Introduction
Atrial natriuretic peptide (ANP) signaling occurs
through NP receptor A (NPR-A) by increasing cyclic guanosine
3′,5′-monophosphate (cGMP) and activating cGMP-dependent protein
kinase (PKG). Activated PKG in turn up-regulates the expression of
genes encoding ion transporters and transcription factors, which
together affect cell growth, apoptosis, proliferation and
inflammation (1–3). NPR-A has been reported to be expressed
in lung, prostate and ovarian cancer. NPR-A expression and
signaling is important for tumor growth, and a NPR-A deficiency has
been shown to protect C57BL/6 mice from lung, skin and ovarian
cancers, suggesting that NPR-A is a new target for cancer therapy
(4). NPR-A has been demonstrated to
be expressed in pre-implantation embryos and embryonic stem (ES)
cells and has a novel role in the maintenance of self-renewal and
the pluripotency of ES cells (5).
Evidence has demonstrated the critical role of
plasma K+ channels in the regulation of tumor cell
proliferation (6,7). It has been shown that the delayed
rectifier potassium channel subunits, voltage-gated potassium
channed (Kv), Kv1.3, Kv1.5, Kv1.6, Kv2.1 and Kv2.2, are expressed
in human gastric cancer cells, and that the downregulation of this
expression significantly inhibits the proliferation of gastric
cancer (8). Gastric cancer is a
common malignant disease worldwide, with a high incidence and
mortality and a five-year relative post-treatment survival rate of
<25%. ANP has been reported to inhibit the proliferation of
various types of cancer (9). The
involvement of guanylyl cyclase (GC)-coupled natriuretic receptors
has been identified, with lower concentrations of ANP able to
stimulate proliferation in neural tumor cell lines with the
involvement of a GC receptor, while higher concentrations of ANP
exert a mitogen-activated protein kinase-dependent
antiproliferative action, which involves a non-GC receptor
(10). Based on clinical data, it
has been suggested that ANP is cardioprotective at a plasma
concentration of 10−9 M (11). Studies have demonstrated the
protective effects of exposure to 10−9 M ANP in
endothelial cells, neural cells and hepatocytes (12–14).
However, higher concentrations of ANP induce apoptosis in
endothelial cells and neonatal rat cardiac myocytes (15,16).
In cardiomyocytes, various effects of ANP have been shown, with the
prevention or induction of apoptosis at concentrations of
10−9 or 10−6 M, respectively. The mechanism
by which 10−6 M ANP promotes cardiomyocyte survival is
the cGMP-dependent nuclear accumulation of zyxin and Akt (11).
As NPR-A is a fairly new target for cancer therapy
(4), NPR-A expression in gastric
cancer has not been investigated. The present study investigated
the effects of the expression of NPR-A on the gastric cancer AGS
cell line, the effects of ANP on the proliferation of AGS and the
role of K+ channels in this ANP-affected
proliferation.
Materials and methods
Chemicals and antibodies
ANP, tetraethylammonium chloride (TEA) and 293B were
obtained from Sigma-Aldrich (St. Louis, MO, USA). The primary
antibody was a rabbit anti-NPR-A polyclonal antibody (1:300
dilution; Abcam, Cambridge, MA, USA). The secondary antibody used
was donkey anti-rabbit Alexa 488 (Molecular Probes, Eugene, OR,
USA). Nuclei were stained with Hoechst 33342.
Human gastric cancer AGS cell
culture
Human gastric adenocarcinoma AGS cells (ATCC,
Manassas, VA, USA) were grown in F-12k (ATCC) supplemented with 10%
fetal bovine serum and 1% penicillin-streptomycin. The cells were
cultured at 37°C with humidified 5% CO2, fed with fresh
medium every third day and split when subconfluent.
Immunofluorescence, RT-PCR and western
blotting
The cells were fixed by incubation for 30 min at
room temperature with 100 μl of freshly prepared 3–4%
paraformaldehyde in PBS (dissolved in boiling PBS and cooled to
room temperature). The cells were then rinsed twice with PBS for 5
min and permeabilized by incubation at room temperature for 30 min
with 3% Triton X-100 in PBS. Subsequently, the cells were rinsed
three times with PBS and incubated for 15 min at room temperature
with block medium (Cas-block). The cells were then incubated for 1
h at room temperature with the primary antibody in PBS containing
3% horse serum. Following incubation, the cells were rinsed three
times with PBS for 5 min and further incubated with a fluorescent
antibody (Anti-rabbit 488) diluted 1:500 in PBS containing 3% horse
serum. The cells were then rinsed three times with PBS and
incubated with 2 μg/ml Hoechst 33342 for 15 min at room
temperature. Next, the cells were rinsed three times in PBS and
mounted onto glass slides. Subsequent to coverslipping, the cells
were visualized at ×400 magnification using fluorescence
microscopy.
RNA was isolated from the human gastric cancer AGS
cell line according to the manufacturer’s instructions (RNeasy Plus
Micro kit, Qiagen, Hilden, Germany). The purity of the RNA was
estimated by the A260/A280 ratio (NanoDrop
1000 Spectrophotometer; Thermo Scientific, Waltham, MA, USA). Total
RNA (200 ng) was reverse transcribed using the oligo dT primer and
superscript III first strand synthesis system (Invitrogen,
Carlsbad, CA, USA). PCR was subsequently performed using human
potassium voltage-gated channel, KQT-like subfamily, member 1
(KCNQ1) primer pairs and platinum Taq DNA polymerase (Invitrogen).
The primers were designed using Primer-Blast provided by the NCBI.
The size of the PCR products was determined by comparison to a 100
bp DNA ladder (Invitrogen) under UV illumination following 1–2%
agarose gel electrophoresis.
Total protein samples from the cell lysates prepared
from the AGS cells were used to assess the expression of NPR-A and
KCNQ1 by western blotting. In brief, 5 μg of protein was
fractionated by SDS-PAGE (4–20% gradient gel; Bio-Rad, Hercules,
CA, USA) and transferred onto a PVDF membrane. The membrane was
probed with a primary antibody against human NPR-A (Abcam,
Cambridge, MA, USA), KCNQ1 HERG, kv2.1, Kv4.1 and Kv1.5 (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) followed by an
HRP-conjugated anti-rabbit secondary antibody (Sigma-Aldrich).
Protein signals were detected using the enhanced chemiluminescence
(ECL) system (Thermo Scientific).
BrdU cell proliferation assay
Cell proliferation was measured using
5-Bromo-2′-deoxy-uridine Labeling and Dectection kit III (Roche
Applied Science, Mannheim, Germany) in accordance with the
manufacturer’s instructions. Briefly, 1×104 cells were
plated into a 96-well plate and grown to confluence. Prior to
incubation with ANP, the cells were serum free for 24 h.
