Introduction
The current guidelines to treat stage II colon
cancer patients include the recommendation that post-operative
adjuvant chemotherapy (ACT) should only be considered for patients
who have a high risk of recurrence (1,2).
Although the definition of high risk has not been completely
established to date, various risk factors have been proposed. The
current American Society of Clinical Oncology (ASCO) Guidelines
recommend the provision of ACT for patients with
inadequately-sampled nodes, T4 lesions, tumor perforation or
poorly-differentiated histology (3). The current European Society for
Medical Oncology (ESMO) Clinical Practice Guidelines define
patients with stage II colon cancer to be at high risk and a
candidate for adjuvant therapy if at least one of the following
characteristics are identified: Lymph nodes sampling <12; a
poorly-differentiated tumor; vascular, lymphatic or perineural
invasion; tumor presentation with obstruction or tumor perforation;
and pT4 stage (4).
In accordance with the ASCO and ESMO guidelines, the
current Japanese guidelines for the treatment of colorectal cancer
recommend adjuvant therapy for patients at a high risk of
recurrence (5). Based on extensive
research, the Japanese Study Group for Post-operative Follow-up of
Colorectal Cancer, a multicenter collaborative study group,
recently recommended ACT for patients who meet two or more of the
following criteria: Extensive venous invasion; <13 dissected
lymph nodes; an age of >50 years; and/or being of the male
gender (6). In a previous
examination of stage II colon cancer patients, it was identified
that the patients who had presented with invasive gross tumors and
elevated pre-operative serum carcinoembryonic antigen (CEA) levels
and who had not undergone ACT were at a high risk of recurrence
(7). Accordingly, in the present
study, the patients with elevated pre-operative serum CEA levels
were identified as candidates for ACT. The majority of studies
conducted prior to the present study were based on a comparison of
data that was collected from patients who had or had not undergone
ACT. As a result, the effect of chemotherapy was not excluded as a
confounding factor in the analysis of risk factors for recurrence.
In addition, the effect of age or gender, which may not be
associated with the malignant potential of colon cancer, cannot be
excluded in the analysis of prognostic factors in terms of overall
survival (OS), commonly used in past studies. To address these
limitations, the time to recurrence (TTR), a relatively objective
measure, was used to determine the prognosis of the stage II colon
cancer patients who had not undergone ACT following a successful
curative resection, and to compare the prognosis with that of
patients who had undergone adjuvant therapy in order to identify
the risk factors for recurrence that may be indicators for adjuvant
therapy (8).
Materials and methods
Patients and specimens
The present retrospective study examined data
collected from 377 patients who were treated at a single medical
institution (Kurume University Hospital, Kurume, Fukuoka, Japan)
between 1982 and 2005 for stage II colon cancer. All patients met
the study criteria of i) having been pathologically diagnosed with
stage II colon cancer, ii) having undergone a curative resection
with lymphadenectomy and iii) not having undergone pre-operative
chemotherapy, radiotherapy or immunotherapy. Of these 377 patients,
163 had undergone ACT and thus constituted the ACT group and 214
patients had not undergone ACT and thus constituted the surgery
alone (SA) group. The primary reasons as to why ACT had not been
administered were refusal to provide informed consent and the
patient age being ≥75 years. Approval for the study was obtained
from the Kurume University Hospital Ethics Committee.
Diagnosis and staging procedures
The pathological factors and stage classification of
colon cancer were determined according to the TNM classification
system developed by the International Union against Cancer (UICC)
(9). Mesenteric lymph nodes had
been removed from the mesenteric adipose tissue for histological
examination immediately after surgery. A pathological examination
of all isolated lymph nodes was performed, with the
histopathological examination being performed using a 5-mm thick
longitudinal whole tissue section. Lymphatic permeation and venous
invasion was determined on the basis of previously defined criteria
(10,11).
ACT
Oral fluoropyrimidines, including tegafur plus
uracil (UFT; 300 mg/day per 1 m2 body area) and
doxifluridine (5′-DFUR; 600 mg/day per 1 m2 body area),
an intermediate metabolite of capecitabine, were the agents that
were administered during a course of post-operative ACT that was
provided for >6 months.
Follow-up schedule and examinations
All patients were monitored as outpatients according
to a regular examination schedule. The final follow-up date for the
present study was April 30, 2011. The post-operative surveillance
consisted of measuring the tumor marker levels, chest radiography
and abdominal ultrasonography, in addition to a physical
examination every 3 to 6 months for the first 3 years, every 6
months for the next 4 years and annually thereafter. Chest and
abdominal computed tomography or magnetic resonance imaging was
performed every 6 to 12 months for the first 3 years and then
annually or when recurrence was suspected thereafter.
Statistical analysis
The TTR was calculated using the day of the first
surgery as the start date and considering recurrence of the primary
cancer or mortality due to the primary cancer as an event. TTR
curves were then generated using the Kaplan-Meier method and the
significance between groups was determined using the log-rank test.
