friedman S, Genin
J, Sarrazin D and Rouesse J: The importance of histologic grade in
long-term prognosis of breast cancer: a study of 1,010 patients,
uniformly treated at the Institut Gustave-Roussy. J Clin Oncol.
5:1378–1386. 1987.PubMed/NCBI
|
|
26.
|
Liu R, Wang X, Chen GY, et al: The
prognostic role of a gene signature from tumorigenic breast-cancer
cells. N Engl J Med. 356:217–226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
van der Lugt NM, Domen J, Linders K, et
al: Posterior transformation, neurological abnormalities, and
severe hematopoietic defects in mice with a targeted deletion of
the bmi-1 protooncogene. Genes Dev. 8:757–769. 1994.
|
|
28.
|
Episkopou V: SOX2 functions in adult
neural stem cells. Trends Neurosci. 28:219–221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Papp B and Müller J: Histone
trimethylation and the maintenance of transcriptional ON and OFF
states by trxG and PcG proteins. Genes Dev. 20:2041–2054. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Leung C, Lingbeek M, Shakhova O, et al:
Bmi1 is essential for cerebellar development and is overexpressed
in human medulloblastomas. Nature. 428:337–341. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Boyer LA, Lee TI, Cole MF, et al: Core
transcriptional regulatory circuitry in human embryonic stem cells.
Cell. 122:947–956. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Yu CC, Lo WL, Chen YW, et al: Bmi-1
regulates snail expression and promotes metastasis ability in head
and neck squamous cancer-derived ALDH1 positive cells. J Oncol.
2011:6092592011.PubMed/NCBI
|
|
33.
|
Song LB, Li J, Liao WT, et al: The
polycomb group protein Bmi-1 represses the tumor suppressor PTEN
and induces epithelial-mesenchymal transition in human
nasopharyngeal epithelial cells. J Clin Invest. 119:3626–3636.
2009. View
Article : Google Scholar
|
|
34.
|
Lu Y, Futtner C, Rock JR, et al: Evidence
that SOX2 overexpression is oncogenic in the lung. PLoS One.
5:e110222010. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Yang S, Zheng J, Ma Y, et al: Oct4 and
Sox2 are overexpressed in human neuroblastoma and inhibited by
chemotherapy. Oncol Rep. 28:186–192. 2012.PubMed/NCBI
|
|
36.
|
Neumann J, Bahr F, Horst D, et al: SOX2
expression correlates with lymph-node metastases and distant spread
in right-sided colon cancer. BMC Cancer. 11:5182011. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Jia X, Li X, Xu Y, et al: SOX2 promotes
tumorigenesis and increases the anti-apoptotic property of human
prostate cancer cell. J Mol Cell Biol. 3:230–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Yoo YM, Jung EM, Choi KC and Jeung EB:
Effect of melatonin on mRNA expressions of transcription factors in
murine embryonic stem cells. Brain Res. 1385:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Bexell D, Gunnarsson S, Siesjö P, Bengzon
J and Darabi A: CD133+ and nestin+
tumor-initiating cells dominate in N29 and N32 experimental
gliomas. Int J Cancer. 125:15–22. 2009.
|
|
40.
|
Sobottka SB and Berger MR: Assessment of
antineoplastic agents by MTT assay: partial underestimation of
antiproliferative properties. Cancer Chemother Pharmacol.
30:385–393. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Cajochen C, Kräuchi K and Wirz-Justice A:
Role of melatonin in the regulation of human circadian rhythms and
sleep. J Neuroendocrinol. 15:432–437. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Armstrong KJ and Niles LP: Induction of
GDNF mRNA expression by melatonin in rat C6 glioma cells.
Neuroreport. 13:473–475. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Feng Z and Zhang JT: Protective effect of
melatonin on beta-amyloid-induced apoptosis in rat astroglioma C6
cells and its mechanism. Free Radic Biol Med. 37:1790–1801. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Liu ZG, Liu L, Xu LH, et al: Bmi-1 induces
radioresistance in MCF-7 mammary carcinoma cells. Oncol Rep.
27:1116–1122. 2012.PubMed/NCBI
|
|
45.
|
Krupkova O Jr, Loja T, Redova M, et al:
Analysis of nuclear nestin localization in cell lines derived from
neurogenic tumors. Tumour Biol. 32:631–639. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Loja T, Chlapek P, Kuglik P, et al:
Characterization of a GM7 glioblastoma cell line showing CD133
positivity and both cytoplasmic and nuclear localization of nestin.
Oncol Rep. 21:119–127. 2009.PubMed/NCBI
|
|
47.
|
Veselska R, Kuglik P, Cejpek P, et al:
Nestin expression in the cell lines derived from glioblastoma
multiforme. BMC Cancer. 6:322006. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Thomas SK, Messam CA, Spengler BA, Biedler
JL and Ross RA: Nestin is a potential mediator of malignancy in
human neuroblastoma cells. J Biol Chem. 279:27994–27999. 2004.
View Article : Google Scholar : PubMed/NCBI
|