Subsequently, the cells were incubated with various concentrations
of ANP for 24 h. The medium was switched to culture medium
containing 10 μM BrdU and the cells were incubated for an
additional 2 h. BrdU incorporation into cellular DNA was measured
using a microplate reader (Safire II; Tecan, Männedorf,
Switzerland). Three independent experiments were performed and each
assay was performed in triplicate.
cGMP assay
cGMP levels were measured as previously described
(17). Briefly, 1×105
cells were plated in each 35-mm dish and grown to confluence. The
cells were washed with 1 ml medium, then incubated for an
additional 15 min at 37°C with or without ANP at concentrations of
10−10, 10−9, 10−8, 10−7
or 10−6 M. The medium was then replaced by 0.3 ml 0.45%
NP-40 (Sigma-Aldrich). Subsequent to a 5-min incubation on ice, the
lysate was removed from the plates and centrifuged for 2 min at 4°C
(12,000 × g). The supernatant was collected and assayed for cGMP
levels using a cGMP ELISA kit (Cell Biolabs, San Diego, CA, USA)
according to the manufacturer’s instructions.
Patch clamp recordings
The voltage clamp technique was performed using
whole-cell configuration at room temperature (22–25°C). The
Tyrode’s solution used in the experiments contained the following:
140 mM NaCl, 5.4 mM KCl, 1.2 mM MgCl2, 5 mM HEPES, 1.8
mMx CaCl2 and 10 mM glucose, which was titrated to pH
7.4 with NaOH. The pipette solution used in the experiments
contained the following: 120 mM K-Asparatate, 10 mM
Na2ATP/2H2O, 2 mM MgCl2, 10 mM
EGTA and 10 mM HEPES, which was titrated to pH 7.4 with KOH. The