Univariate and multivariate analyses of the clinicopathological
factors, including the CEA level associated with the TTR, were
performed using Cox’s proportional hazards model. The analysis of
the differences in the clinicopathological factors between the
groups was performed using Fisher’s exact test and Student’s
t-test. All statistical analyses were performed using JMP version
9.0.2 software (SAS Institute, Inc., Cary, NC, USA) and P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient clinicopathological data
The clinicopathological data of the patients are
presented in Table I. The tumors
were located in the left colon (descending, sigmoid or rectosigmoid
colon) in 225 patients and the right colon (cecum, ascending colon
or transverse colon) in 152 patients. The classification of the
tumor according to the gross tumor type was invasive in 39 patients
and non-invasive in 338 patients. An increased pre-operative CEA
level was observed in 136 patients (36.1%), 25 (18.4%) of who later
experienced recurrence. In addition, a CEA level of twice the
cut-off value was observed in 68 patients (18.0%), 15 (22.1%) of
whom later experienced recurrence (Table II). The tumor diameter was greater
than the median tumor diameter of 55 mm in 203 patients.
 | Table IComparison of patient data by
treatment group. |
Table I
Comparison of patient data by
treatment group.
| Variable | ACT group | SA group | P-value |
|---|
| Age, years (mean ±
SD) | 63.3±9.4 | 67.7±11.7 | 0.001a |
| Gender, n |
| Male | 104 | 141 | 0.744 |
| Female | 59 | 73 | |
| Tumor location,
n |
| Left colon | 96 | 129 | 0.832 |
| Right colon | 67 | 85 | |
| Gross tumor type,
n |
| Invasive | 19 | 20 | 0.498 |
| Non-invasive | 144 | 194 | |
| Pre-operative CEA
level, n |
| ≥NL | 56 | 80 | 0.448 |
| <NL | 107 | 128 | |
| Pre-operative CEA
level, n |
| ≥NL×2 | 30 | 38 | 0.999 |
| <NL×2 | 133 | 170 | |
| Pre-operative CA19-9
level, n |
| ≥NL | 12 | 20 | 0.690 |
| <NL | 55 | 73 | |
| Tumor size, n |
| ≥Median | 96 | 107 | 0.096 |
| <Median | 67 | 107 | |
| Number of dissected
LNs, n |
| <12 | 9 | 31 | 0.006a |
| ≥12 | 154 | 183 | |
| Histology, n |
| Others | 19 | 18 | 0.301 |
| Well/mod | 144 | 196 | |
| T factor |
| T4 | 63 | 74 | 0.450 |
| T3 | 100 | 140 | |
| Adjacent organ
invasion, n |
| Positive | 19 | 23 | 0.869 |
| Negative | 144 | 191 | |
| Bowel obstruction,
n |
| Positive | 10 | 15 | 0.836 |
| Negative | 153 | 199 | |
| Lymphatic
permeation, n |
| Extensive | 20 | 25 | 0.874 |
| Slight | 143 | 189 | |
| Venous invasion,
n |
| Extensive | 8 | 25 | 0.026a |
| Slight | 155 | 189 | |
 | Table IIComparison of patient data by
recurrence status. |
Table II
Comparison of patient data by
recurrence status.
| Variable | Recurrent
cases | Non-recurrent
cases | P-value |
|---|
| Age, years
(mean±SD) | 66.0±11.8 | 65.8±10.8 | 0.927 |
| Gender, n |
| Male | 34 | 211 | 0.420 |
| Female | 14 | 118 | |
| Tumor location,
n |
| Left colon | 33 | 192 | 0.208 |
| Right colon | 15 | 137 | |
| Gross tumor-type,
n |
| Invasive | 9 | 30 | 0.070 |
| Non-invasive | 39 | 299 | |
| Pre-operative CEA
level, n |
| ≥NL | 25 | 111 | 0.024a |
| <NL | 23 | 212 | |
| Pre-operative CEA
level, n |
| ≥NL×2 | 15 | 53 | 0.026a |
| <NL×2 | 33 | 270 | |
| Pre-operative
CA19-9 level, n |
| ≥NL | 3 | 29 | 0.385 |
| <NL | 6 | 122 | |
| Tumor size, n |
| ≥Median | 24 | 179 | 0.643 |
| <Median | 24 | 150 | |
| Number of dissected
LNs, n |
| <12 | 7 | 33 | 0.321 |
| ≥12 | 41 | 296 | |
| Histology, n |
| Others | 5 | 32 | 0.799 |
| Well/mod | 43 | 297 | |
| T factor, n |
| T4 | 18 | 119 | 0.873 |
| T3 | 30 | 210 | |
| Adjacent organ
invasion, n |
| Positive | 9 | 33 | 0.085 |
| Negative | 39 | 296 | |
| Bowel obstruction,
n |
| Positive | 6 | 19 | 0.112 |
| Negative | 42 | 310 | |
| Lymphatic
permeation, n |
| Extensive | 10 | 35 | 0.055 |
| Slight | 38 | 294 | |
| Venous invasion,
n |
| Extensive | 7 | 26 | 0.165 |
| Slight | 41 | 303 | |
| Treatment, n |
| SA | 34 | 180 | 0.042a |
| ACT | 14 | 149 | |
Among the total patients, there were 337 in whom ≥12
lymph nodes had been sampled and the histological examination
indicated that the tumor was well-differentiated in 276 patients,
moderately-differentiated in 64 and poorly-differentiated/other in
37. A total of 25 patients were diagnosed with a pre-operative
bowel obstruction; these patients were defined as those who
required the placement of an oral or transanal decompression tube
or had undergone emergency surgery for pre-operative bowel
obstruction. Perforations had been observed in two of these
patients, who were therefore included in the group of patients with
bowel obstructions. Extensive lymphatic permeation was observed in
45 patients and extensive venous invasion in 33 patients.
