Introduction
Renal cell carcinoma (RCC) accounts for 80–90% of
cases of kidney cancer. The incidence rate of RCC is generally
higher in more developed countries than in less developed countries
(1). The rate of RCC is 2-fold
higher in males than in females and increases with age. RCC is a
pathogenically heterogeneous disease and clear cell RCC (ccRCC)
accounts for 70–80% of cases of kidney cancer. Epidemiological
studies have identified that hypertension, obesity, cigarette
smoking and exposure to trichloroethylene are environmental risk
factors of RCC; however, the impact of these factors may vary among
populations (2–4). Environmental factors and their
interaction with genetic factors are hypothesized to affect the
risk of RCC (5,6). Single nucleotide polymorphisms (SNPs)
in cancer growth-related cytokines, including vascular endothelial
growth factor, Dickkopf-3 in the Wnt pathway and blood pressure
genes, are associated with RCC risk (7–9). SNPs
of metabolic enzymes, including cytochrome P450 mono-oxygenases,
N-acetyltransferase and glutathione S-transferase, are associated
with RCC risk in Caucasians; although, these correlations have not
been replicated in Chinese individuals (4,10),
indicating that the association between genetic predisposition and
RCC risk alters among various populations. However, a number of
studies using candidate-gene approaches have not yielded a
conclusive result (4–10).
Surgical resection remains the curative therapy for
RCC patients; although, patients treated surgically have 5- and
10-year relative survival rates of 72 and 63%, respectively
(11). Previous studies have shown
that specific clinical and molecular factors have predictive values
for postoperative prognosis: Advanced tumor stages; circulating
molecules, including C-reactive protein and erythrocyte polyamines;
molecules in the tumor, including L1 cell adhesion molecules; and
chromosomal variations, including 10q and 13q deletions, and D9S168
micro-satellite alterations. These factors are capable of
predicting a poor postoperative prognosis for RCC (12–16).
Genetic predispositions, including the Dickkopf-2 rs17037102
polymorphism, interleukin-4 haplotype -589T-33T, MDM2
rs2279744 polymorphism and miRNA-related genetic polymorphisms are
closely associated with RCC clinical outcome (8,17–19).
A previous genome-wide association study (GWAS) in
RCC cases and controls of European background revealed that two
loci mapped to EPAS1, encoding hypoxia inducible factor
(HIF)-2α, on 2p21 (rs11894252 and rs7579899), a locus on 11q13.3
(rs7105934) and a locus mapped to SCARB1, encoding the
scavenger receptor class B, member 1, on 12q24.31 (rs4765623) were
significantly associated with RCC susceptibility (20). rs7105934 at 11q13.3 was detected to
modulate the binding and function of HIF-2α at a transcriptional
enhancer of CCND1 (encoding cyclin D1) (21). In the present study, the
correlations between SNPs identified in the present GWAS and RCC
susceptibility were validated, and the role of SNPs in the
postoperative prognosis of RCC was investigated in a Chinese
population.
Materials and methods
Study subjects
A total of 400 pathologically confirmed, sporadic
RCC patients diagnosed between November 1998 and November 2011 at
the Department of Urology (Changhai Hospital, Second Military
Medical University, Shanghai, China) were involved in the present
study. In addition, 806 controls that received comprehensive
physical examinations, including type-B ultrasonic and blood tests,
and were confirmed to be healthy at the Physical Examination Center
of Changhai Hospital between 2006 and 2011, were also involved.
Individuals who did not have notable metastasis at the time of
surgery and did not receive adjuvant therapy after surgery were
followed up. Follow-up was initiated 6 months after surgery,
performed by telephone or interviews in person at the Outpatient
Department every 3 months, in accordance with standard
epidemiological procedures. The median follow-up period was 34.0
months (range, 3.0–90.9 months). All participants were of Chinese
ethnic origin, and the study protocol conformed to the ethical
guidelines of the 1975 Declaration of Helsinki and was approved by
the Institutional Review Board of the Second Military Medical
University (Shanghai, China). All participants provided written
informed consent.
Genotyping of genetic polymorphisms
Genomic DNA was extracted from blood samples using
QIAquick PCR purification kits (Qiagen, Hilden, Germany).
rs4765623, rs7105934, rs7579899 and rs1867785 (highly correlated
with rs11894252) were genotyped using fluorescent-probe qPCR in a
LightCycler™ 480 (Roche Diagnostics, Mannheim, Germany).
Primers and probes (TaqMan or Minor Groove Binder) were designed
and synthesized by GeneCore BioTechnologies Co., Ltd. (Shanghai,
China), and the sequences of the primers and probes are shown in
Table I. Each reaction mixture
contained 0.2 μmol/l primers and probes, as well as 0.1–0.5
μg purified templates in the Premix Ex Taq™
reaction system (Takara Bio, Inc., Shiga, Japan). Three duplicative
samples were run with a template-free control.
 | Table I.Primers and probes for genotyping the
four single nucleotide polymorphisms. |
Table I.
