Introduction
Colorectal cancer (CRC) is the leading cause of
cancer-related morbidity and mortality in Taiwan, with ~10,000 new
cases and 4,200 mortalities reported each year. Colon cancer
progresses via a multistep process known as the adenoma to
carcinoma sequence, which has histological and molecular
consequences (1). Over 140 years
ago, the German pathologist, Rudolf Virchow hypothesized that
chronic colonic inflammation was a risk factor predisposing
individuals to colon carcinogenesis (2,3).
During chronic inflammation, constitutive cellular activation and
release of proinflammatory factors damages otherwise healthy
neighboring epithelial cells, promoting carcinogenesis by damaging
targets and pathways crucial for normal tissue homeostasis
(4).
Marked cyclooxygenase-2 (COX-2) expression is
detected in cancer and inflammatory cells, the vascular endothelium
and fibroblasts of the cancer lesions. COX enzymes produce a number
of substances, including prostaglandins (bioactive lipid
molecules), that function as major effectors of cancer initiation
and progression (5–7). It is widely accepted that the
deregulation of the COX-2 signaling pathway affects colorectal
tumorigenesis. COX-2 is commonly overexpressed in early neoplastic
lesions in the colon and rectum and its expression has been shown
to correlate with cell proliferation, differentiation,
tumorigenesis and the inhibition of the mitochondrial apoptotic
pathway (8). The mechanism of COX-2
induction in these tumors is not fully understood, however, COX-2
expression may be stimulated by proinflammatory cytokines, growth
factors, tumor promoters or mutagenic substances under inflammatory
and tumor growth conditions (9,10).
A number of previous studies have identified that
COX-2 protein expression is higher in normal colonic mucosa than in
tumor tissue (6,11). However, by contrast, other studies
have demonstrated that COX-2 expression is absent in normal colonic
mucosa but high in tumor tissue, and that the long-term use of
non-steroidal anti-inflammatory drugs lowers the risk of developing
CRC by 40–50% (12). The mechanism
underlying the effect of COX-2 on tumor growth has not been
determined, but it is hypothesized that stromal and tumor-derived
COX-2 affect tumor angiogenesis and/or immune function (13). In the current study, COX-2
expression in tumor tissue and the adjacent normal mucosa were
compared to define the extent of COX-2 expression in the tumor
microenvironment.
Peroxisome proliferator activated receptor γ
(PPAR-γ) functions as a nuclear receptor with antitumor and
anti-inflammatory effects. It has been hypothesized that the
majority of PPAR-γ is restricted to adipose tissue and that its
activation inhibits the nuclear translocation of nuclear factor
(NF)-κB (14). Numerous studies
have shown that the PPAR-γ ligand has a therapeutic effect on
colitis and an antineoplastic effect on CRC (15–18).
PPAR-γ is highly expressed in normal colonic mucosa, colon cancer
cell lines and tumors (19).
In the present study, we hypothesized that the
expression of COX-2 in the normal mucosa affects the expression of
the COX-2 gene in the adjacent tumor tissue. A total of 49 pairs of
CRC tissues and adjacent normal mucosa specimens were investigated
for COX-2 and PPAR-γ expression and the correlation between COX-2
and PPAR-γ expression and survival rate was evaluated. In addition,
nine colon cancer cell lines were investigated.
Materials and methods
Patients
To determine the levels of COX-2 and PPAR-γ
expression in human CRC tissue and adjacent normal tissue (5 cm
from the tumor margin), 49 specimen pairs (98 specimens) were
evaluated by immunohistochemistry (IHC) and western blot analysis.
The samples were obtained from patients who had received curative
surgery for early-stage, primary CRC at the National Cheng Kung
University Hospital (Tainan, Taiwan) between January 2000 and
December 2001. Patient characteristics are shown in Table I. This study was approved by the
Institutional Review Board of The National Cheng Kung University
Hospital (Tainan, Taiwan).
 | Table ICharacteristics of 49 CRC
patients. |
Table I
Characteristics of 49 CRC
patients.
| Characteristics | Value |
|---|
| Age, years |
| Median | 61 |
| Range | 34–75 |
| Performance status,
n |
| 0–1 | 47 |
| 2 | 2 |
| Gender, n |
| Male | 27 |
| Female | 22 |
| Histological
differentiation, n |
| Well | 10 |
| Moderate | 33 |
| Poor | 6 |
| Primary tumor origin,
n |
| Colon-Sigmoid | 34 |
| Rectum | 15 |
| Tumor statusa, n |
| T1-T2 | 6 |
| T3-T4 | 43 |
| Nodal statusa, n |
| 0 | 30 |
| 1 | 15 |
| 2 | 4 |
| Stagea, n |
| II | 30 |
| III | 16 |
| IV | 3 |
Cell lines
Cell lines derived from human colon carcinomas at
various stages were purchased from American Type Culture Collection
(ATCC; Manassas, VA, USA). HT29 cells (grade I colorectal
adenocarcinoma), HT116 cells (colorectal carcinoma) and Daudi cells
(B lymphoblasts) were maintained in DMEM with 10% fetal bovine
serum (FBS). Caco2 (colorectal adenocarcinoma) and T84 (metastatic
carcinoma) cells were maintained in DMEM with 20 and 5% FBS,
respectively. SW116 (Dukes A), SW480 (Dukes B) and SW620 (Dukes C)
cells (all from colorectal adenocarcinomas) were maintained in L-15
medium with 10% FBS. C205 (Dukes D) cells (colorectal
adenocarcinoma and ascites metastasis) were maintained in RPMI-1640
medium with 10% FBS.
