Introduction
Lung cancer is mostly diagnosed at an advanced stage
and is the leading cause of mortality caused by malignancies
worldwide (1,2). Of all types of lung tumors, non-small
cell lung cancer (NSCLC) accounts for ~80%, with adenocarcinoma as
the most prevalent subtype (3,4).
Furthermore, NSCLC, particularly lung adenocarcinoma, often
presents with pleural metastasis and even malignant pleural
effusion (MPE) at the time of diagnosis, which is an indicator of
poor prognosis for patients (1).
Although the pleural metastasis of lung adenocarcinoma occurs with
high frequency and indicates a poor prognosis, the underlying
genetic mechanism remains largely unclear.
Epidermal growth factor receptor (EGFR), a
transmembrane glycoprotein with tyrosine kinase activity, is a
major regulator of several signaling pathways (5). EGFR activation and/or overexpression
often leads to signal transduction cascades, which in turn
contribute to cell proliferation, angiogenesis, cancer invasion and
metastasis (6). In humans, EGFR is
frequently overexpressed in 50–81% of NSCLC and such overexpression
has been demonstrated to be associated with cancer susceptibility,
metastasis, survival prognosis and chemotherapy response (7–14).
Numerous mutations and variants in the EGFR gene have also
been characterized in human lung tumors, of which a number have
been demonstrated to be associated with EGFR overexpression or
activation (7–9). EGFR mutations, which are mostly
limited to the first four exons, occur more often in lung cancer
patients with adenocarcinoma histology, Asian origin, female gender
and a non-smoking background (13,15).
Additionally, several functional variants in the EGFR gene,
including CA-SSR1 (CA repeat in intron 1 of EGFR), -216G/T and
R497K, have also been detected with higher frequency in lung
cancer, as well as other tumors, and these variants often result in
increased promoter activity and EGFR transcription (16–18).
Therefore, it has been proposed that genetic alterations in the
EGFR gene may be associated with the development and metastasis of
lung cancers (11,12,19).
-216G/T (rs712829), a functional polymorphism in the
EGFR promoter, is located in the Sp1 recognition site where
multiple protein factors and transcriptional start sites have been
identified (20–22). Since the Sp1 binding site is a
region that is critical for the regulation of EGFR
transcription (23–25), the replacement of G by T at position
-216 increases promoter activity by 30%, thereby resulting in a
higher EGFR expression level (18,22,26).
In clinical studies, it has been shown that -216G/T may be
associated with inherited susceptibility to cancers, as well as
other common diseases (22,27). Furthermore, studies have also
observed that -216G/T was able to predict drug response and that
the NSCLC patients with at least one -216T allele exhibited
significant improvements with regard to the effects of gefitinib
treatment on survival time (28,29).
Although evidence indicates that -216G/T may be correlated with the
development, treatment response and survival prognosis of lung
cancer patients, its role in cancer metastasis remains largely
unknown.
Based on previous findings, we proposed that -216G/T
in the EGFR promoter may be associated with an increased
risk of the pleural metastasis of lung adenocarcinoma. Therefore,
in the present study, -216G/T genotyping and immunohistochemical
detection of EGFR protein expression was performed in two
cohorts of patients with primary lung adenocarcinoma and pleural
metastasis respectively, with the aim of determining the
association between -216G/T variants in the EGFR gene and
the risk of the pleural metastasis of lung adenocarcinoma.
Materials and methods
Patient information
A total of 638 patients, including 326 cases of
primary lung adenocarcinoma and 312 matched cases with pleural
metastasis, were enrolled into the study between May 2008 and April
2011 at Shandong Provincial Hospital (Shandong, China). All the
subjects enrolled in the study were at stage IV according to the
revised TNM staging system for NSCLC announced by the International
Association for the Study of Lung Cancer (IASLC) (30). The diagnoses for all the patients,
including that of pleural metastasis, were confirmed by
pathological and/or cytological examination. The clinical
information from these patients, including age, gender, smoking
history, cancer stage and pathology/cytology examination result,
was recorded. The enrolled patients were categorized into smokers
and those who had never smoked according to their smoking history.