Primers and probes for genotyping the
four single nucleotide polymorphisms.
| Variation | Primers 5′-3′ | Probes |
|---|
| rs7105934 | G/A |
GAGGAATGATGAACAAACTGTGGTA |
FAM-CCAAAATGCATCGTGCTAAGAAGCC-TAMRA |
|
CAGAACATCACATAAATGGAATCATACA |
HEX-TCCAAAATGCATCATGCTAAGAAGCC-TAMRA |
| rs1867785 | A/G |
GGACTTCTCTCTCCCTTCACCCT |
FAM-AAATTAGCTTCGTTGACCTCAGCCAGC-TAMRA |
|
TCCTGTGTTTCCAAGAGTTCTCAGA |
HEX-ATTAGCTTCGTCGACCTCAGCCAGC-TAMRA |
| rs7579899 | A/G |
ACACAGCCAAATCCAAGTCAGA |
FAM-ACACCCTGTACAAAGCACTGCGACC-TAMRA |
|
TGACCAAACACTAGGAAAGGAGAAG |
HEX-ACACCCTGTACAGAGCACTGCGACC-TAMRA |
| rs4765623 | C/T |
GGTCTCGCGCATGTGTCA | FAM-
AGTACAGCCACCTCGGAGAGCCACT-TAMRA |
|
CCAGATGCGTTCAGCAGTTC | HEX-
AGTACAGCCACCTTGGAGAGCCACTG-TAMRA |
Western blotting
Protein was extracted from the adjacent renal tissue
of fresh RCC specimens and quantified using cell lysis buffer and
Enhanced BCA Protein Assay kits (Beyotime Institute of
Biotechnology, Jiangsu, China). Extracted proteins were resolved by
routine PAGE gels and transferred onto polyvinylidene difluoride
membranes. The membranes were incubated overnight with rabbit
monoclonal antibodies against cyclin D1 (1:1,000; Epitomics Inc.,
Burlingame, CA, USA) and β-ctin (1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA), washed and incubated with
HRP-conjugated anti-rabbit or anti-mouse IgG (Cell Signaling
Technology, Inc.) for 2 h. Peroxidase activity was developed by ECL
detection reagent (GE Healthcare, Amersham, UK) according to the
manufacturer’s instructions. The signal intensity of each band was
quantified using Genetools software, version 4.02 (Syngene,
Cambridge, UK). The relative intensity of each sample was
calculated as the intensity of the cyclin D1 band (raw
value-background noise)/the intensity of the β-actin band (raw
value-background noise).
Immunohistochemistry
Antibodies against cyclin D1 (1:100) were used for
immunohistochemistry of RCC specimens in accordance with methods
previously described for western blotting (22). Sections were independently assessed
by four researchers who were blinded to the clinical information.
Immunostaining intensity was scored as follows: Negative (−),
<5% positive cancer cells; weak (+), 5–20% positive; moderate
(++), 20–60% positive; and strong (+++), >60% positive. There
was >95% agreement among the researchers and disagreements were
resolved by consensus. A total of 90 paraffin-embedded samples were
used in immunohistochemical assay.
Statistical analysis
Differences in categorical variables were evaluated
using the χ2 test. Hardy-Weinberg equilibrium (HWE) was
examined online (http://ihg.gsf.de/ihg/snps.html) and linkage
disequilibrium (LD) analyses for the SNPs involved in the current
study and those identified in previous GWAS (23,24)
were performed using online Haploview 4.2 software (http://hapmap.ncbi.nlm.nih.gov). Differences in
the relative intensities of the western blotting bands among the
samples of various genotypes were analyzed using the Student’s
t-test. Unconditional logistic regression analysis was used to
obtain an odds ratio (OR) for each SNP with RCC and a 95%
confidence interval (CI). Overall survival was analyzed using the
Kaplan-Meier method and the log-rank test was used to compare
survival curves. Forward stepwise multivariate Cox regression
analysis (Pentry=0.05 and Premoval=0.10) was
performed to determine the factors contributing independently to
RCC prognosis. The correlation between immunostaining scores for
cyclin D1 and RCC stage and the rs7105934 genotypes was determined
by Spearman’s rank correlation analysis, respectively. All
statistical tests were two-sided and conducted using SPSS for
Windows, version 16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Correlation between the four SNPs and RCC
risk
Table II shows the
characteristics of the RCC patients and healthy controls from the
present study. The gender distribution was not significantly
different between the RCC patients and healthy controls; however,
the healthy controls were significantly older than the RCC patients
(median ± standard deviation; 60.1±12.8 vs. 57.1±12.8 years;
P<0.001). The genotyping call rate was 100% for each SNP, and in
the healthy controls, rs4765623, rs7105934, rs7579899 and rs1867785
conformed to HWE (P=0.481, 0.891, 0.352 and 0.373, respectively).