IHC
IHC was performed as described previously (20). Tissue sections were incubated at
room temperature (RT) for 2 h with monoclonal antibodies against
COX-2 and PPAR-γ (Thermo Fisher Scientific, Cheshire, UK). The
optimal dilution (1:100–1:200) was determined using human kidney
tissue as a positive control. The StrAviGen Super Sensitive
MultiLink kit (BioGenex Laboratories, Inc., San Ramon, CA, USA) was
used to detect the resulting immune complex. Peroxidase activity
was visualized using an aminoethyl carbazole substrate kit (Zymed
Laboratories, Inc., San Francisco, CA, USA). Sections were
counterstained with hematoxylin and non-immune mouse immunoglobulin
was used in place of the primary antibody to serve as a control.
Since no significant differences in staining intensity were
identified, only the proportion of tumor cells that were stained
was evaluated. The staining of COX-2 and PPAR-γ was scored as
negative if <10% of the tumor cells showed membranous
immunoreactivity (21).
Western blot analysis
The cells were lysed with WCE buffer containing 20
mM 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (pH
7.9), 5% octylphenoxypolyethoxyethanol CA-630, 7.5% glycerol, 150
mM NaCl, 1 mM EDTA, 210 μg/ml NaF, 1 mM
Na3VO4, 1 mM dithiothreitol, 1 μg/ml
leupeptin, 1 μg/ml pepstatin, 1 μg/ml aprotinin and 0.5 mM
phenylmethanesulfonylfluoride. For the western blot analysis,
proteins were resolved in an 8–12% SDS-PAGE gel and
electrotransferred to a polyvinylidene fluoride membrane according
to standard procedure. Following blocking for 1 h with 5% skimmed
dry milk in TBS-T buffer (2.4 g Tris, 8.8 g NaCl and 1 ml Tween 20)
dissolved in 1 l deionized H2O (pH 7.4), the blot was