glass pipette electrodes were made from Corning 7056 glass
capillaries (Warner Instruments, Hammed, CT, USA) with a pipette
resistance of 2–3 MΩ in the bath solution. All recordings were
initiated at least 10 min after the rupture of the membrane.
Signals were measured with an Axopatch 700A amplifier using pCLAMP
9 software (Molecular Devices, Sunnyvale, CA, USA), with a Bessel
low-pass filter (cut-off frequency, 10 kHz) and a sampling
frequency of 10 kHz.
Statistical analysis
All data were analyzed using Clampfit (Axon
Instruments, Sunnyvale, CA, USA) and Igor software (WaveMetrics,
Lake Oswego, OR, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
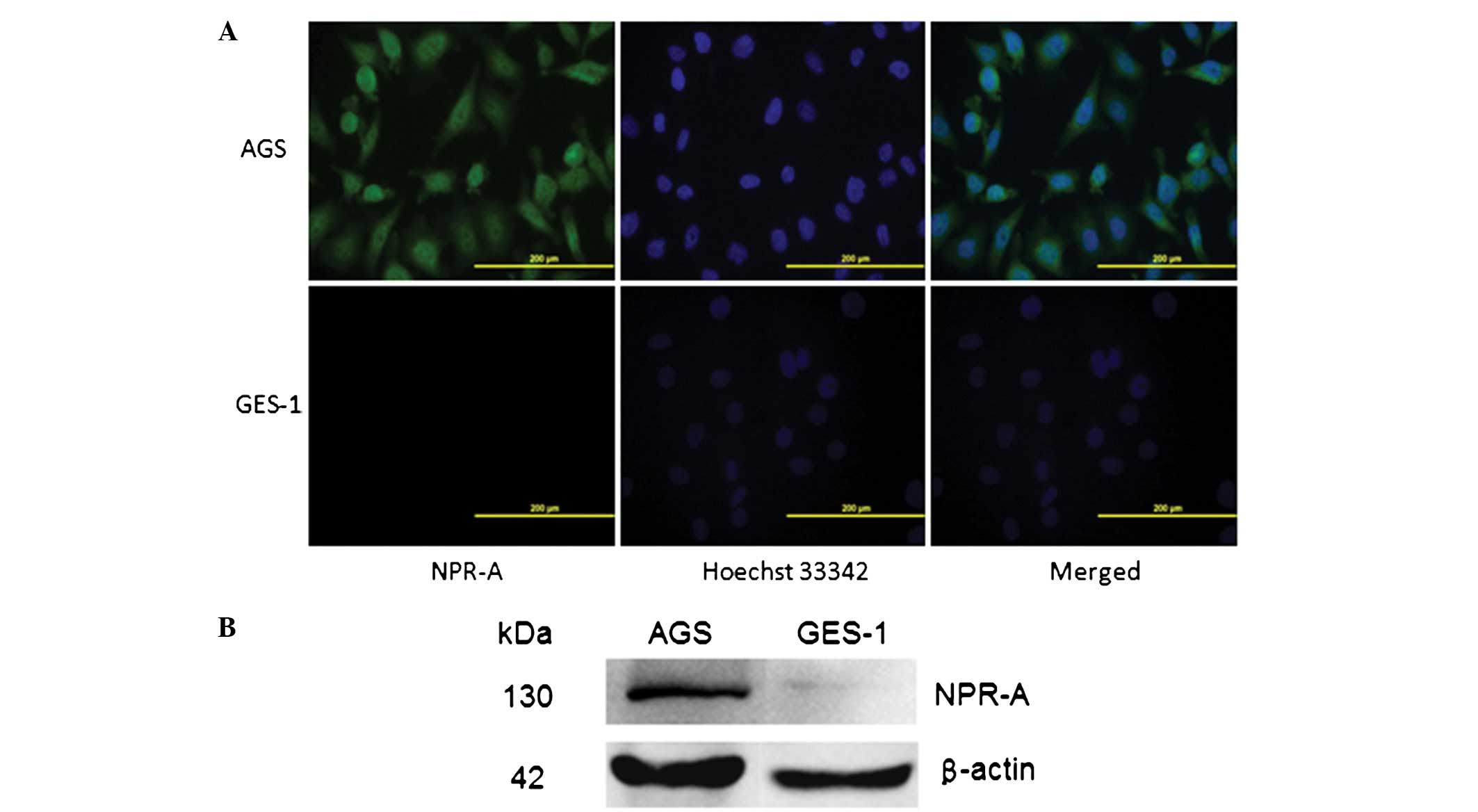
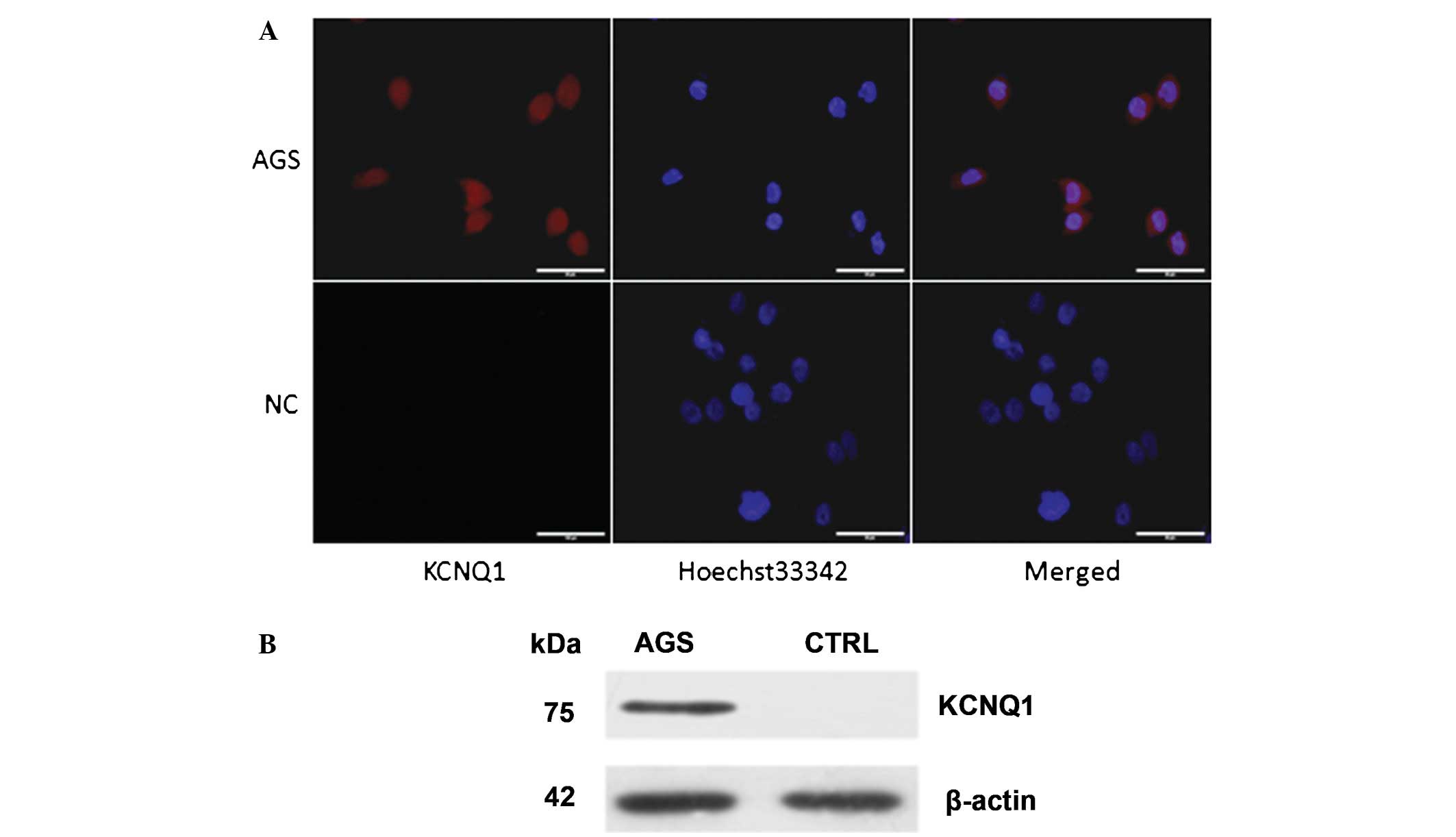
NPR-A is expressed in the human gastric
cancer AGS cell line
NPR-A expression was evaluated by western blotting
and immunofluorescence in the human gastric cancer AGS cells and
was compared with human gastric epithelial immortalized GES-1
cells. The results of the western blot analysis and
immunofluorescence showed that NPR-A was expressed abundantly in
the human gastric cancer AGS cells, but not in the human gastric
epithelial immortalized GES-1 cells (Fig. 1).
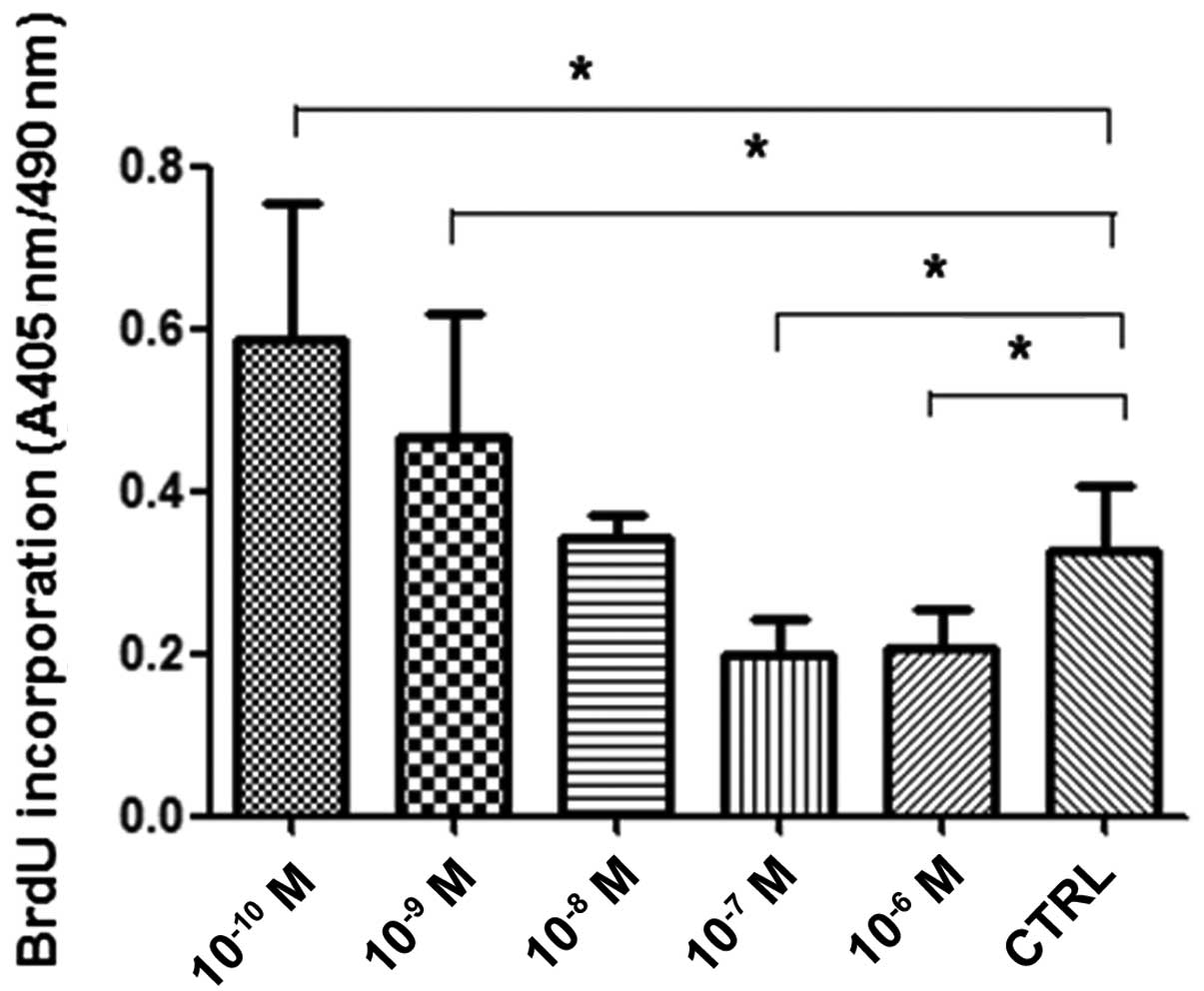
Effect of ANP on the proliferation of the
AGS cells
The effect of ANP on AGS cell proliferation was
evaluated by comparing the BrdU incorporation into replicating DNA
in the human gastric cancer AGS cells. The results showed that
10−10 and 10−9 M ANP significantly promoted
the proliferation of the AGS cells (Fig. 2, P<0.05, n=3), while