The overall median follow-up duration was 98 months.
Recurrence was observed in 48 patients (12.7%), of whom 14 (8.6%)
were in the ACT group and 34 (15.9%) were in the SA group. A
statistical analysis of the results indicated a significant
difference between the two groups in terms of recurrence (P=0.042;
Table II), age (P=0.001), number
of dissected lymph nodes if <12 nodes had been sampled (P=0.006)
and the extent of venous invasion (P=0.026; Table I).
Univariate and multivariate analyses
The results of the univariate analysis, which used
Cox’s proportional hazards model to examine the TTR of the SA
group, revealed that the CEA levels were twice the cut-off value
and that bowel obstruction and extensive lymphatic permeation were
significant risk factors for recurrence (Table III). The statistical analysis of
the significance of a CEA level above the cut-off value, twice the
cut-off value and three times the cut-off value indicated that a
level that was twice the cut-off value had the highest hazard ratio
(HR) and the least significant association. Therefore, a CEA level
of twice the cut-off value was used in the subsequent analyses. The
multivariate analysis of the parameters that were identified as
significant in the univariate analysis confirmed that a CEA level
of twice the cut-off value and bowel obstruction were significant
risk factors for recurrence.
 | Table IIIUnivariate and multivariate analyses
of the TTR of the SA group. |
Table III
Univariate and multivariate analyses
of the TTR of the SA group.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (≥75 years vs.
<75 years) | 1.561 | 0.760–3.087 | 0.218 | | | |
| Gender (male vs.
female) | 1.556 | 0.753–3.527 | 0.240 | | | |
| Tumor location
(left vs. right colon) | 1.177 | 0.592–2.457 | 0.647 | | | |
| Gross tumor-type
(invasive vs. non-invasive) | 2.518 | 0.941–5.684 | 0.064 | | | |
| CEA (≥NL vs.
<NL) | 2.324 | 1.183–4.649 | 0.015a | | | |
| CEA (≥NL×2 vs.
<NL×2) | 3.353 | 1.513–7.165 | 0.004a | 3.840 | 1.674–8.629 | 0.002a |
| CEA (≥NL×3 vs.
<NL×3) | 2.958 | 1.349–6.013 | 0.008a | | | |
| CA19-9 (≥NL vs.
<NL) | 1.828 | 0.262–8.496 | 0.492 | | | |
| Tumor size (≥
median vs. <median) | 0.765 | 0.383–1.503 | 0.437 | | | |
| Number of dissected
lymph nodes (<12 vs. >12) | 1.898 | 0.760–4.132 | 0.158 | | | |
| Histology (others
vs. well/mod) | 0.983 | 0.236–2.751 | 0.977 | | | |
| T factor (T4 vs.
T3) | 1.059 | 0.496–2.129 | 0.876 | | | |
| Bowel obstruction
(yes vs. no) | 3.482 | 1.301–7.859 | 0.016a | 6.284 | 2.024–16.47 | 0.003a |
| Lymphatic
permeation (extensive vs. slight) | 2.720 | 1.150–5.748 | 0.025a | 2.523 | 0.911–6.017 | 0.072 |
| Venous invasion
(extensive vs. slight) | 1.654 | 0.562–3.923 | 0.328 | | | |
Effect of ACT on patients with risk
factors for recurrence
Subsequent investigation of the effect of
post-operative ACT in patients with the previously mentioned risk
factors for recurrence showed no significant differences in the
recurrence rate in patients with and without a CEA level of twice
the cut-off value in the ACT group (P=0.999). However, the
recurrence rate was significantly higher in patients with a CEA
level of twice the cut-off value in the SA group (P=0.003). In
addition, the rate of recurrence in patients in the ACT group who
had presented with a CEA level of twice the cut-off value was
identified to be significantly lower than that of patients in the
SA group (P=0.008; Table IV).
 | Table IVCorrelation between serum CEA levels
of twice the cut-off value and recurrence. |
Table IV
Correlation between serum CEA levels
of twice the cut-off value and recurrence.
| ACT groupa | SA groupb |
|---|
|
|
|
|---|
| CEA | ≥NL×2c | <NL×2 | ≥NL×2c | <NL×2 |
|---|
| Recurrence |
| Yes, n (%) | 2 (6.7) | 12 (9.0) | 13 (34.2) | 21 (12.4) |
| No, n (%) | 28 (93.3) | 121 (91.0) | 25 (65.8) | 149 (87.6) |
No significant differences were identified in the
rate of recurrence between patients in the ACT group who were and
who were not diagnosed with a pre-operative bowel obstruction
(P=0.999). In contrast, within the SA group, the rate of recurrence
was shown to be significantly higher in patients who were diagnosed
with pre-operative bowel obstructions than in those who were not
(P=0.018; Table V). A comparison of
the patients in the ACT and SA groups who were diagnosed with a
pre-operative bowel obstruction revealed that the rate of
recurrence of the patients in the ACT group was lower than that of
the patients in the SA group (P=0.051; Table V).
 | Table VCorrelation between bowel obstruction
and recurrence. |
Table V
Correlation between bowel obstruction
and recurrence.