probed with the primary antibodies overnight at 4°C. Next, the blot
was incubated with peroxidase-conjugated secondary antibody for 1 h
at RT followed by detection of the protein with enhanced
chemiluminescence reagents and exposure to X-ray film.
Statistical analysis
Statistical significances between COX-2 and PPAR-γ
expression and clinical and pathological parameters were assessed
using the χ2 or Mann-Whitney U tests. Kaplan-Meier
curves were used to assess the effect of COX-2 and PPAR-γ
expression on disease-free and overall survival. Overall survival
was defined as the time between surgery and patient mortality due
to CRC. Individuals who succumbed to additional causes or survived
to the last follow-up were censored. All P-values were based on a
two-tailed statistical analysis and P<0.05 was considered to
indicate a statistically significant difference. The correlation
between COX-2 and PPAR-γ was evaluated by linear regression
analysis.
Results
COX-2 and PPAR-γ expression in colorectal
tumor specimens, as determined by western blotting
The levels of COX-2 and PPAR-γ expression in the
paired specimens from 49 patients were measured by western blot
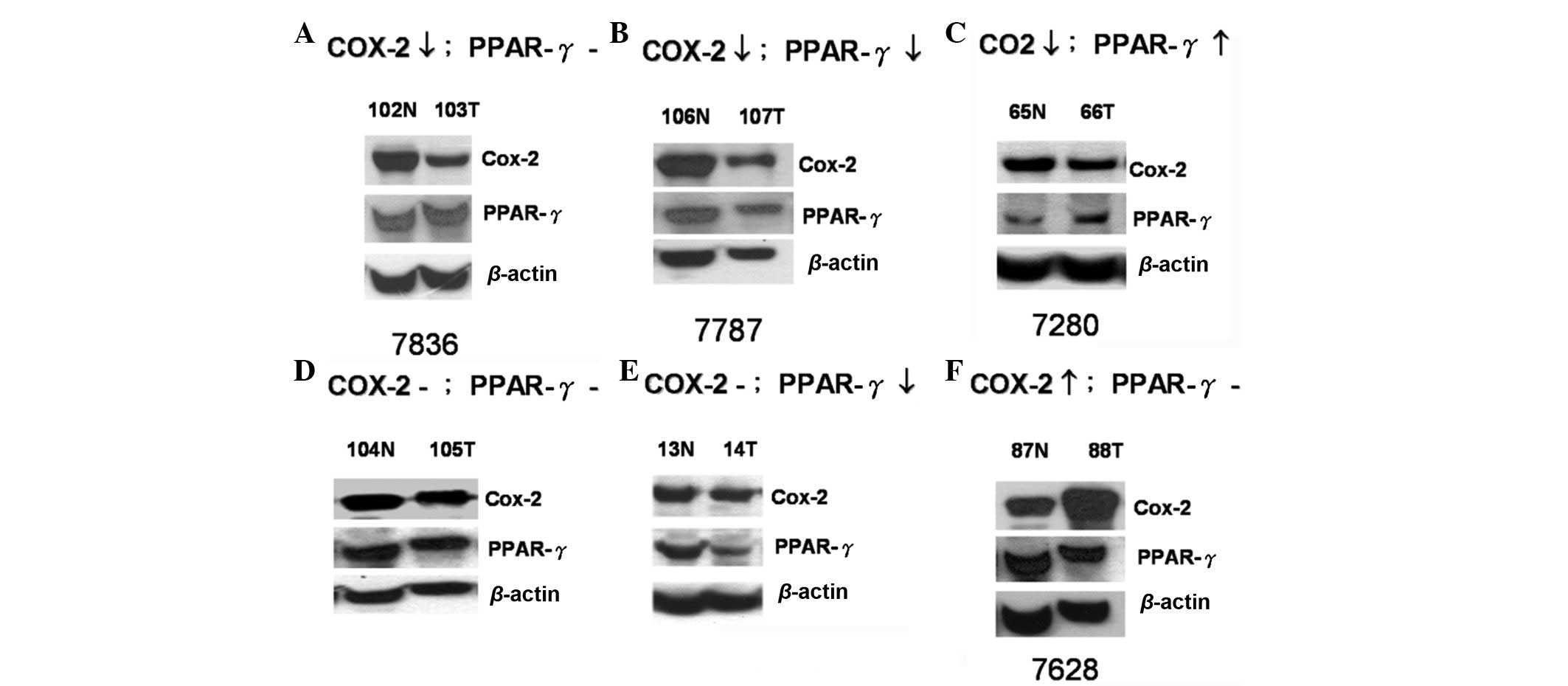
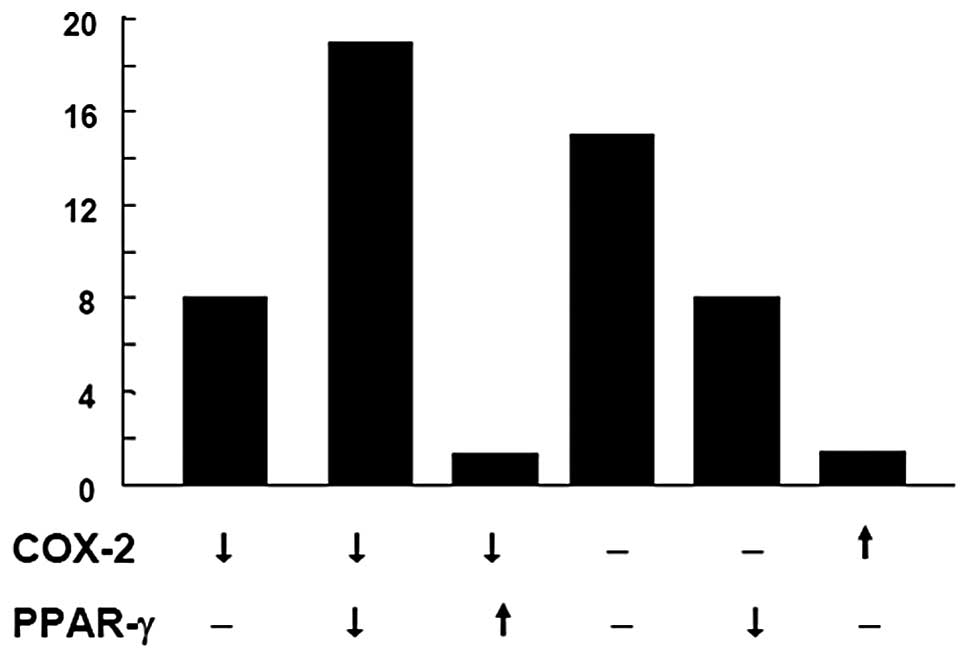
analysis. The expression profiles were categorized into six groups:
i) COX-2 decreased, PPAR-γ unchanged (8; 16.3%); ii) COX-2
decreased, PPAR-γ decreased (18; 36.7%); iii) COX-2 decreased,
PPAR-γ increased (1; 2.04%); iv) COX-2 unchanged, PPAR-γ unchanged
(14; 28.6%); v) COX-2 unchanged, PPAR-γ decreased (7; 14.3%); and
vi) COX-2 increased, PPAR-γ unchanged (1; 2.04%; Fig. 1). The quantified data of the six
groups are shown in Fig. 2. In
summary, the highest percentage of colon cancer specimens showed