10−7 and 10−6 M ANP significantly inhibited
the proliferation of the AGS cells (Fig. 2; P<0.05; n=3). No significant
differences were detected between the 10−8 M ANP and
control groups (Fig. 2; P>0.05;
n=3). The results in the AGS cells of the present study were
similar to those Kato et al observed in cardiomyocytes
(11). Lower concentrations of ANP
promote the proliferation of AGS cells, while higher concentrations
decrease the proliferation of AGS cells.
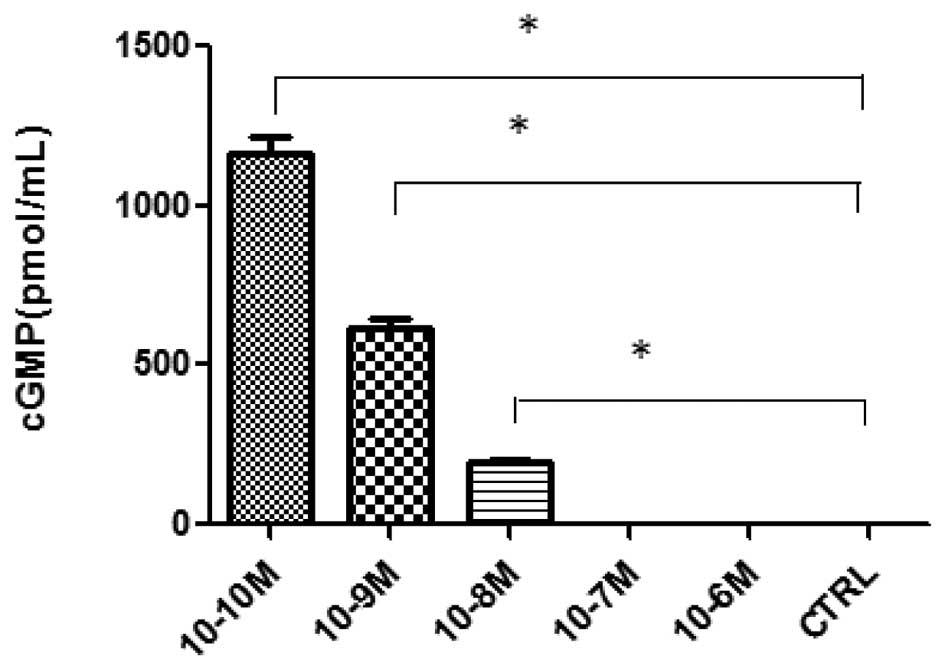
cGMP assay
A cGMP ELISA kit assay was used to investigate
ANP-induced changes in the cGMP levels. Fig. 3 shows that the incubation of the AGS
cells with 10−10, 10−9 and 10−8 M
ANP significantly increased the level of cGMP activity compared
with the control (P<0.05; n=3), while no significant differences
were observed in the 10−7 and 10−6 M ANP
groups (P>0.05; n=3).
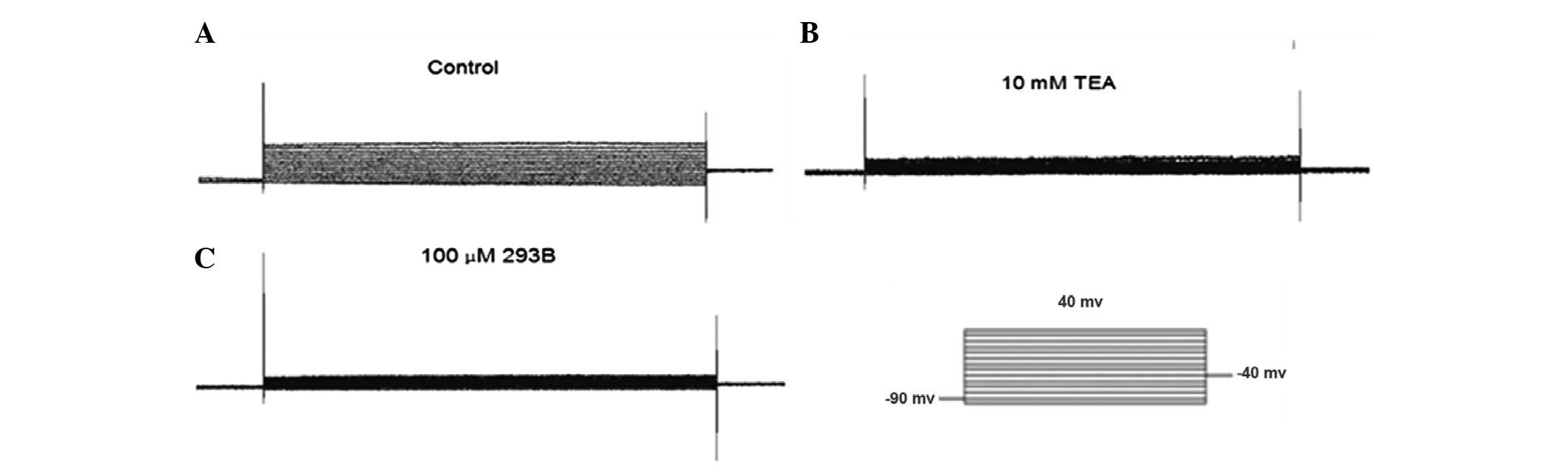
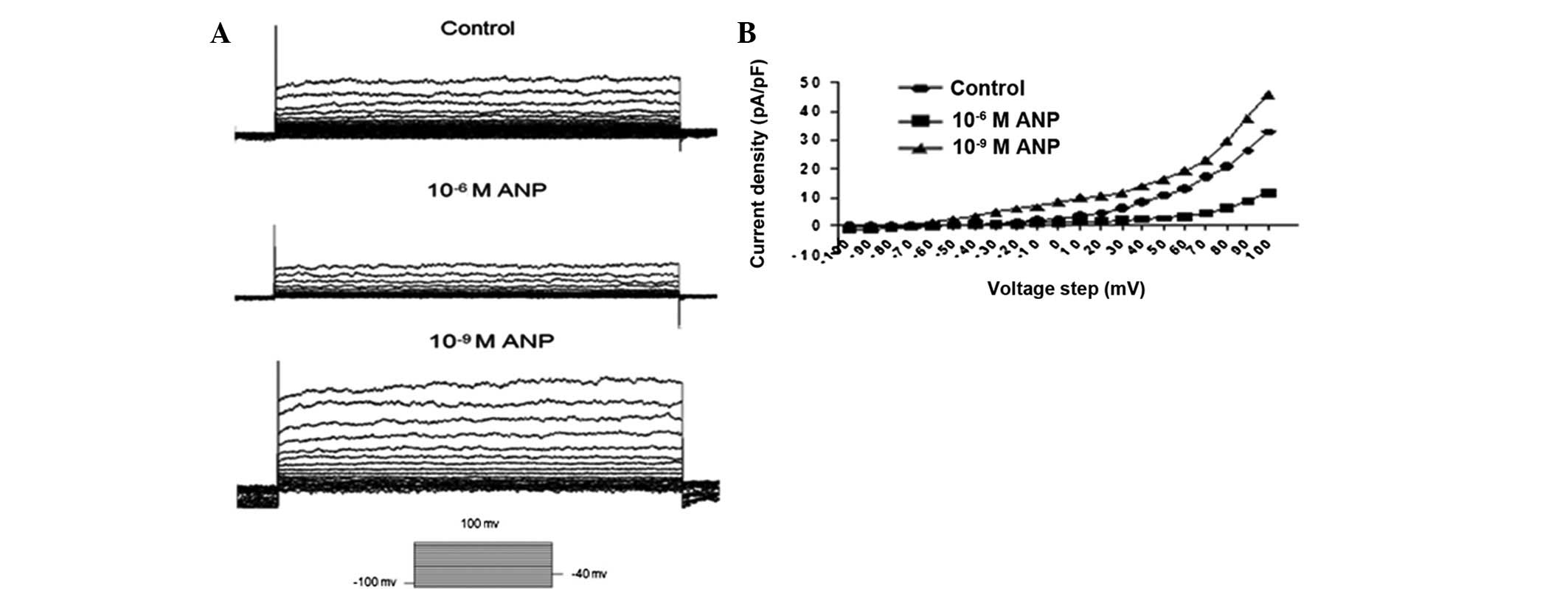
Kv in AGS cells
In agreement with previous studies (8), the AGS cells exhibited a prominent
voltage-gated outward K+ current, while the membrane
potential was depolarized from a holding potential of −90 to 40 mV
(Fig. 4). This current was blocked
completely by 10 mM TEA and 100 μM 293B (Fig. 4). These results suggest that the
K+ current (IK) in the AGS cells was via a
TEA- and 293B-sensitive IK.
IK channels of AGS cells
revealed by immunofluorescence and western blot analysis
It has been reported that KCNQ1 (18), HERG (19–22),
Kv1.3 (8), Kv1.5 (8,23),
Kv1.6 (8), Kv2.1 (8), Kv2.2 (8), KCNE2 (24,25),
Eag1 (26) and KATP (27) are the main IK channels of