| ACT groupa | SA groupb |
|---|
|
|
|
|---|
| Bowel
obstruction | Yesc | No | Yesc | No |
|---|
| Recurrence |
| Yes, n (%) | 0 (0.0) | 14 (9.2) | 6 (40.0) | 28 (14.1) |
| No, n (%) | 10 (100) | 139 (90.8) | 9 (60.0) | 171 (85.9) |
A multivariate analysis confirmed that a CEA level
of twice the cut-off value and a diagnosis of a pre-operative bowel
obstruction were significant factors in predicting the rate of
recurrence. Based on these results, the 377 patients were divided
into two groups. Those who had presented with at least one of the
risk factors (CEA level of twice the cut-off value and/or a
diagnosis of a pre-operative bowel obstruction) were assigned to
the high-risk group (n=86) and those who had presented with neither
risk factor were assigned to the low-risk group (n=291). A
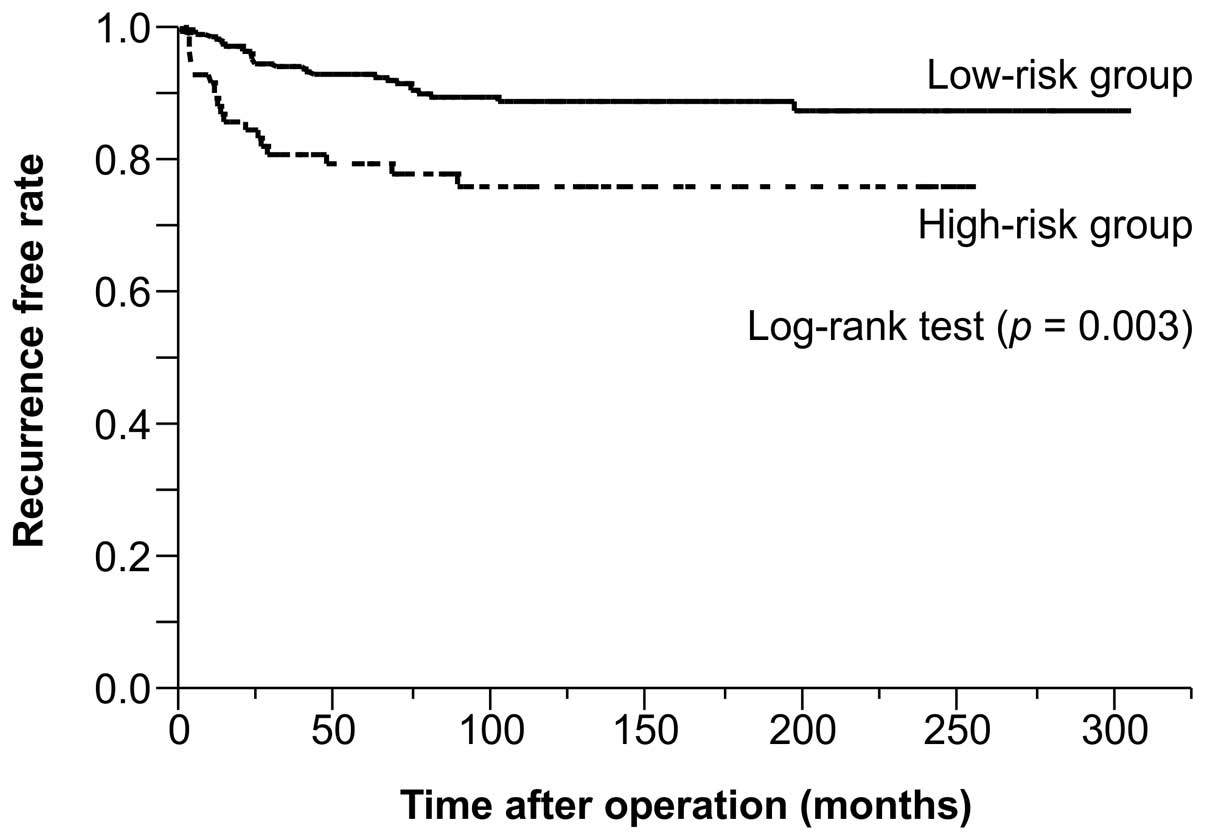
between-group comparison, which calculated the TTR using the
Kaplan-Meier method, revealed a significantly lower rate of
recurrence in the low-risk group (Fig.