LD analysis indicated that rs7579899 was markedly correlated with
rs1867785 (r2=0.988 in this study population), whereas
rs1867785 was not in LD with rs12617313 or rs9679290
(r2<0.05 in the HapMap Chinese population), the SNPs
identified on chromosome 2p21 by a previous GWAS (24). Table
III shows the correlation between the SNPs and RCC risk. Of the
four SNPs, rs7105934 only was found to significantly correlate with
RCC risk. The dominant model or A allele at rs7105934 was
significantly correlated with a reduced risk of RCC following
adjustments for age and gender, with a power of 77.4%. As ccRCC is
the major histological type, the correlation between the four SNPs
and ccRCC risk was examined, and rs7105934 only was found to
correlate with the risk of ccRCC. Compared with the GG genotype,
the GA + AA genotype of rs7105934 was significantly correlated with
a reduced ccRCC risk with an OR of 0.60 (95% CI, 0.40–0.92; P=
0.018), following adjustments for age and gender. By contrast, the
A allele of rs7105934 was inversely associated with ccRCC risk
following adjustments (OR, 0.62; 95% CI, 0.41–0.92; P=0.018).
 | Table II.Demographic and clinicopathological
characteristics of the study subjects. |
Table II.
Demographic and clinicopathological
characteristics of the study subjects.
| Characteristics | Cases, n (%) | Controls, n (%) | P-value |
|---|
| Age, years | | | |
| ≤40 | 31 (7.8) | 36 (4.5) | 0.008 |
| 40–60 | 210 (52.5) | 380 (47.1) | |
| 60–80 | 147 (36.8) | 352 (43.7) | |
| >80 | 12 (3.0) | 38 (4.7) | |
| Gender | | | |
| Male | 274 (68.5) | 548 (68.0) | 0.858 |
| Female | 126 (31.5) | 258 (32.0) | |
| Histology | | | |
| Clear cell | 373 (93.3) | - | |
| Papillary | 12 (3.0) | - | |
| Chromophobe | 8 (2.0) | - | |
| Unclassified | 7 (1.8) | - | |
| AJCC (2002)
stage | | | |
| I | 324 (81.0) | - | |
| II | 33 (8.3) | - | |
| III | 43 (10.8) | - | |
 | Table III.Correlation between the four SNPs and
risk of RCC following adjustment for age and gender. |
Table III.
Correlation between the four SNPs and
risk of RCC following adjustment for age and gender.
| Genotype | Cases, n (%) | Controls, n
(%) | OR (95% CI) | P-valuea |
|---|
| rs7105934 | | | | |
| GG | 364 (91.0) | 700 (86.8) | 1.00
(reference) | |
| GA | 35 (8.8) | 102 (12.7) | 0.65
(0.43–0.97) | 0.036 |