AGS cells. The patch clamp results showed that the K+
current in the AGS cells was a 293B-sensitive IK. 293B
is the inhibitor of the KCNQ1 channel (28). The expression of KCNQ1 in the AGS
cells was investigated by immunofluorescence and western blotting.
The results of the western blot analysis and immunofluorescence
showed that KCNQ1 is expressed abundantly in human gastric cancer
AGS cells (Fig. 5).
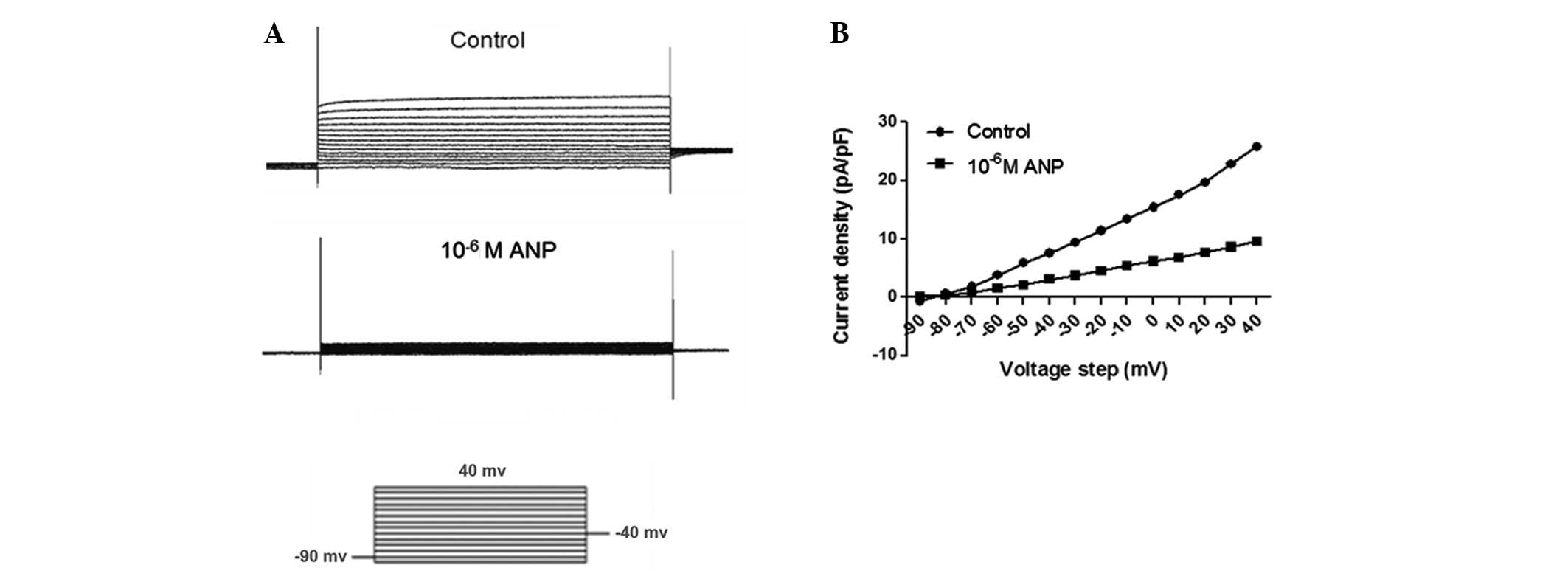
ANP modulates the voltage-gated outward
K+ current in AGS cells
Using the BrdU cell proliferation assay, lower
concentrations of ANP (10−10 and 10−9 M) were
observed to promote the proliferation of the AGS cells, while
higher concentrations of ANP (10−7 and 10−6
M) decreased proliferation. According to the present data and
previous studies, 10−9 M ANP was selected as the lower
concentration, while 10−6 M ANP was used as the higher
concentration for the patch clamp study. The effect of ANP on
voltage-dependent steady-state activation and inactivation was then
studied at concentrations of 10−9 and 10−6 M
ANP. The steady-state activation of IK was elicited
using the appropriate voltage protocols as follows: IK
was evoked by a 500 msec depolarizing pulse from a first pulse
potential of −90 to 40 mV, in 10 mV steps at 10 sec intervals
(Figs. 6A and 7A). The TEA- and 293B-sensitive
K+ current was significantly increased by
10−9 M ANP (n=12, P<0.05), while 10−6 M
ANP significantly decreased the TEA- and 293B-sensitive
K+ current (n=12, P<0.05). By plotting normalized
conductance as a function of the command potential, the