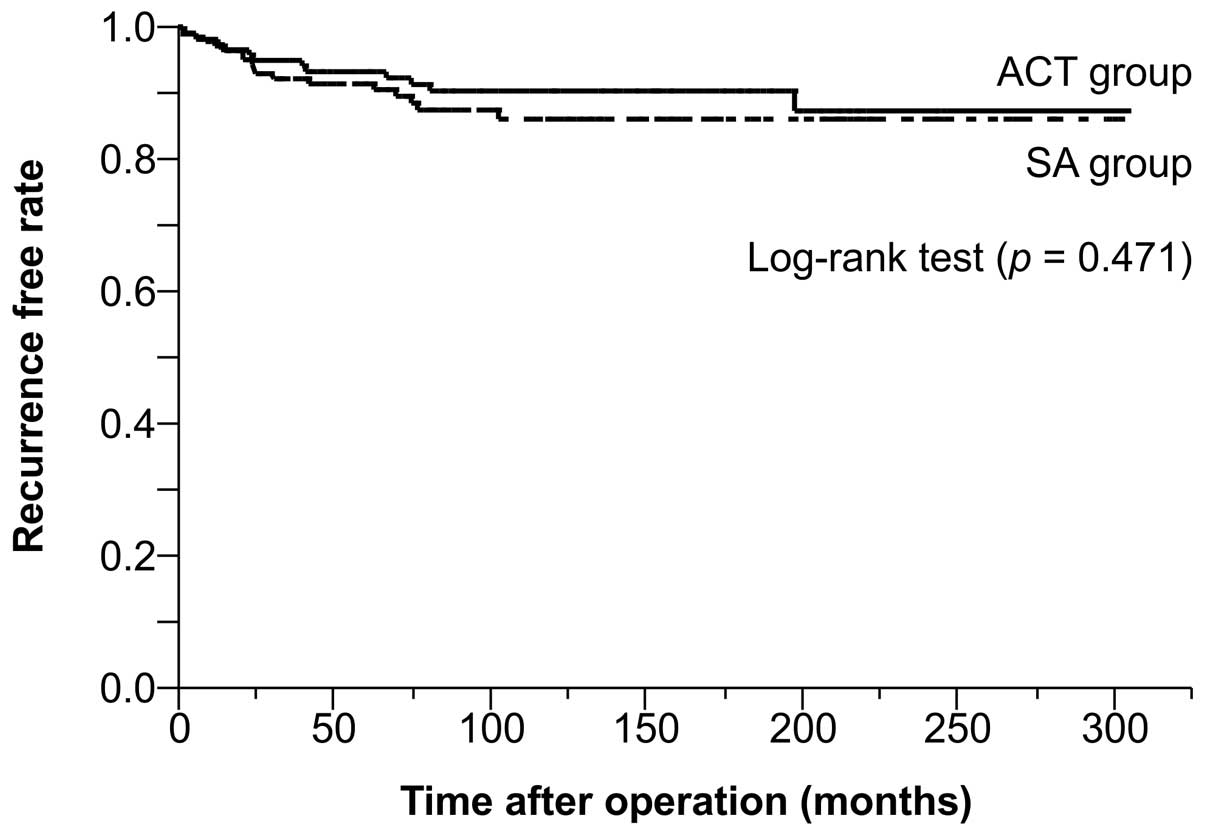
1). A within-group analysis of the low-risk group indicated no
significant differences in the rate of recurrence between the
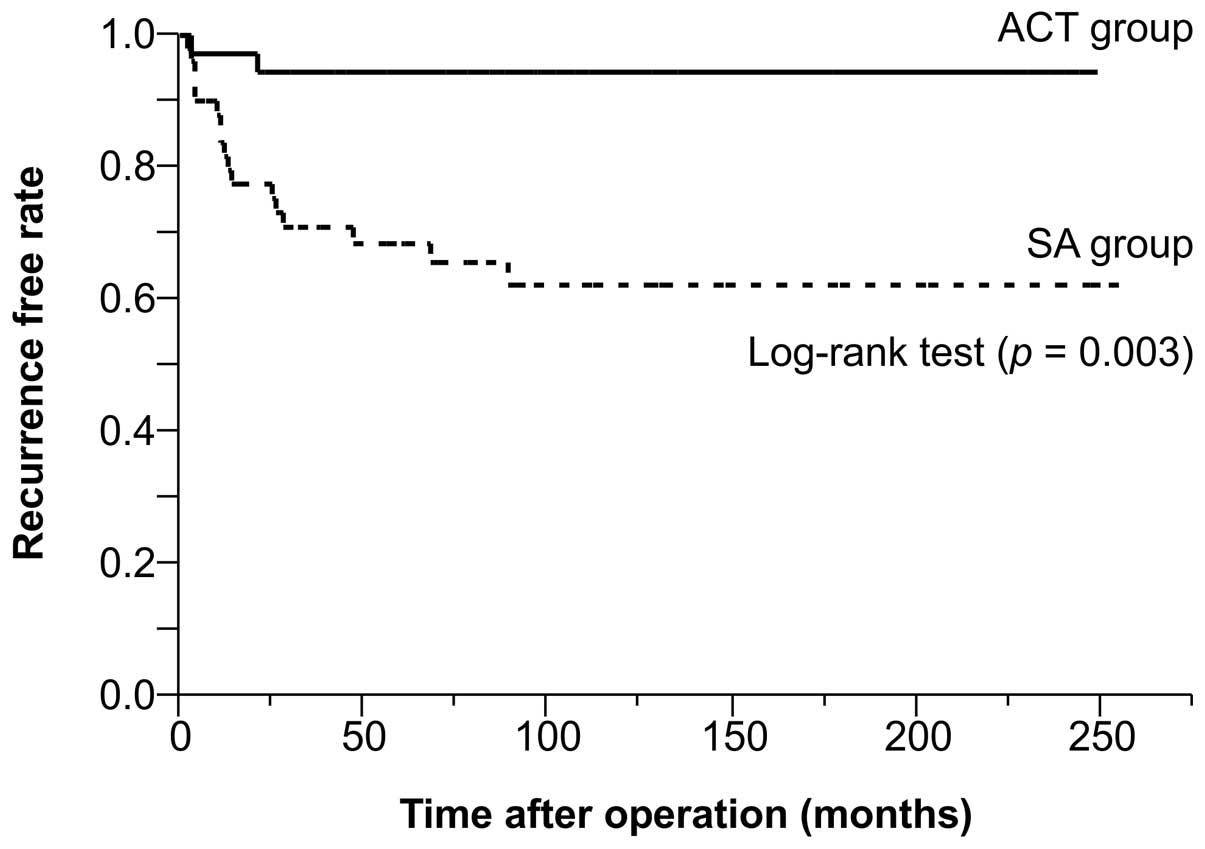
low-risk patients in the ACT and SA groups (Fig. 2). However, the within-group analysis
indicated a significantly lower risk in the high-risk patients in
the ACT group than in the high-risk patients in the SA group
(Fig. 3).