decreased expression (18; 36.7%) or no change in expression (14;
28.6%) of COX-2 and PPAR-γ. Only 2.04% of specimens showed
increased COX-2 expression in the tumor tissues, which is
inconsistent with a previous study (9).
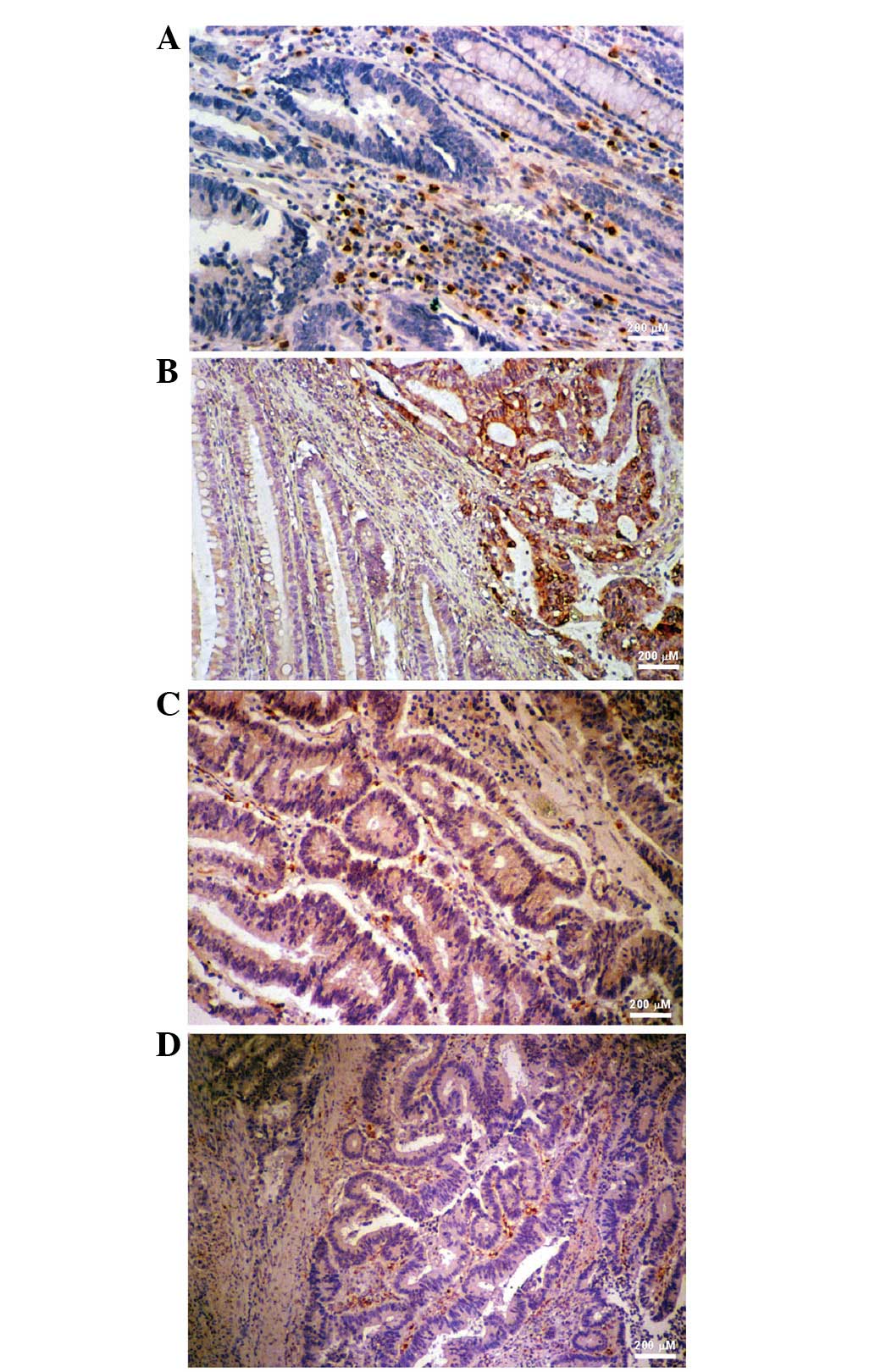
COX-2 expression in colorectal tumor
specimens determined by IHC
COX-2 staining was strong in the adjacent stromal
cells of specimen #7280, but weak within the tumor tissue (Fig. 3A), which was consistent with the
results of the western blot analysis (Fig. 1). IHC of specimen #7628 showed that
COX-2 was overexpressed in the gland cells of the tumor tissue but
not in the normal and stromal cells (Fig. 3B), which was also consistent with
the western blot analysis (Fig. 1).
COX-2 staining in specimen #7787 was marked in the gland and
stromal cells of the colorectal tumor specimen (Fig. 3C) and COX-2 expression was higher in
the normal tissue compared with the tumor tissue (Fig. 1). In specimen #7836, COX-2
expression was higher in the surrounding stromal cells (Fig. 3D) and normal tissue (Fig. 1) than in the tumor tissue, as
determined by IHC staining and western blotting, respectively. The
majority of results from the current study show a higher expression
of COX-2 in the adjacent normal tissues and stromal cells than in
the tumor tissue.
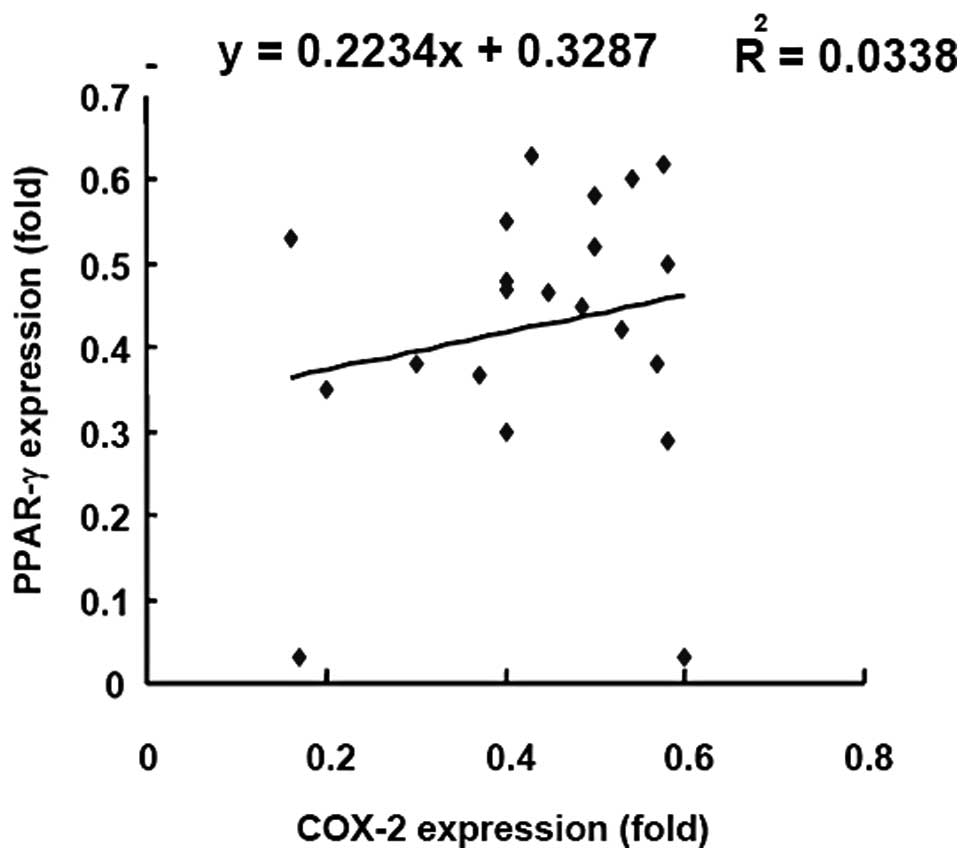
Correlation between COX-2 and PPAR-γ
expression
To investigate the correlation between the
expression of COX-2 and PPAR-γ, the expression levels were
investigated in specimens from 21 CRC patients by linear regression
analysis (Fig. 4). The R-value of
the linear regression line was 0.03 indicating that there was no
linear correlation between COX-2 and PPAR-γ expression.