IK activation curve was obtained. As shown in Figs. 6B and 7B, the activation curve was significantly
shifted towards the left by the application of 10−9 M
ANP. The activation curve was significantly shifted towards the
right by the application of 10−6 M ANP (Fig. 6B).
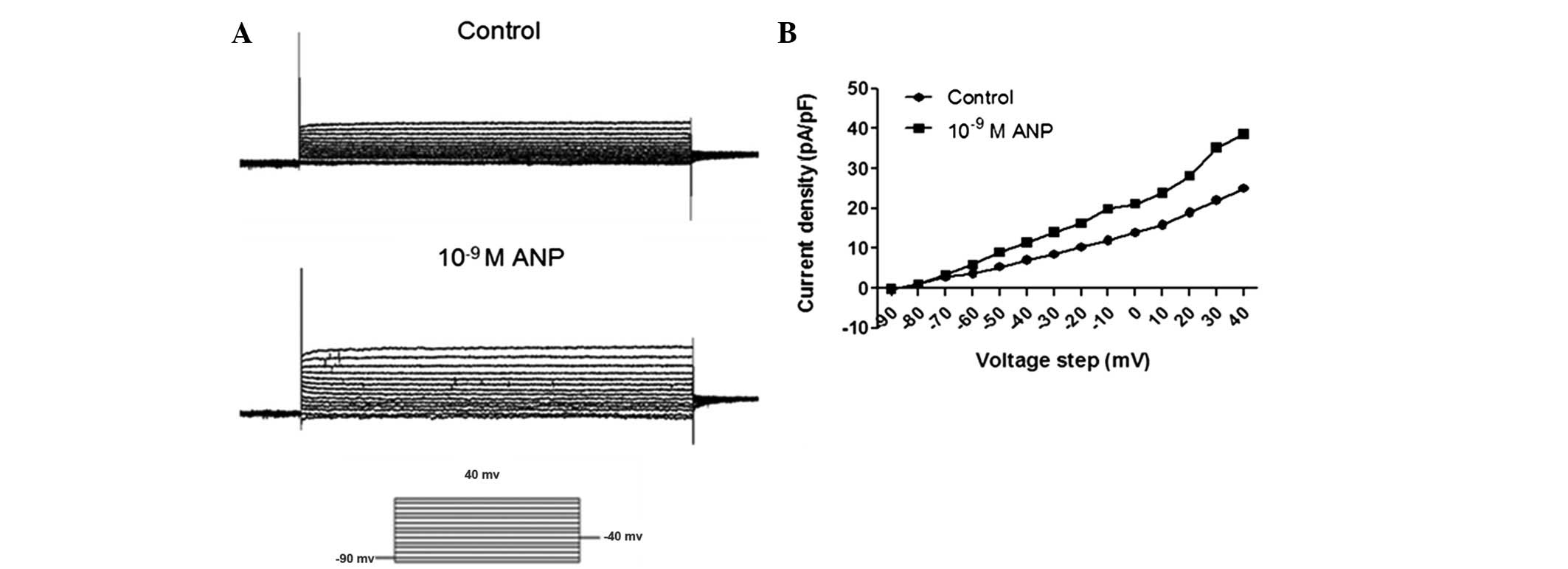
In the BrdU cell proliferation assay, the AGS cells
were incubated at various concentrations of ANP for 24 h. In the
patch clamp study, the AGS cells were only treated for 3–5 min to
investigate the effects of various concentrations of ANP on the
voltage-gated outward K+ current. Next the AGS cells
were incubated with various concentrations of ANP for 24 h.
Subsequent to this, the AGS cells were used for a patch clamp
study. IK was evoked by a 500 msec depolarizing pulse
from a first pulse potential of −90 to 40 mV, in 10 mV steps at 10
sec intervals (Fig. 8A). The
results were similar to when the AGS cells were treated for 3–5 min
to investigate the effects of various concentrations of ANP on the
voltage-gated outward K+ current. The TEA- and
293B-sensitive K+ current was significantly increased by
10−9 M ANP (n=12, P<0.05), while 10−6 M
ANP significantly decreased the TEA- and 293B-sensitive
K+ current (n=12, P<0.05). By plotting the normalized
conductance as a function of the command potential, the
IK activation curve was obtained. As shown in Figs. 7B and 8B, the activation curve was significantly
shifted towards the left by the application of 10−9 M
ANP, while the activation curve was significantly shifted towards
the right by the application of 10−6 M ANP.