Discussion
Patients with stage II colon cancer who are only
treated with surgery are generally considered to have a better
prognosis if a curative resection is possible. Previous studies
have estimated the recurrence and 5-year OS rates following the
treatment of stage II colon cancer to be 7.9–22 and 75–92.5%,
respectively, and have shown that ~20% of patients experience
recurrence within 5 years (1,12,13).
In Japan, the recurrence and 5-year OS rates are estimated to be
13.3–13.8 and 83.7%, respectively (6,14).
While these rates are comparable with the 12.7 and 82.6% rates
identified for recurrence and OS, respectively, in the present
study, when all the patients were analyzed, the rates did not
reflect the significant differences in the recurrence and 5-year OS
rates between patients in the ACT group (8.6% and 91.0%) and the SA
group (15.9% and 76.1%; P<0.0001). Although the improved
prognosis of the ACT group may have been due to selection bias,
identifying the high-risk factors for recurrence is fundamental for
determining the form of adjuvant therapy that is most likely to be
effective.
Among the various endpoints that may be used in a
study of adjuvant therapy, Punt et al recommended the use of
the disease-free survival (DFS) rate as the primary endpoint and
the TTR as the secondary endpoint (8). However, as the present study aimed to
identify the high-risk factors for recurrence, the TTR was used as
the endpoint instead of DFS or OS rates to exclude the effect of
mortality due to other causes or other cancers. With regard to the
correlation between prognosis and gender, previous studies have
revealed that while there are no significant differences in OS or
disease-specific survival (DSS) rates between male and female colon
cancer patients (15,16), male colon cancer patients have a
poorer prognosis (6,17–19),
experience a significantly higher rate of post-operative mortality
and are more likely to succumb to adverse cardiovascular events
(17). These findings indicate that
colon tumors have the same biological malignant potential in male
and female patients, but that males are more vulnerable to surgical
stress (16). Nevertheless, female
colorectal cancer patients have also been identified to have a poor
prognosis (19), and thus a
consensus remains to be achieved with regard to the correlation
between gender and prognosis. With regard to the correlation
between prognosis and age, elderly patients have been identified to
have low rates of survival, which has been attributed to the
consideration of all mortalities as events and the low rate of
administration of adjuvant therapy to this population (16). However, such attribution is
controversial, as a poor prognosis has also been reported in
younger patients (20).
In the case of all events, gender and age are highly
likely to affect the inherent biological behavior of the patient,
rather than any difference in the biological malignant potential of
the tumor. It is thus unclear whether gender and age should be
considered high-risk factors, simply as statistically significant
differences have been observed between males and females and among
various age groups. Based on this reasoning, the TTR was considered
a more acceptable measure in the present analysis of the risk of
recurrence than the OS or DFS rates, for which gender or age may
have been a confounding risk factor.
In a previous study on stage II colon cancer
patients, the invasive gross tumor type, elevated pre-operative
serum CEA level and lack of adjuvant therapy were identified as
risk factors for a shorter relapse-free survival (7). However, as the patients in the ACT
group had shown a better prognosis than those in the SA group,
regardless of whether the patients presented with a normal or
elevated pre-operative CEA level, the CEA level was considered to
be a significant factor in the assessment of indication for
post-operative ACT. In contrast, a CEA level of twice the cut-off
value and a diagnosis of pre-operative bowel obstruction were
identified as independent factors for a shorter TTR in the present
study.
Previous studies have identified several
pathological factors, including gross tumor type (6,7), depth
of invasion (21–23), tumor histology (23) and vascular invasion (6,21,23),
and surgical factors, including the number of dissected lymph nodes
(6,22), as high-risk factors in stage II
colon cancer. Researchers generally interpret the results that are
associated with the risk factors that are assessed objectively,
including age and gender, in a similar manner. However, the results
with regard to the pathological risk factors that are assessed
subjectively, including tumor histology and vascular invasion, as
well as those regarding the gross tumor type and number of
dissected lymph nodes, may be interpreted in a different manner. To
avoid inconsistent interpretations of the present results, a serum
CEA level of twice the cut-off value and a diagnosis of
pre-operative bowel obstruction, which are indicators that are
relatively objective and reflective of a high risk prior to
surgery, were used in the analysis of the TTR in the present
study.
Several studies have also considered pre-operative
bowel obstruction to be a risk factor for recurrence in stage II
colon cancer patients (23–25). However, as these studies often used
various definitions of pre-operative bowel obstruction, leading to
wide variations in the rate of obstruction of between 9.8 and 47%
(23–26), it is difficult to assess the utility
of this symptom as a risk factor. Pre-operative bowel obstruction
is generally defined using clinical signs, including arrested
flatus/bowel movement, abdominal distension and vomiting and
radiological findings, such as intestinal dilatation (23). The incidence of pre-operative bowel
obstruction among the patients examined in the present study was
7.0%, a lower incidence than reported in previous studies. This
finding may be attributed to the use of a more restrictive and
objective definition of bowel obstruction; specifically, bowel
obstruction was diagnosed if patients required emergency
decompression via placement of a decompression tube or emergency
surgery. This is a more objective means of assessment compared with
the evaluation of clinical signs and radiological findings.