| AA | 1 (0.3) | 4 (0.5) | 0.47
(0.05–4.25) | 0.500 |
| GG | 364 (91.0) | 700 (86.8) | 1.00
(reference) | |
| GA + AA | 36 (9.0) | 106 (13.2) | 0.64
(0.43–0.96) | 0.029 |
| G allele | 763 (95.4) | 1502 (93.2) | 1.00
(reference) | |
| A allele | 37 (4.6) | 110 (6.8) | 0.65
(0.44–0.95) | 0.028 |
| rs1867785 | | | | |
| AA | 288 (72.0) | 561 (69.6) | 1.00
(reference) | |
| AG | 105 (26.3) | 227 (28.2) | 0.90
(0.69–1.19) | 0.472 |
| GG | 7 (1.8) | 18 (2.2) | 0.76
(0.31–1.86) | 0.552 |
| AA | 288 (72.0) | 561 (69.6) | 1.00
(reference) | |
| AG + GG | 112 (28.0) | 245 (30.4) | 0.89
(0.69–1.17) | 0.411 |
| A allele | 681 (85.1) | 1349 (83.7) | 1.00
(reference) | |
| G allele | 119 (14.9) | 263 (16.3) | 0.90
(0.71–1.14) | 0.381 |
| rs7579899 | | | | |
| AA | 287 (71.8) | 560 (69.5) | 1.00
(reference) | |
| AG | 106 (26.5) | 228 (28.3) | 0.91
(0.69–1.19) | 0.495 |
| GG | 7 (1.8) | 18 (2.2) | 0.76
(0.31–1.86) | 0.554 |
| AA | 287 (71.8) | 560 (69.5) | 1.00
(reference) | |
| AG + GG | 113 (28.3) | 246 (30.5) | 0.90
(0.69–1.17) | 0.432 |
| A allele | 680 (85.0) | 1348 (83.6) | 1.00
(reference) | |
| G allele | 120 (15.0) | 264 (16.4) | 0.90
(0.71–1.14) | 0.399 |
| rs4765623 | | | | |
| CC | 136 (34.0) | 268 (33.3) | 1.00
(reference) | |
| CT | 188 (47.0) | 385 (47.8) | 0.97
(0.74–1.28) | 0.845 |
| TT | 76 (19.0) | 153 (19.0) | 0.97
(0.68–1.37) | 0.844 |
| CC | 136 (34.0) | 268 (33.3) | 1.00
(reference) | |
| CT + TT | 264 (66.0) | 538 (66.7) | 0.97
(0.75–1.25) | 0.822 |
| C allele | 460 (57.5) | 921 (57.1) | 1.00
(reference) | |
| T allele | 340 (42.5) | 691 (42.9) | 0.98
(0.83–1.17) | 0.826 |
Correlation between SNPs and RCC
prognosis
Of the RCC patients, 194/400 completed the follow-up
and were included in the survival analysis. It was identified that
rs7105934 GA + AA genotype was significantly correlated with an
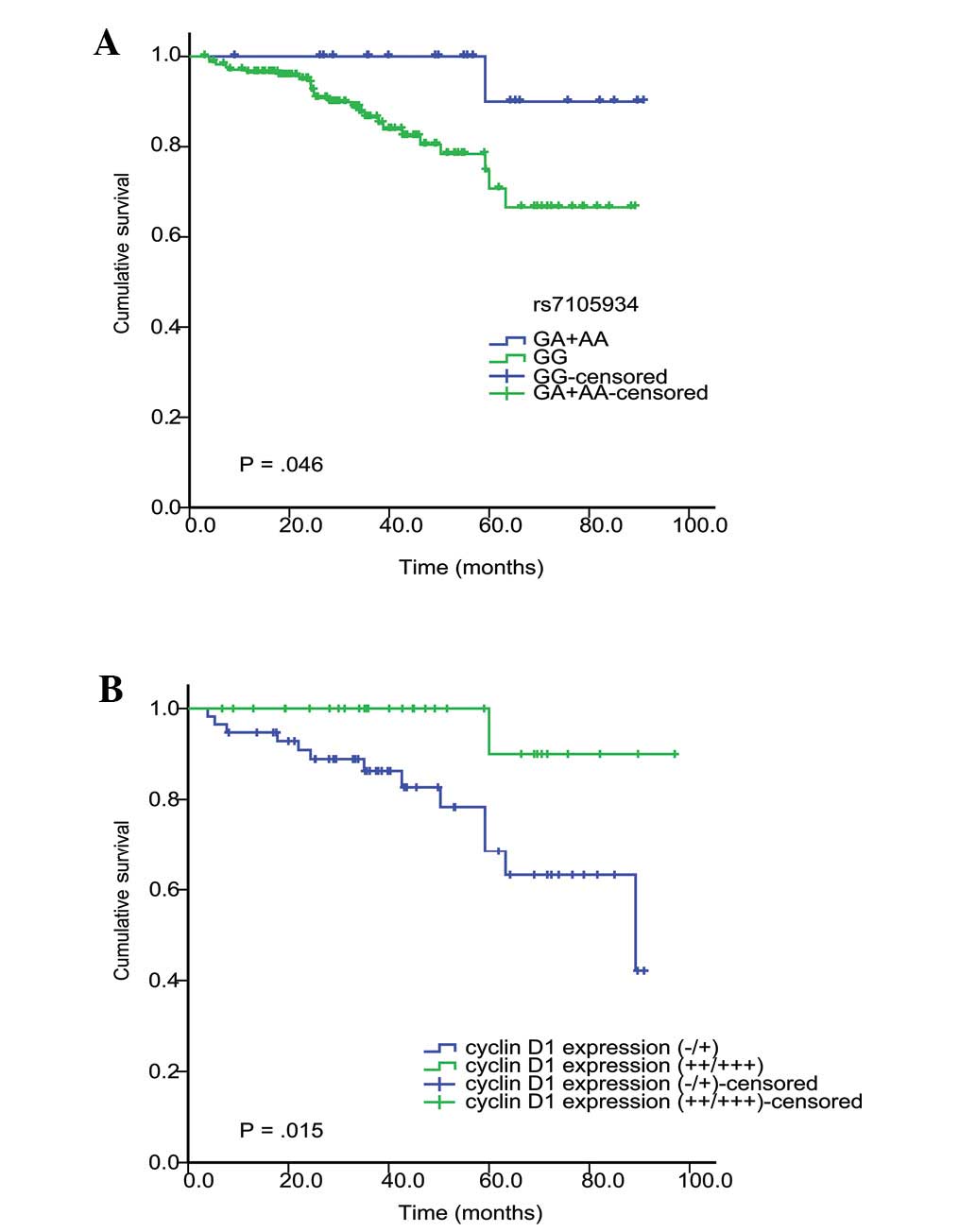
improved postoperative prognosis of RCC (P=0.046; Kaplan-Meier and
log-rank test; Fig. 1A). Table IV presents the results of stepwise
multivariate Cox regression analysis, indicating that patients with
the rs7105934 GA + AA genotype have a decreased risk of mortality
(HR, 0.12; 95% CI, 0.016–0.93; P=0.042). In addition, advanced
tumor stage was also found to independently predict poor survival
rates in RCC.