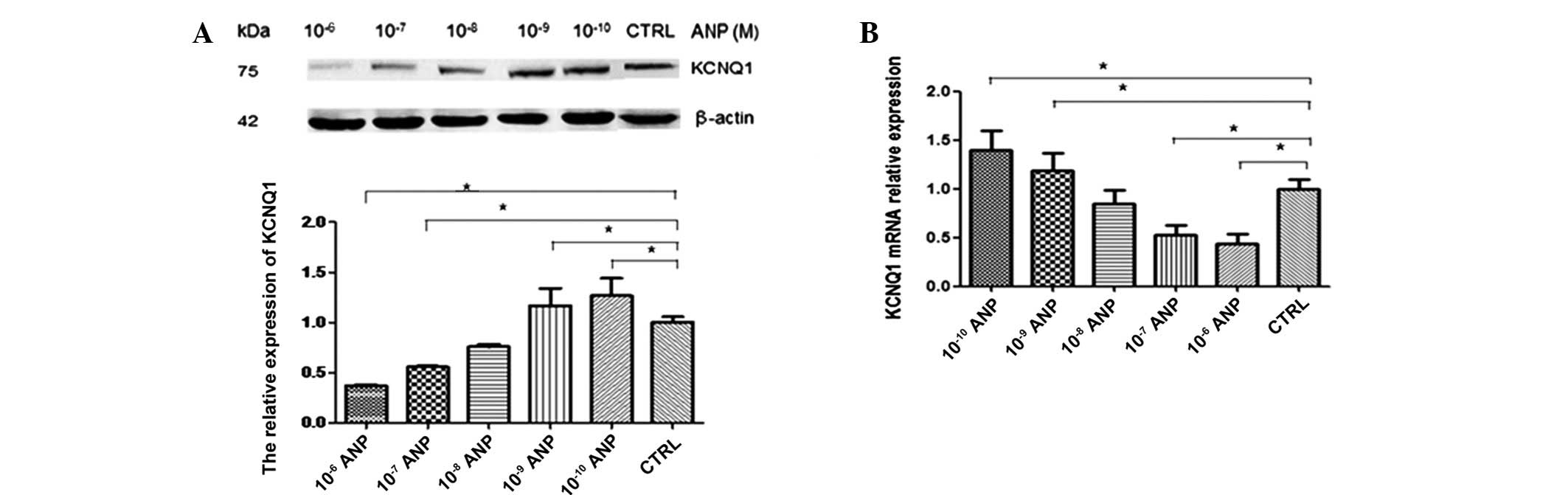
Effect of ANP on the expression of
KCNQ1
The AGS cells were treated with various
concentrations of ANP for 24 h. KCNQ1 protein expression levels
were detected by western blotting. Data are expressed as the mean ±
SEM of each group of cells from three separate experiments. As
shown in Fig. 9A, 10−10
and 10−9 M ANP significantly upregulated the expression
of KCNQ1 at the protein level (n=3; P<0.05), while
10−7 and 10−6 M ANP significantly
downregulated expression (n=3; P<0.05). KCNQ1 mRNA expression
levels were detected by qPCR. The qPCR results were similar to
those obtained by western blotting. As shown in Fig. 9B, 10−10 and
10−9 M ANP significantly upregulated the expression of
KCNQ1 at the mRNA level (n=3; P<0.05), while 10−7 and
10−6 M ANP significantly downregulated expression (n=3;
P<0.05).
Discussion
Using immunofluorescence, BrdU assays and whole-cell
patch clamp recording, it was revealed that NPR-A is expressed in
the human gastric cancer AGS cell line and that lower and higher
concentrations of ANP have opposing effects on the proliferation of
AGS cells. The voltage-gated outward K+ current was
demonstrated to be involved in the anti-proliferative effect of
higher concentrations of ANP and the pro-proliferative effect of
lower concentrations of ANP.
NPR-A is the receptor for ANP and brain NP (BNP).
ANP and BNP belong to the NP family, which regulates mammalian
blood volume and blood pressure. ANP signaling through
NPR-A/cGMP/PKG activates various downstream effectors involved in
cell growth, apoptosis, proliferation and inflammation (2). NPR-A has been reported to be expressed
in lung, prostate and ovarian cancer. NPR-A expression and
signaling is important for tumor growth and its deficiency has be
shown to protect C57BL/6 mice from lung, skin and ovarian cancers,
suggesting that NPR-A is a new target for cancer therapy (4,29). In
the present study, using the immunofluorescence method, it was
demonstrated that NPR-A is expressed in AGS cells.
In neural tumor cell lines, the involvement of
guanylyl cyclase (GC)-coupled natriuretic receptors has been
identified, with lower concentrations of ANP able to stimulate
proliferation with the involvement of a GC receptor, while higher
concentrations of ANP exert a mitogen-activated protein
kinase-dependent antiproliferative action, which involves a non-GC
receptor (10). Another study
demonstrated similar results in cardiomyocytes (11). In the present study, as NPR-A is
expressed in AGS cells, the effect of ANP was investigated on the
proliferation of the AGS cells. The results obtained were similar
to those from the neural tumor cell lines and cardiomyocyte studies
(10,11).
Since plasma K+ channels are critical in
the regulation of tumor cell proliferation, typical high
(10−6 M) and low (10−9 M) concentrations of
ANP were used to investigate its effect on the K+
channels of AGS cells. The results showed that 10−6 M
ANP significantly decreased the TEA- and 293B-sensitive K+ current,
while 10−9 M ANP significantly increased the TEA- and
293B-sensitive K+ current.
According to a review of the literature and the
present study results, this K+ current is
293B-sensitive. 293B is the inhibitor of the KCNQ1 channel.
Consequently, the decision was made to focus on KCNQ1. The
expression of KCNQ1 was investigated in the AGS cells by
immunofluorescence and western blotting. The results of the western
blot analysis and immunofluorescence showed that KCNQ1 is expressed
abundantly in human gastric cancer AGS cells. According to the
present data and results from previous studies, 10−9 M
ANP was selected as the lower concentration, while 10−6
M ANP was selected as the higher concentration for the patch clamp
study. The patch clamp results showed that 10−9 M ANP
significantly increased the TEA- and 293B-sensitive K+ current,
while 10−6 M ANP significantly decreased the TEA- and
293B-sensitive K+ current. To investigate the role of
KCNQ1 in the effects of various concentrations of ANP on the
proliferation of AGS cells, the AGS cells were treated with various
concentrations of ANP for 24 h. KCNQ1 protein and mRNA expression
levels were detected by western blotting and qPCR, respectively.
The results showed that, at the protein and mRNA level,
10−10 and 10−9 M ANP significantly
upregulated the expression of KCNQ1, while 10−7 and
10−6 M ANP significantly downregulated expression.
The present data indicated that lower and higher
concentrations of ANP have opposing effects on the proliferation of
AGS cells through cGMP-dependent or -independent pathways. KCNQ1
upregulation and downregulation by lower and higher concentrations
of ANP, respectively, have separate effects on the promotion and
inhibition of proliferation.