Generally, patients with colon cancer who develop pre-operative
bowel obstructions tend to experience distant metastasis. Although
the mechanism by which metastasis develops is unclear (23), infiltration of micro tumor cells
into the lymphatic vessels or veins and circulation due to
increased intestinal pressure have been hypothesized.
CEA is a glycoprotein that was discovered by Gold
and Freedman in 1965 and is present in the digestive tract of the
fetus and the tumor tissue of endodermally-derived digestive organs
(27). Although CEA was once
considered to be a specific marker for gastrointestinal cancer, it
is now recognized as a more general tumor marker. The pre-operative
CEA level is now known to be associated with prognosis (7,21,28–30).
Although a number of studies have established the cut-off value
between a normal and high CEA level as 5 ng/ml (7,21,28),
others have observed it to range between 10 ng/ml (29) and 15 ng/ml (30). In an experimental model, a
CEA-producing tumor was identified to be more capable of liver
metastasis than a non-CEA-producing tumor (31). Therefore, the higher the CEA level,
the higher the malignant potential of the tumor. Patients with an
elevated pre-operative CEA level may display micrometastases,
particularly in the liver.
The 2006 ASCO Guidelines indicate that a
pre-operative serum CEA level of >5 ng/ml is associated with a
poor prognosis. However, this factor has not been adopted as an
indicator for the assessment of indication for post-operative ACT
due to insufficient data to support its use (32). Although the previous ESMO Clinical
Practice Guidelines indicated a high serum CEA level as a high-risk
factor for stage II colon cancer (33), the latest guidelines do not
(4). As such, it remains
controversial to consider a high CEA level as a high-risk factor or
as an indicator for ACT. Nevertheless, using CEA levels offers the
advantage of objectivity in an assessment, as an objective
indicator is unlikely to be assessed differently by various
interpreters.
Based on these previous findings and indications, we
propose that stage II colon cancer patients who have undergone a
curative resection should be considered to be at a high risk for
recurrence if they present with a pre-operative CEA level of twice
the cut-off value and/or with a pre-operative bowel obstruction. In
the present study, patients in the low-risk group (n=291) who had
presented with neither indicator were shown not have benefited from
adjuvant therapy. In contrast, the patients in the high-risk group
(n=86), who presented with one or both indicators and had undergone
adjuvant therapy were shown to have experienced a significantly
improved prognosis than those in the high-risk group who had not
undergone adjuvant therapy; this was manifested by the 5-year
recurrence-free rate of 94.4% observed in the ACT group compared
with 71.1% identified in the AS group. In accordance with the
proposal and results of the present study, patients who present
with a pre-operative serum CEA level of twice the cut-off value or
with a pre-operative bowel obstruction should be considered as
candidates for adjuvant chemotherapy. Future studies into the
prognostic risk factors for stage II colon cancer should examine
the validity of this proposal by conducting a prospective
investigation of the benefit of ACT for stage II colon cancer
patients who present with the two proposed high-risk factors.
Further research should also build on the findings using standard
procedures at a single institution to obtain objective data to
examine the correlation between prognosis and a variety of
clinicopathological factors.
References
|
1
|
No authors listed. Efficacy of adjuvant
fluorouracil and folinic acid in B2 colon cancer: International
Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2)
Investigators. J Clin Oncol. 17:1356–1363. 1999.PubMed/NCBI
|
|
2
|
Moertel CG, Fleming TR, Macdonald JS, et
al: Intergroup study of fluorouracil plus levamisole as adjuvant
therapy for stage II/Dukes’ B2 colon cancer. J Clin Oncol.
13:2936–2943. 1995.PubMed/NCBI
|
|
3
|
Benson AB 3rd, Schrag D, Somerfield MR, et
al: American Society of Clinical Oncology recommendations on
adjuvant chemotherapy for stage II colon cancer. J Clin Oncol.
22:3408–3419. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Labianca R, Nordlinger B, Beretta GD,
Brouquet A and Cervantes A; ESMO Guidelines Working Group. Primary
colon cancer: ESMO Clinical Practice Guidelines for diagnosis,
adjuvant treatment and follow-up. Ann Oncol. 21(Suppl 5): v70–v77.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Watanabe T, Itabashi M, Shimada Y, et al;
Japanese Society for Cancer of the Colon and Rectum. Japanese
Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010
for the treatment of colorectal cancer. Int J Oncol. 1–29. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sato H, Maeda K, Sugihara K, et al:
High-risk stage II colon cancer after curative resection. J Surg
Oncol. 104:45–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ogata Y, Murakami H, Sasatomi T, et al:
Elevated preoperative serum carcinoembrionic antigen level may be
an effective indicator for needing adjuvant chemotherapy after
potentially curative resection of stage II colon cancer. J Surg
Oncol. 99:65–70. 2009. View Article : Google Scholar
|
|
8
|
Punt CJ, Buyse M, Köhne CH, et al:
Endpoints in adjuvant treatment trials: a systematic review of the
literature in colon cancer and proposed definitions for future
trials. J Natl Cancer Inst. 99:998–1003. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumours. 7th edition.