Relative ratio of tumor-to-normal tissue
COX-2 expression correlates with high recurrence rate and poor
prognosis
In the multivariate logistic regression analysis,
the recurrence of CRC was identified to significantly correlate
with COX-2 expression (tumor tissue vs. normal tissue; P=0.015;
n=49; cut-off value, 0.6; Table
II). The correlation between COX-2 expression and tumor
recurrence was independent of age, gender, histological
differentiation, primary tumor origin, tumor size and nodal status,
as determined by univariate logistic regression analysis (Table II). High COX-2 expression in the
tumor tissues (specimen #7628; Figs.
1 and 3B) also correlated with
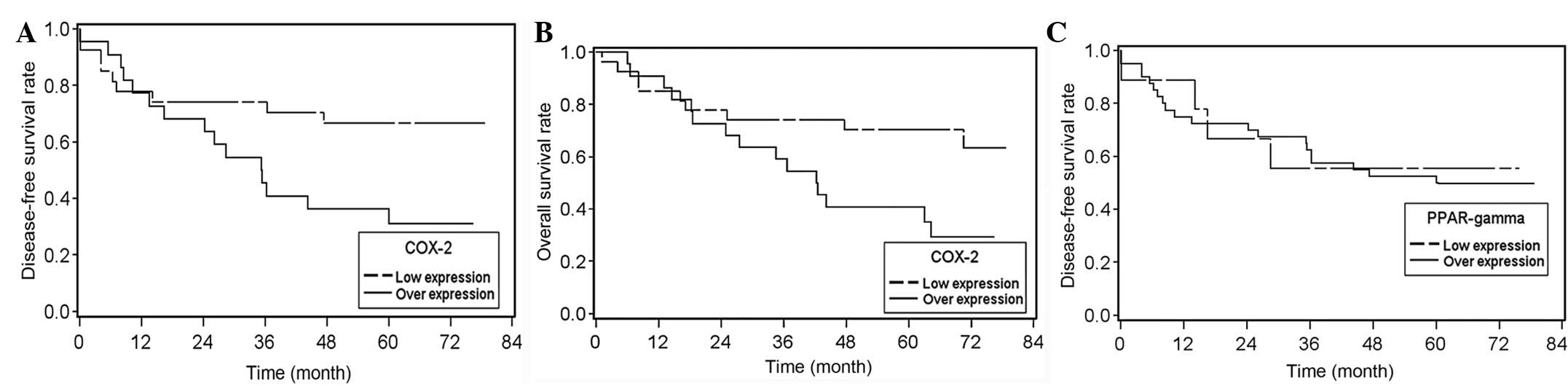
poor disease-free and overall survival rates. Disease-free and
overall survival times were significantly lower in patients with a
high tumor-to-normal tissue COX-2 expression ratio when compared
with that of subjects with a low tumor-to-normal tissue COX-2
expression ratio (P=0.03; Fig. 5A and
5B). However, no correlation was identified between PPAR-γ
expression and disease-free survival (P=0.23; Fig. 5C). In summary, COX-2 overexpression
in tumors correlates with recurrence and poor survival, however
PPAR-γ overexpression does not.
 | Table IICorrelation between COX2 expression
and various prognostic factors of colorectal cancer patients. |
Table II
Correlation between COX2 expression
and various prognostic factors of colorectal cancer patients.
| COX2a, n | |
|---|
|
| |
|---|
| Variables | ≥0.6 | <0.6 | P-value |
|---|
| Patients | 22 | 27 | |
| Gender |
| Male | 12 | 15 | 0.944 |
| Female | 10 | 12 | |
| Histological
differentiation |
| Well | 5 | 5 | 0.883 |
| Moderate | 14 | 19 | |
| Poor | 3 | 3 | |
| Primary tumor
origin |
| Colon-sigmoid | 15 | 19 | 0.869 |
| Rectum | 7 | 8 | |
| Tumor
statusb |
| T1-2 | 2 | 5 | 0.138 |
| T3-4 | 20 | 22 | |
| Nodal
statusb |
| 0 | 13 | 17 | 0.965 |
| 1 | 7 | 8 | |
| 2 | 2 | 2 | |
| Pathological
stage |
| II | 13 | 17 | 0.835 |
| III | 8 | 8 | |
| IV | 1 | 2 | |
| Recurrence |
| Yes | 15 | 9 | 0.015 |
| No | 7 | 18 | |
Levels of COX-2 and PPAR-γ expression in
nine colon cancer cell lines
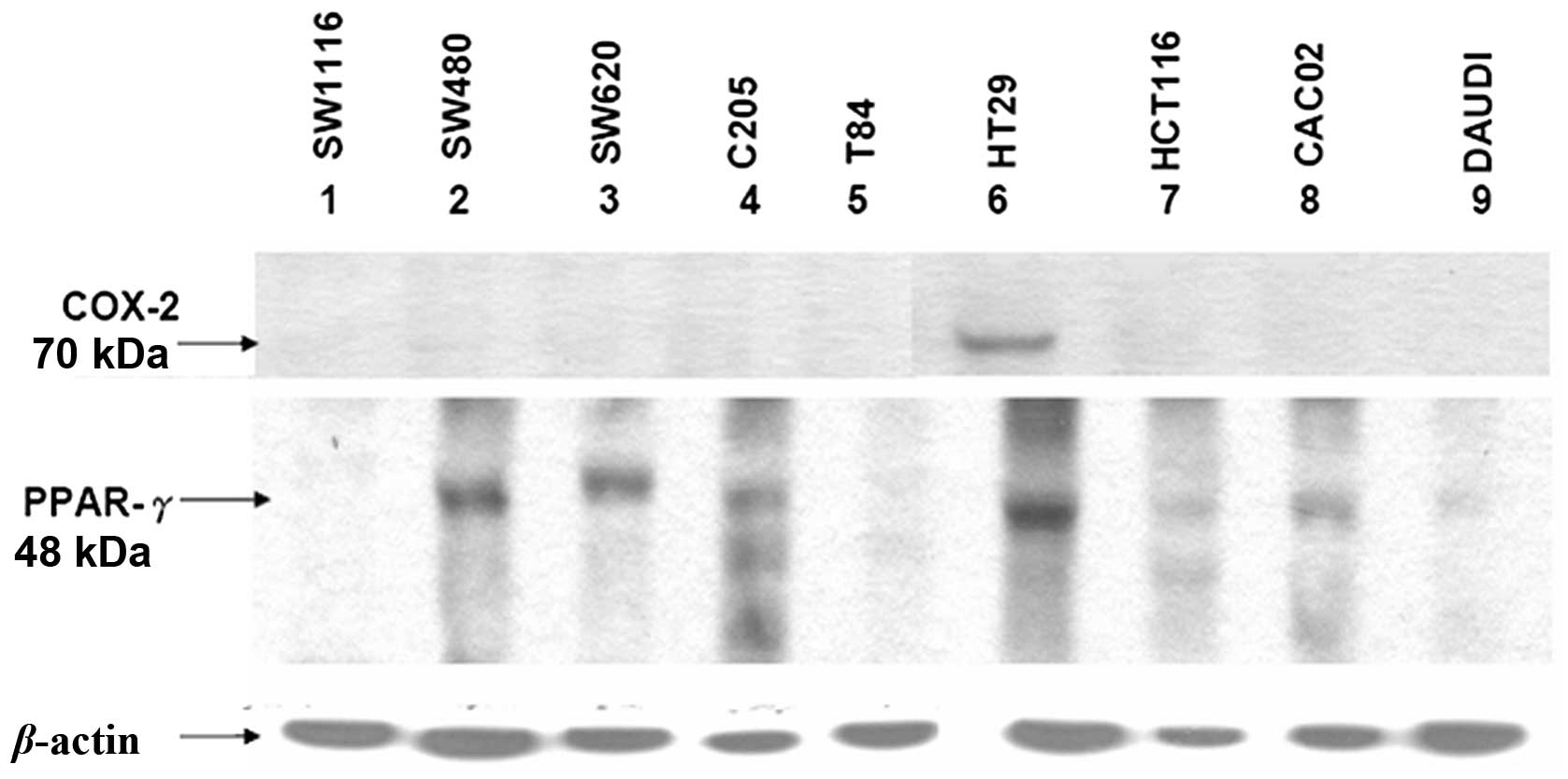
To evaluate the levels of COX-2 and PPAR-γ
expression in nine CRC cell lines, namely SW116, SW480, SW620,
C205, T84, HT29, HCT116, CACO-2 and DAuD1, representing various
grades of malignancy, the total protein extracted from these lines
was evaluated by western blotting using monoclonal anti-COX-2 and
-PPAR-γ antibodies (Fig. 6). One