 | Table IV.Factors independently associated with
overall survival of RCC patients in stepwise multivariate Cox
regression analysis. |
Table IV.
Factors independently associated with
overall survival of RCC patients in stepwise multivariate Cox
regression analysis.
| Cases evaluated,
n | Cases of RCC
mortality, n | HR (95% CI) | P-value |
|---|
| rs7105934 | | | | |
| GG | 172 | 25 | 1.00
(reference) | |
| GA + AA | 22 | 1 | 0.12
(0.016–0.93) | 0.042 |
| AJCC (2002)
stage | | | | |
| I | 147 | 7 | 1.00
(reference) | |
| II | 12 | 3 | 5.79
(1.47–22.82) | 0.012 |
| III | 35 | 16 | 15.72
(6.36–38.84) | <0.001 |
Correlation between rs7105934 genotypes
and cyclin D1 expression
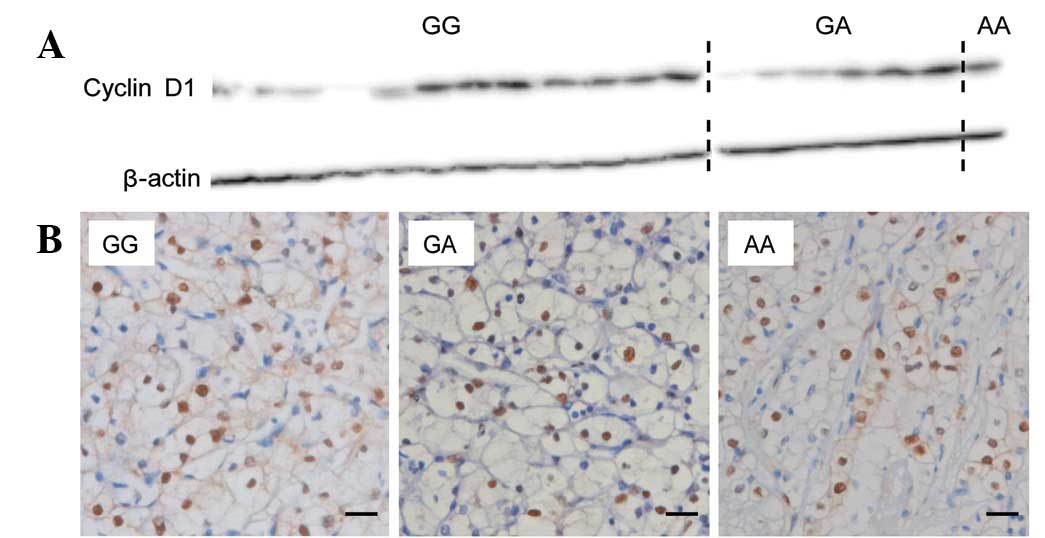
Expression of cyclin D1 protein in the adjacent
renal tissue of fresh RCC specimens was examined using western
blotting. No significant differences were identified in the
relative intensity of cyclin D1 protein between patients with the
GG genotype and those with the GA + AA genotype of rs7105934
(P=0.514; Fig. 2A). Cyclin D1
expression in the tumors was examined using immunohistochemistry
(Fig. 2B). Immunostaining
intensities of cyclin D1 in the cancer cells of the RCC patient
specimens did not correlate significantly among the three genotype
groups of rs7105934 (P=0.844) or the GG and GA + AA genotypes of
rs7105934 (P=0.884). However, immunostaining intensities of cyclin
D1 in the cancer cells was inversely correlated with advanced tumor
stage (P=0.046; Spearman’s correlation coefficient, −0.211;
Table V).
 | Table V.Correlation between rs7105934
genotypes or AJCC stage and cyclin D1 expression in ccRCC
specimens. |
Table V.