Wiley-Blackwell; New York, NY: 2009
|
|
10
|
Shirouzu K, Isomoto H, Morodomi T and
Kakegawa T: Carcinomatous lymphatic permeation. Prognostic
significance in patients with rectal carcinoma - a long term
prospective study. Cancer. 75:4–10. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shirouzu K, Isomoto H, Kakegawa T and
Morimatsu M: A prospective clinicopathologic study of venous
invasion in colorectal cancer. Am J Surg. 162:216–222. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schrag D, Rifas-Shiman S, Saltz L, Bach PB
and Begg CB: Adjuvant chemotherapy use for Medicare beneficiaries
with stage II colon cancer. J Clin Oncol. 20:3999–4005. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gertler R, Rosenberg R, Schuster T and
Friess H: Defining a high-risk subgroup with colon cancer stages I
and II for possible adjuvant therapy. Eur J Cancer. 45:2992–2999.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kobayashi H, Mochizuki H, Sugihara K, et
al: Characteristics of recurrence and surveillance tools after
curative resection for colorectal cancer: a multicenter study.
Surgery. 141:67–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Manfredi S, Bouvier AM, Lepage C, Hatem C,
Dancourt V and Faivre J: Incidence and patterns of recurrence after
resection for cure of colonic cancer in a well defined population.
Br J Surg. 93:1115–1122. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
van Leeuwen BL, Påhlman L, Gunnarsson U,
Sjövall A and Martling A: The effect of age and gender on outcome
after treatment for colon carcinoma. A population-based study in
the Uppsala and Stockholm region. Crit Rev Oncol Hematol.
67:229–236. 2008.PubMed/NCBI
|
|
17
|
McArdle CS, McMillan DC and Hole DJ: Male
gender adversely affects survival following surgery for colorectal
cancer. Br J Surg. 90:711–715. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wichmann MW, Müller C, Hornung HM,
Lau-Werner U and Schildberg FW; Colorectal Cancer Study Group.
Gender differences in long-term survival of patients with
colorectal cancer. Br J Surg. 88:1092–1098. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oñate-Ocaña LF, Montesdeoca R,
López-Graniel CM, et al: Identification of patients with high-risk
lymph node-negative colorectal cancer and potential benefit from
adjuvant chemotherapy. Jpn J Clin Oncol. 34:323–328.
2004.PubMed/NCBI
|
|
20
|
Chou CL, Chang SC, Lin TC, et al:
Differences in clinicopathological characteristics of colorectal
cancer between younger and elderly patients: an analysis of 322
patients from a single institution. Am J Surg. 202:574–582. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Quah HM, Chou JF, Gonen M, et al:
Identification of patients with high-risk stage II colon cancer for
adjuvant therapy. Dis Colon Rectum. 51:503–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burdy G, Panis Y, Alves A, Nemeth J,
Lavergne-Slove A and Valleur P: Identifying patients with T3–T4
node-negative colon cancer at high risk of recurrence. Dis Colon
Rectum. 44:1682–1688. 2001.
|
|
23
|
Lin CC, Lin JK, Chang SC, et al: Is
adjuvant chemotherapy beneficial to high risk stage II colon
cancer? Analysis in a single institute. Int J Colorectal Dis.
24:665–676. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mulcahy HE, Toner M, Patchett SE, Daly L
and O’Donoghue DP: Identifying stage B colorectal cancer patients
at high risk of tumor recurrence and death. Dis Colon Rectum.
40:326–331. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chin CC, Wang JY, Changchien CR, Huang WS
and Tang R: Carcinoma obstruction of the proximal colon cancer and
long-term prognosis - obstruction is a predictor of worse outcome
in TNM stage II tumor. Int J Colorectal Dis. 25:817–822. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Katoh H, Yamashita K, Wang G, Sato T,
Nakamura T and Watanabe M: Prognostic significance of preoperative
bowel obstruction in stage III colorectal cancer. Ann Surg Oncol.
18:2432–2441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gold P and Freedman SO: Demonstration of
tumor-specific antigens in human colonic carcinomata by
immunological tolerance and absorption techniques. J Exp Med.
121:439–462. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harrison LE, Guillem JG, Paty P and Cohen
AM: Preoperative carcinoembryonic antigen predicts outcomes in
node-negative colon cancer patients: a multivariate analysis of 572
patients. J Am Coll Surg. 185:55–59. 1997. View Article : Google Scholar
|
|
29
|
Wolmark N, Fisher B, Wieand HS, et al: The
prognostic significance of preoperative carcinoembryonic antigen
levels in colorectal cancer. Results from NSABP (National Surgical
Adjuvant Breast and Bowel Project) clinical trials. Ann Surg.
199:375–382. 1984. View Article : Google Scholar
|
|
30
|
Sener SF, Imperato JP, Chmiel J, Fremgen A
and Sylvester J: The use of cancer registry data to study
preoperative carcinoembryonic antigen level as an indicator of
survival in colorectal cancer. CA Cancer J Clin. 39:50–57. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wagner HE, Toth CA, Steele GD Jr and
Thomas P: Metastatic potential of human colon cancer cell lines:
relationship to cellular differentiation and carcinoembryonic
antigen production. Clin Exp Metastasis. 10:25–31. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Locker GY, Hamilton S, Harris J, et al;
ASCO. ASCO 2006 update of recommendations for the use of tumor
markers in gastrointestinal cancer. J Clin Oncol. 24:5313–5327.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Van Cutsem EJ and Oliveira J; ESMO
Guidelines Working Group. Colon cancer: ESMO clinical
recommendations for diagnosis, adjuvant treatment and follow-up.
Ann Oncol. 19(Suppl 2): ii29–ii30. 2008.
|