colon cancer cell line, HT29, expressed COX-2. By contrast, PPAR-γ
expression varied in the nine cancer cell lines. The expression of
PPAR-γ was high in four of the colon cancer cell lines, while
SW480, SW620, C205 and HT29 were demonstrated to be have
insignificant or undetectable expression in five. Overall, the
majority of the CRC cell lines expressed extremely low levels of
COX-2, which was consistent with the results from the CRC patients
(Fig. 1).
Discussion
In the present study, the majority of the patients
with colon cancer exhibited low levels of COX-2 expression in the
tumor tissues and high levels of COX-2 expression in the adjacent
normal tissues, as determined by western blotting and IHC staining.
However, a high ratio of tumor-to-normal tissue COX-2 expression
was shown to correlate with high recurrence rates and poor
prognosis. In addition, previous studies have shown that tumor
stromal cells contribute to COX-2 expression in CRC, indicating
that normal and tumor cells may contribute to an increase in
prostaglandin levels within the tumor microenvironment and the
subsequent development of cancer (22). Previously, Charalambous et al
reported that COX-2 expression in stromal cells correlates with the
clinical severity of CRC (11). In
general, COX-2 is not detectable in normal and premalignant
colorectal epithelium and it has been hypothesized to be confined
to subepithelial cells, including fibroblasts, in non-malignant
colonic tissue. Fibroblasts and additional mesenchymal cells,
including stromal cells, are the source of COX-2 in normal and
premalignant colorectal tissues. The moderately higher rate of
COX-2 transcription in fibroblasts leads to a corresponding
increase in prostaglandin E2 synthesis. The effect of prostaglandin
E2 is amplified progressively via the robust stabilization of COX-2
mRNA (22). Intestinal epithelial
cells with high expression levels of the COX-2 gene have altered
adhesion properties, resist apoptosis and exhibit a marked decrease
in retinoblastoma kinase activity, which correlates with the
activation of cyclin-dependent kinase 4 (23). Carcinogenesis has previously been
reported to correlate with the transformation of normal stroma into
a ‘reactive’ stromal phenotype (24). In the current study, COX-2
expression was extremely low in ~75% of tumor tissues and higher in
the stromal cells of adjacent normal tissues. The COX-2 expression
of cancer cells in vivo may be affected by the
microenvironment of the tissue surrounding the tumors.