Acknowledgements
The present study was supported by the
Chinese National Natural Science Foundation Projects (NSFC,
81072053).
References
|
1.
|
Pedram A, Razandi M, Kehrl J and Levin ER:
Natriuretic peptides inhibit G protein activation. Mediation
through cross-talk between cyclic GMP-dependent protein kinase and
regulators of G protein-signaling proteins J Biol Chem.
275:7365–7372. 2000.PubMed/NCBI
|
|
2.
|
Silberbach M and Roberts CT Jr:
Natriuretic peptide signalling: molecular and cellular pathways to
growth regulation. Cell Signal. 13:221–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Fiscus RR: Involvement of cyclic GMP and
protein kinase G in the regulation of apoptosis and survival in
neural cells. Neurosignals. 11:175–190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kong X, Wang X, Xu W, et al: Natriuretic
peptide receptor a as a novel anticancer target. Cancer Res.
68:249–256. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Abdelalim EM and Tooyama I: NPR-A
regulates self-renewal and pluripotency of embryonic stem cells.
Cell Death Dis. 2:e1272011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Zhanping W, Xiaoyu P, Na C, Shenglan W and
Bo W: Voltage-gated K+ channels are associated with cell
proliferation and cell cycle of ovarian cancer cell. Gynecol Oncol.
104:455–460. 2007.
|
|
7.
|
Spitzner M, Ousingsawat J, Scheidt K,
Kunzelmann K and Schreiber R: Voltage-gated K+ channels support
proliferation of colonic carcinoma cells. FASEB J. 21:35–44.
2007.
|
|
8.
|
Lan M, Shi Y, Han Z, et al: Expression of
delayed rectifier potassium channels and their possible roles in
proliferation of human gastric cancer cells. Cancer Biol Ther.
4:1342–1347. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Vesely DL: Atrial natriuretic peptides:
anticancer agents. J Investig Med. 53:360–365. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Lelièvre V, Pineau N, Hu Z, et al:
Proliferative actions of natriuretic peptides on neuroblastoma
cells. Involvement of guanylyl cyclase and non-guanylyl cyclase
pathways. J Biol Chem. 276:43668–43676. 2001.PubMed/NCBI
|
|
11.
|
Kato T, Muraski J, Chen Y, et al: Atrial
natriuretic peptide promotes cardiomyocyte survival by
cGMP-dependent nuclear accumulation of zyxin and Akt. J Clin
Invest. 115:2716–2730. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Cottart CH, Nivet-Antoine V, Do L, et al:
Hepatic cytoprotection by nitric oxide and the cGMP pathway after
ischaemia-reperfusion in the rat. Nitric Oxide. 9:57–63. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Fiscus RR, Tu AW and Chew SB: Natriuretic
peptides inhibit apoptosis and prolong the survival of
serum-deprived PC12 cells. Neuroreport. 12:185–189. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Matsumura T, Kugiyama K, Sugiyama S, et
al: Neutral endopeptidase 24.11 in neutrophils modulates protective
effects of natriuretic peptides against neutrophils-induced
endothelial cytotoxity. J Clin Invest. 97:2192–2203. 1996.
View Article : Google Scholar
|
|
15.
|
Suenobu N, Shichiri M, Iwashina M, Marumo
F and Hirata Y: Natriuretic peptides and nitric oxide induce
endothelial apoptosis via a cGMP-dependent mechanism. Arterioscler
Thromb Vasc Biol. 19:140–146. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Han B, Fixler R, Beeri R, Wang Y, Bachrach
U and Hasin Y: The opposing effects of endothelin-1 and C-type
natriuretic peptide on apoptosis of neonatal rat cardiac myocytes.
Eur J Pharmacol. 474:15–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Bader B, Butt E, Palmetshofer A, et al: A
cGMP-dependent protein kinase assay for high throughput screening
based on time-resolved fluorescence resonance energy transfer. J
Biomol Screen. 6:255–264. 2001. View Article : Google Scholar
|
|
18.
|
Elso CM, Lu X, Culiat CT, et al:
Heightened susceptibility to chronic gastritis, hyperplasia and
metaplasia in Kcnq1 mutant mice. Hum Mol Genet. 13:2813–2821. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Shao XD, Wu KC, Hao ZM, Hong L, Zhang J
and Fan DM: The potent inhibitory effects of cisapride, a specific
blocker for human ether-a-go-go-related gene (HERG) channel, on
gastric cancer cells. Cancer Biol Ther. 4:295–301. 2005. View Article : Google Scholar
|
|
20.
|
Shao XD, Wu KC, Guo XZ, Xie MJ, Zhang J
and Fan DM: Expression and significance of HERG protein in gastric
cancer. Cancer Biol Ther. 7:45–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ding XW, Yang WB, Gao S, et al: Prognostic
significance of hERG1 expression in gastric cancer. Dig Dis Sci.
55:1004–1010. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Zhang R, Tian P, Chi Q, et al: Human
ether-à-go-go-related gene expression is essential for cisplatin to
induce apoptosis in human gastric cancer. Oncol Rep. 27:433–440.
2012.
|
|
23.
|
Han Y, Shi Y, Han Z, Sun L and Fan D:
Detection of potassium currents and regulation of multidrug
resistance by potassium channels in human gastric cancer cells.
Cell Biol Int. 31:741–747. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Yanglin P, Lina Z, Zhiguo L, et al: KCNE2,
a down-regulated gene identified by in silico analysis, suppressed
proliferation of gastric cancer cells. Cancer Lett. 246:129–138.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Roepke TK, Purtell K, King EC, La Perle
KM, Lerner DJ and Abbott GW: Targeted deletion of Kcne2 causes
gastritis cystica profunda and gastric neoplasia. PLoS One.
5:e114512010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Ding XW, Luo HS, Jin X, Yan JJ and Ai YW:
Aberrant expression of Eag1 potassium channels in gastric cancer
patients and cell lines. Med Oncol. 24:345–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Qian X, Li J, Ding J, Wang Z, Duan L and
Hu G: Glibenclamide exerts an antitumor activity through reactive
oxygen species-c-jun NH2-terminal kinase pathway in human gastric
cancer cell line MGC-803. Biochem Pharmacol. 76:1705–1715. 2008.
View Article : Google Scholar
|
|
28.
|
Lerche C, Bruhova I, Lerche H, et al:
Chromanol 293B binding in KCNQ1 (Kv7.1) channels involves
electrostatic interactions with a potassium ion in the selectivity
filter. Mol Pharmacol. 71:1503–1511. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Wang X, Raulji P, Mohapatra SS, et al:
Natriuretic peptide receptor a as a novel target for prostate
cancer. Mol Cancer. 10:562011. View Article : Google Scholar : PubMed/NCBI
|