Correlation between rs7105934
genotypes or AJCC stage and cyclin D1 expression in ccRCC
specimens.
| Cyclin D1
expression
| P-value |
rsa |
|---|
| − | + | ++/+++ |
|---|
| rs7105934 | | | | | |
| GG | 24 | 25 | 27 | 0.844 | 0.021 |
| GA | 5 | 3 | 5 | | |
| AA | 0 | 0 | 1 | | |
| GG | 24 | 25 | 27 | 0.884 | 0.016 |
| GA + AA | 5 | 3 | 6 | | |
| AJCC (2002)
stage | | | | | |
| I | 16 | 15 | 26 | 0.046 | −0.211 |
| II | 3 | 3 | 2 | | |
| III | 10 | 10 | 5 | | |
Correlation between cyclin D1 expression
in cancer cells and RCC prognosis
The association between cyclin D1 expression in
cancer cells and RCC prognosis was first evaluated using
Kaplan-Meier analysis. Moderate to high expression (++/+++) of
cyclin D1 in cancer cells predicted an improved postoperative
prognosis of RCC when compared with that of negative or weak
expression (−/+) (P=0.015; Kaplan-Meier analysis and the log-rank
test; Fig. 1B). Multivariate Cox
regression analysis indicated that moderate and high expression
(++/+++) of cyclin D1 in cancer cells independently predicted an
improved postoperative prognosis (HR, 0.13; 95% CI, 0.02–0.96;
P=0.045).
Discussion
In the present study, the correlation between four
SNPs identified in a previous GWAS study of a European population
and the development and prognosis of RCC in a Chinese population
was investigated. In the current study, the healthy controls and
RCC patients were matched by gender, but the healthy controls were
significantly older than the RCC patients. This age contrast may
have prevented misclassification bias, as RCC incidence increases
with age (2). The current study
identified that, of the four SNPs validated, only rs7105934
significantly correlated with the risk of RCC, particularly ccRCC,
in the Chinese population. An additional study investigating the
association between rs7579899, rs7105934 and rs4765623 and RCC risk
in an additional group of Chinese individuals has been reported
(25). Similar to the results of
the current study, the study identified that only rs7105934 of the
genotyped SNPs correlated with a reduced risk of RCC. Therefore,
genetic predisposition of RCC appears to differ between the two
ethnic groups and rs7105934 may confer genetic susceptibility to
RCC in a number of populations.
An important observation of the present study was
that the rs7105934 GA + AA genotype is an independent factor for
improved RCC prognosis, with an HR of 0.12 (95% CI, 0.02–0.93), in
contrast to advanced tumor stage. The genetic risk factor of RCC
development may also modulate RCC outcome. rs7105934 is situated at
a transcriptional enhancer ∼220-kb telomeric to the cyclin D1
encoding gene, CCND1 (20).
SNPs 5-kb centromeric to rs7105934 modulate the binding and
function of HIF-2α at the enhancer for CCND1. The minor
(RCC-protective) allele at 11q13.3 disrupts HIF binding, DNA
accessibility and interaction with the transcriptional apparatus at
the CCND1 enhancer, and alters the allelic balance of
CCND1 gene expression (21).
The rs7105934 GA genotype appears to be associated with lower
levels of CCND1 mRNA in adjacent renal tissue when compared
with that of the GG genotype (25).
Therefore, the correlation between the GA + AA genotype and an
improved prognosis of RCC may be caused by low expression of cyclin
D1 in tumors. Of note, the present study identified that moderate
to high expression of cyclin D1 is an independent predictor of an
improved postoperative prognosis in RCC. No significant differences
were identified in cyclin D1 protein expression between specimens
with the GG genotype and those with the GA + AA genotype in
adjacent renal tissue and in the tumor tissue. In addition, the
intensity of cyclin D1 immunostaining did not correlate with the
rs7105934 genotype, and these results were not consistent with the
hypothesized involvement of rs7105934 genotypes in cyclin D1
expression in RCCs. Thus, cyclin D1 and the rs7105934 SNP may both
be significant in the carcinogenesis and postoperative prognosis of
RCC, but function independently. Further studies are required to
validate and investigate the correlation between rs7105934
genotypes and the expression of cyclin D1 and other candidate
target proteins in RCCs.