Prostaglandin I2 production by stromal cells promotes the survival
of colonocytes through PPAR-γ activation. This mechanism may aid
the maintenance of cells in normal crypts and the clonal expansion
of mutant colonocytes during tumorigenesis (22). In the present study, of the nine
colon cancer cell lines representing various grades of malignancy,
only HT29 showed increased COX-2 expression, indicating that
expression is negatively regulated in the majority of CRC cell
lines. However, the underlying mechanism remains unclear. Higher
COX-2 expression in the microenvironment adjacent to the tumor may
affect the expression of COX-2 in the tumor cells.
The majority of colorectal adenomas and carcinomas
are characterized by chromosomal instability and a progressive loss
of heterozygosity. By contrast, in 15–20% of colorectal neoplasms,
induction occurs via a distinct genetic pathway characterized by
microsatellite instability and loss of expression of a DNA mismatch
repair enzyme, commonly hMLH1 or hMSH2 (25). Overall, the results of the present
study show that 33% of defective mismatch repair was identified in
colorectal tumors with low or absent COX-2 staining (P<0.05).
Additional features have also been identified to be predictive of
low COX-2 staining, including marked infiltration of the tumor by
lymphocytes and solid/cribriform or signet ring histological
patterns (25). These
investigations indicate that CRC with molecular and phenotypic
characteristics of defective DNA mismatch repair express lower
levels of COX-2. The clinical implications of this biological
distinction remain unknown, but must be considered when
investigating the efficacy of COX-2 inhibitors for chemoprevention
in patients whose tumors may arise in the setting of defective DNA
mismatch repair (25).
The growth and differentiation of colon cancer cells
are also modulated by PPAR-γ. PPARs are transcription factors that
regulate molecular events in normal and cancer cells (26). A number of COX enzymes produce
specific eicosanoids that have previously been shown to activate
transcription mediated by PPAR-γ. The expression of PPAR-γ is
largely restricted to adipose tissue and a marked increase in
PPAR-γ RNA levels has been identified in colon tumors compared with
paired normal mucosa. PPAR-γ protein expression has been previously
reported in 4/5 colon tumor samples (27).
However, the levels of PPAR-γ expression in the nine
colon cancer cell lines of the present study were variable. The
patterns of COX-2 and PPAR-γ expression in the colon cancer
patients were classified into six types and the majority of the
specimens showed decreased or unchanged expression levels of COX-2
and PPAR-γ. However, one specimen showed increased expression of
COX-2 with unchanged expression of PPAR-γ, whilst a second showed
increased expression of PPAR-γ with unchanged expression of COX-2.
In addition, no linear correlation between COX-2 and PPAR-γ
expression was identified in the 21 colon cancer specimens,
demonstrating that the expression of COX-2 and PPAR-γ is not
essential for colon cancer formation.
The roles of PPAR-γ, COX-2 and p-IκB-α (important
molecular targets for colon cancer chemoprevention) in stromal
remodeling were investigated by comparing the expression of these
molecules in the tumor and surrounding normal colonic mucosa of
stromal myofibroblasts, macrophages and endothelial cells. COX-2
expression was upregulated by NF-κB in the stromal myofibroblasts
surrounding the colon adenocarcinomas and the expression was
identified to markedly correlate with p-IκB-α expression
(P<0.001). No correlation between PPAR-γ, COX-2 or p-IκB-α
expression and the stage or differentiation status of the
adenocarcinomas was identified (24). In addition, no correlation was shown
between PPAR-γ and COX-2 expression.
In conclusion, the observations of the current study
indicated that COX2 expression in normal tissue adjacent to tumors
may be important for colon cancer carcinogenesis, despite the
correlation between a higher ratio of tumor-to-normal tissue COX-2
expression and poor prognosis in CRC.
Acknowledgements
The present study was supported by Landmark Project
Grant A25 of the National Cheng Kung University funded by the
Ministry of Education in Taiwan and from the National Science
Council, Taiwan (no. 96-2628-B-006-003-MY3).
References
|
1
|
Vogelstein B, Fearon ER, Hamilton SR, et
al: Genetic alterations during colorectal-tumor development. N Engl
J Med. 319:525–532. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruce WR, Wolever TM and Giacca A:
Mechanisms linking diet and colorectal cancer: the possible role of
insulin resistance. Nutr Cancer. 37:19–26. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balkwill F and Mantovani A: Inflammation
and cancer: back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hussain SP, Hofseth LJ and Harris CC:
Radical causes of cancer. Nat Rev Cancer. 3:276–285. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mariani F, Sena P, Marzona L, Riccio M,
Fano R, Manni P, et al: Cyclooxygenase-2 and Hypoxia-Inducible
Factor-1alpha protein expression is related to inflammation, and
up-regulated since the early steps of colorectal carcinogenesis.
Cancer Lett. 279:221–229. 2009. View Article : Google Scholar
|
|
6
|
Adegboyega PA, Ololade O, Saada J, Mifflin
R, Di Mari JF and Powell DW: Subepithelial myofibroblasts express
cyclooxygenase-2 in colorectal tubular adenomas. Clin Cancer Res.
10:5870–5879. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hawcroft G, Ko CW and Hull MA:
Prostaglandin E2-EP4 receptor signalling promotes tumorigenic
behaviour of HT-29 human colorectal cancer cells. Oncogene.
26:3006–3019. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun Y, Tang XM, Half E, Kuo MT and
Sinicrope FA: Cyclooxygenase-2 overexpression reduces apoptotic
susceptibility by inhibiting the cytochrome c-dependent apoptotic
pathway in human colon cancer cells. Cancer Res. 62:6323–6328.
2002.PubMed/NCBI
|
|
9
|
Eberhart CE, Coffey RJ, Radhika A,
Giardiello FM, Ferrenbach S and DuBois RN: Up-regulation of
cyclooxygenase 2 gene expression in human colorectal adenomas and
adenocarcinomas. Gastroenterology. 107:1183–1188. 1994.PubMed/NCBI
|
|
10
|
Levy BD, Clish CB, Schmidt B, Gronert K
and Serhan CN: Lipid mediator class switching during acute
inflammation: signals in resolution. Nat Immunol. 2:612–619. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Charalambous MP, Lightfoot T, Speirs V,
Horgan K and Gooderham NJ: Expression of COX-2, NF-kappaB-p65,
NF-kappaB-p50 and IKKalpha in malignant and adjacent normal human
colorectal tissue. Br J Cancer. 101:106–115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sano H, Kawahito Y, Wilder RL, Hashiramoto
A, Mukai S, Asai K, et al: Expression of cyclooxygenase-1 and −2 in
human colorectal cancer. Cancer Res. 55:3785–3789. 1995.
|
|
13
|
Williams CS, Mann M and DuBois RN: The
role of cyclooxygenases in inflammation, cancer and development.
Oncogene. 18:7908–7916. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang M, Deng CS, Zheng JJ and Xia J:
Curcumin regulated shift from Th1 to Th2 in trinitrobenzene
sulphonic acid-induced chronic colitis. Acta Pharmacol Sin.
27:1071–1077. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamazaki K, Shimizu M, Okuno M,
Matsushima-Nishiwaki R, Kanemura N, Araki H, et al: Synergistic
effects of RXR alpha and PPAR gamma ligands to inhibit growth in
human colon cancer cells - phosphorylated RXR alpha is a critical
target for colon cancer management. Gut. 56:1557–1563. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kitamura S, Miyazaki Y, Shinomura Y, Kondo
S, Kanayama S and Matsuzawa Y: Peroxisome proliferator-activated
receptor gamma induces growth arrest and differentiation markers of
human colon cancer cells. Jpn J Cancer Res. 90:75–80. 1999.
View Article : Google Scholar
|
|
17
|
Kohno H, Suzuki R, Sugie S and Tanaka T:
Suppression of colitis-related mouse colon carcinogenesis by a
COX-2 inhibitor and PPAR ligands. BMC Cancer. 5:462005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuri M, Sasahira T, Nakai K, Ishimaru S,
Ohmori H and Kuniyasu H: Reversal of expression of
15-lipoxygenase-1 to cyclooxygenase-2 is associated with
development of colonic cancer. Histopathology. 51:520–527. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sarraf P, Mueller E, Jones D, King FJ,
DeAngelo DJ, Partridge B, et al: Differentiation and reversal of
malignant changes in colon cancer through PPARgamma. Nat Med.
4:1046–1052. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tseng YS, Tzeng CC, Huang CY, Chen PH,
Chiu AW, Hsu PY, et al: Aurora-A overexpression associates with
Ha-ras codon-12 mutation and blackfoot disease endemic area in
bladder cancer. Cancer Lett. 241:93–101. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng HL, Trink B, Tzai TS, Liu HS, Chan
SH, Ho CL, Sidransky D and Chow NH: Overexpression of c-met as a
prognostic indicator for transitional cell carcinoma of the urinary
bladder: a comparison with p53 nuclear accumulation. J Clin Oncol.
20:1544–1550. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cutler NS, Graves-Deal R, LaFleur BJ, Gao
Z, Boman BM, Whitehead RH, et al: Stromal production of
prostacyclin confers an antiapoptotic effect to colonic epithelial
cells. Cancer Res. 63:1748–1751. 2003.PubMed/NCBI
|
|
23
|
DuBois RN, Shao J, Tsujii M, Sheng H and
Beauchamp RD: G1 delay in cells overexpressing prostaglandin
endoperoxide synthase-2. Cancer Res. 56:733–737. 1996.PubMed/NCBI
|
|
24
|
Vandoros GP, Konstantinopoulos PA,
Sotiropoulou-Bonikou G, Kominea A, Papachristou GI, Karamouzis MV,
et al: PPAR-gamma is expressed and NF-kB pathway is activated and
correlates positively with COX-2 expression in stromal
myofibroblasts surrounding colon adenocarcinomas. J Cancer Res Clin
Oncol. 132:76–84. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karnes WE Jr, Shattuck-Brandt R, Burgart
LJ, DuBois RN, Tester DJ, Cunningham JM, et al: Reduced COX-2
protein in colorectal cancer with defective mismatch repair. Cancer
Res. 58:5473–5477. 1998.PubMed/NCBI
|
|
26
|
Zuo X, Wu Y, Morris JS, Stimmel JB,
Leesnitzer LM, Fischer SM, Lippman SM and Shureiqi I: Oxidative
metabolism of linoleic acid modulates PPAR-beta/delta suppression
of PPAR-gamma activity. Oncogene. 25:1225–1241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
DuBois RN, Gupta R, Brockman J, Reddy BS,
Krakow SL and Lazar MA: The nuclear eicosanoid receptor, PPARgamma,
is aberrantly expressed in colonic cancers. Carcinogenesis.
19:49–53. 1998. View Article : Google Scholar : PubMed/NCBI
|