In the present study, cyclin D1 expression in RCC
cells was confirmed to inversely correlate with advanced stages of
RCC and represent an independent predictor of improved prognosis of
RCC. The results of the current study are similar to those of
previous studies reporting that high cyclin D1 expression
significantly correlates with an improved prognosis, while low
expression of cyclin D1 correlates with a poor prognosis of ccRCC
(26–28). The expression of cyclin D1 is
markedly correlated with that of p27 in ccRCC; low p27 expression
independently predicts a poor prognosis of RCC, whilst low cyclin
D1 expression appears to shorten the survival rate of RCC patients
(29). These differences may
reflect variations in the cyclin D1-related cell biology of various
cancer types. Cyclin D1 is an oncoprotein that activates the
G1-S transition of the cell cycle and functions as a
downstream effector of β-catenin signaling. Downregulation of
β-catenin expression may contribute to the malignant character of
RCC and result in tumor progression (30). Cyclin D1 is upregulated in primary
ccRCCs, which may contribute to cell proliferation in primary RCCs,
and its importance may diminish at later stages of RCC progression
due to specific complex mechanisms, including
epithelial-mesenchymal transition.
Several limitations of the present study must be
noted: i) follow-up information was only available for 194 study
subjects and the majority of individuals without follow-up
information were from other provinces of China, which made regular
contact difficult; ii) patients were excluded in cases where
postoperative therapy was administered, including interferon-α and
interleukin-2 treatments, during the follow-up period; iii)
epidemiological studies of specific risk factors, including smoking
and alcohol consumption, were incomplete and thus not included in
the analysis, resulting in loss of data; and iv) SNPs were
validated from only one GWAS study (20) and novel RCC-related SNPs on 12p11.23
and 2p21 (23,24) require validation, as these SNPs are
not in LD with the four SNPs validated for the present study.
Overall, the present study showed that, of the four
RCC-related SNPs identified in a European population, only
rs7105934 correlated with RCC risk in a Chinese population. The GA
+ AA genotype was significantly correlated with reduced risk and an
improved postoperative prognosis of RCC. Therefore, rs7105934 may
confer genetic susceptibility to RCC in extended populations. No
significant differences were identified in the levels of cyclin D1
expression in adjacent renal tissue and RCC cells between patients
with the rs7105934 GG genotype and those with the GA + AA genotype.
Cyclin D1 expression in RCC cells was inversely correlated with
advanced stages of RCC. In addition, moderate to high expression of
cyclin D1 was an independent predictor of improved postoperative
prognosis of RCC. However, the results do not support the
hypothesis that rs7105934 genotypes are involved in the expression
of cyclin D1 in the kidneys. Cyclin D1 and the rs7105934 SNP may
represent significant factors in RCC, however, their effects appear
to be independent. Although validation of these observations is
required in independent populations, the current study highlights a
novel genetic marker that is capable of predicting the
postoperative prognosis of RCC.
Acknowledgements
The authors would like to thank
Guoping Wang, Liye Ma and Shuang Jiang for their assistance with
sample collection. The present study was supported by grants from
the National Natural Science Foundation of China (nos. 30873041,
81101928 and 81025015).
References
|
1.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Chow WH, Dong LM and Devesa SS:
Epidemiology and risk factors for kidney cancer. Nat Rev Urol.
7:245–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Southard EB, Roff A, Fortugno T, et al:
Lead, calcium uptake, and related genetic variants in association
with renal cell carcinoma risk in a cohort of male Finnish smokers.
Cancer Epidemiol Biomarkers Prev. 21:191–201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Wang G, Hou J, Ma L, et al: Risk factor
for clear cell renal cell carcinoma in Chinese population: a
case-control study. Cancer Epidemiol. 36:177–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Moore LE, Brennan P, Karami S, et al:
Glutathione S-transferase polymorphisms, cruciferous vegetable
intake and cancer risk in the Central and Eastern European Kidney
Cancer Study. Carcinogenesis. 28:1960–1964. 2007. View Article : Google Scholar
|
|
6.
|
Moore LE, Boffetta P, Karami S, et al:
Occupational trichloroethylene exposure and renal carcinoma risk:
evidence of genetic susceptibility by reductive metabolism gene
variants. Cancer Res. 70:6527–6536. 2010. View Article : Google Scholar
|
|
7.
|
Bruyère F, Hovens CM, Marson MN, et al:
VEGF polymorphisms are associated with an increasing risk of
developing renal cell carcinoma. J Urol. 184:1273–1278.
2010.PubMed/NCBI
|
|
8.
|
Hirata H, Hinoda Y, Nakajima K, et al: Wnt
antagonist gene polymorphisms and renal cancer. Cancer.
115:4488–4503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Andreotti G, Boffetta P, Rosenberg PS, et
al: Variants in blood pressure genes and the risk of renal cell
carcinoma. Carcinogenesis. 31:614–620. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Longuemaux S, Deloménie C, Gallou C, et
al: Candidate genetic modifiers of individual susceptibility to
renal cell carcinoma: a study of polymorphic human
xenobiotic-metabolizing enzymes. Cancer Res. 59:2903–2908.
1999.
|
|
11.
|
Aben KK, Luth TK, Janssen-Heijnen ML,
Mulders PF, Kiemeney LA and van Spronsen DJ: No improvement in
renal cell carcinoma survival: a population-based study in the
Netherlands. Eur J Cancer. 44:1701–1709. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Sun M, Shariat SF, Cheng C, et al:
Prognostic factors and predictive models in renal cell carcinoma: a
contemporary review. Eur Urol. 60:644–661. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lughezzani G, Karakiewicz PI, Bigot P, et
al: The prognostic value of erythrocyte polyamines in the
preoperative evaluation of patients with renal cell carcinoma. Eur
J Cancer. 46:1927–1935. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Doberstein K, Wieland A, Lee SB, et al:
L1-CAM expression in ccRCC correlates with shorter patients
survival times and confers chemoresistance in renal cell carcinoma
cells. Carcinogenesis. 32:262–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kojima T, Shimazui T, Horie R, et al:
FOXO1 and TCF7L2 genes involved in metastasis and poor prognosis in
clear cell renal cell carcinoma. Genes Chromosomes Cancer.
49:379–389. 2010.PubMed/NCBI
|
|
16.
|
Li X, Tan X, Yu Y, et al: D9S168
microsatellite alteration predicts a poor prognosis in patients
with clear cell renal cell carcinoma and correlates with the
down-regulation of protein tyrosine phosphatase receptor delta.
Cancer. 117:4201–4211. 2011. View Article : Google Scholar
|
|
17.
|
Kleinrath T, Gassner C, Lackner P,
Thurnher M and Ramoner R: Interleukin-4 promoter polymorphisms: a
genetic prognostic factor for survival in metastatic renal cell
carcinoma. J Clin Oncol. 25:845–851. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Hirata H, Hinoda Y, Kikuno N, et al: MDM2
SNP309 polymorphism as risk factor for susceptibility and poor
prognosis in renal cell carcinoma. Clin Cancer Res. 13:4123–4129.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Lin J, Horikawa Y, Tamboli P, Clague J,
Wood CG and Wu X: Genetic variations in microRNA-related genes are
associated with survival and recurrence in patients with renal cell
carcinoma. Carcinogenesis. 31:1805–1812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Purdue MP, Johansson M, Zelenika D, et al:
Genome-wide association study of renal cell carcinoma identifies
two susceptibility loci on 2p21 and 11q13.3. Nat Genet. 43:60–65.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Schödel J, Bardella C, Sciesielski LK, et
al: Common genetic variants at the 11q13.3 renal cancer
susceptibility locus influence binding of HIF to an enhancer of
cyclin D1 expression. Nat Genet. 44:420–425. 2012.PubMed/NCBI
|
|
22.
|
Chang W, Ma L, Lin L, et al:
Identification of novel hub genes associated with liver metastasis
of gastric cancer. Int J Cancer. 125:2844–2853. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Wu X, Scelo G, Purdue MP, et al: A
genome-wide association study identifies a novel susceptibility
locus for renal cell carcinoma on 12p11.23. Hum Mol Genet.
21:456–462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Han SS, Yeager M, Moore LE, et al: The
chromosome 2p21 region harbors a complex genetic architecture for
association with risk for renal cell carcinoma. Hum Mol Genet.
21:1190–1200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Cao Q, Qin C, Ju X, et al: Chromosome
11q13.3 variant modifies renal cell cancer risk in a Chinese
population. Mutagenesis. 27:345–350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Hedberg Y, Davoodi E, Roos G, Ljungberg B
and Landberg G: Cyclin-D1 expression in human renal-cell carcinoma.
Int J Cancer. 84:268–272. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Phuoc NB, Ehara H, Gotoh T, et al:
Immunohistochemical analysis with multiple antibodies in search of
prognostic markers for clear cell renal cell carcinoma. Urology.
69:843–848. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Allory Y, Matsuoka Y, Bazille C,
Christensen EI, Ronco P and Debiec H: The L1 cell adhesion molecule
is induced in renal cancer cells and correlates with metastasis in
clear cell carcinomas. Clin Cancer Res. 11:1190–1197.
2005.PubMed/NCBI
|
|
29.
|
Migita T, Oda Y, Naito S and Tsuneyoshi M:
Low expression of p27(Kip1) is associated with tumor size and poor
prognosis in patients with renal cell carcinoma. Cancer.
94:973–979. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Bilim V, Kawasaki T, Katagiri A, Wakatsuki
S, Takahashi K and Tomita Y: Altered expression of beta-catenin in
renal cell cancer and transitional cell cancer with the absence of
beta-catenin gene mutations. Clin Cancer Res. 6:460–466.
2000.PubMed/NCBI
|