The detailed characteristics of all the patients are listed in
Table I. This study was approved by
the institutional review board of Shandong Provincial Hospital and
informed consent was obtained from all patients.
 | Table IClinical characteristics of the
patients. |
Table I
Clinical characteristics of the
patients.
| Clinicopathological
parameters | Primary lung
adenocarcinoma (n=326) | Pleural metastasis
(n=312) | P-valuea |
|---|
|
|
|---|
| No. of cases | % | No. of cases | % |
|---|
| Age (years) | | | | | 0.335 |
| <60 | 191 | 58.5 | 171 | 54.8 | |
| ≥60 | 135 | 41.5 | 141 | 45.2 | |
| Gender | | | | | 0.473 |
| Male | 166 | 50.9 | 150 | 48.1 | |
| Female | 160 | 49.1 | 162 | 51.9 | |
| Smoking status | | | | | 0.583 |
| Never | 180 | 55.2 | 179 | 57.4 | |
| Smoker | 146 | 44.8 | 133 | 42.6 | |
| Differentiation
grade | | | | | 0.434 |
| Well | 36 | 11.0 | 42 | 13.5 | 0.225 |
| Moderate | 143 | 43.9 | 122 | 39.1 | |
| Poor | 147 | 45.1 | 148 | 47.4 | |
Sample preparation
Peripheral blood samples were collected
consecutively from all the enrolled patients and tissue samples
were also obtained from a number who had received a bronchoscopic
biopsy during diagnosis. All blood samples were retained for the
genotyping of genetic variants in the EGFR gene and the tissues for
evaluating EGFR expression. The peripheral blood was collected into
EDTA-coated tubes and DNA was extracted with a commercial DNA
extraction kit (Keyuan Biotechnology Development Center, Beijing,
China) according to the manufacturer’s instructions. Tissue samples
were routinely fixed in 10% buffered formalin and embedded in
paraffin for diagnosis and the examination of EGFR protein
expression.
-216G/T genotyping
PCR applications of the -216G/T variants in the
EGFR gene were performed with the forward,
5′-GCTTGGTCCTCTTCGGCATCT-3′ and reverse, 5′-CCGTCTTGACCAGTCGCTTA-3′
primers. The PCR reaction was set up in a 50 μl volume containing
25 μl Master Mix (Tiangen Biotech Company, Beijing, China), 2 μl
forward and reverse primers, 25 ng/4 μl DNA template and 19 μl
nuclease-free water. PCR reactions were run with the following
cycling conditions: pre-denaturation at 94°C for 5 min,
denaturation at 94°C for 30 sec, annealing from 68 to 60°C
decreasing at 1°C/cycle for 8 cycles and at 59°C for 30 cycles,
extension at 72°C for 30 sec and a final extension for 7 min, with
a total of 38 cycles. The PCR products were sequenced directly in
the sense and antisense directions using an ABI373 instrument
(Applied Biosystems, Foster City, CA, USA).
Immunohistochemical staining
Paraffin-embedded tissues were subjected to
immunohistochemical staining with EGFR antibody using a
streptavidin-biotin immunoperoxidase kit (BioGenex, Fremont, CA,
USA) according to the manufacturer’s instructions. In brief,
following antigen retrieval and blocking of endogenous peroxidase
activity, tissue slides (5 μm) were incubated with EGFR monoclonal
antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at a
1:500 dilution, overnight at 4°C in a moist chamber. Subsequent to
being washed in PBS, the slides were sequentially incubated with
the secondary antibody for 45 min at room temperature, stained with
diaminobenzidine tetrahydrochloride (DAB) and finally
counterstained with hematoxylin. Staining without the primary
antibody was employed to create a negative control.
The level of EGFR expression was evaluated by
multiplying the positive cell rate and staining intensity, as
reported in previous studies (31,32).
In brief, positive cell rates of 0, 1–10, 11–50 and 51–100% were
scored as 0, 1, 2, 3 and 4, respectively, while staining intensity
grades of 0, 1, 2 and 3 referred to negative, weak positive,
moderately positive and markedly positive staining for EGFR,
respectively, as described previously (33). EGFR expression was assessed by two
independent investigators who were blinded to the clinical data.
Discrepancies were solved by discussion.
Statistical analysis
All statistical analyses were performed using the
SPSS 10.0 statistical software package (SPSS, Inc., Chicago, IL,
USA). The categorical variables were analyzed using the
χ2 test and Fisher’s exact test, as appropriate. Odds
ratios (ORs) and their 95% confidence intervals (CIs) were
estimated and adjusted by logistic regression analysis for the
clinicopathological factors. EGFR expression data were analyzed
statistically with the Mann-Whitney U test. A two-sided value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical characteristics of the
patients
In total, 638 patients were enrolled in the present
study, including 326 cases of primary lung adenocarcinoma and 312
matched cases with pleural metastasis. The characteristics of the
enrolled subjects are presented in Table I. Between the primary lung
adenocarcinoma and metastatic groups, the distributions of
clinicopathological factors were not significantly different
(Table I). The χ2 test
showed that the genotype distribution of -216G/T was in agreement
with the Hardy-Weinberg equilibrium (P>0.05) in the two
groups.
Genotype/allele frequencies of
-216G/T
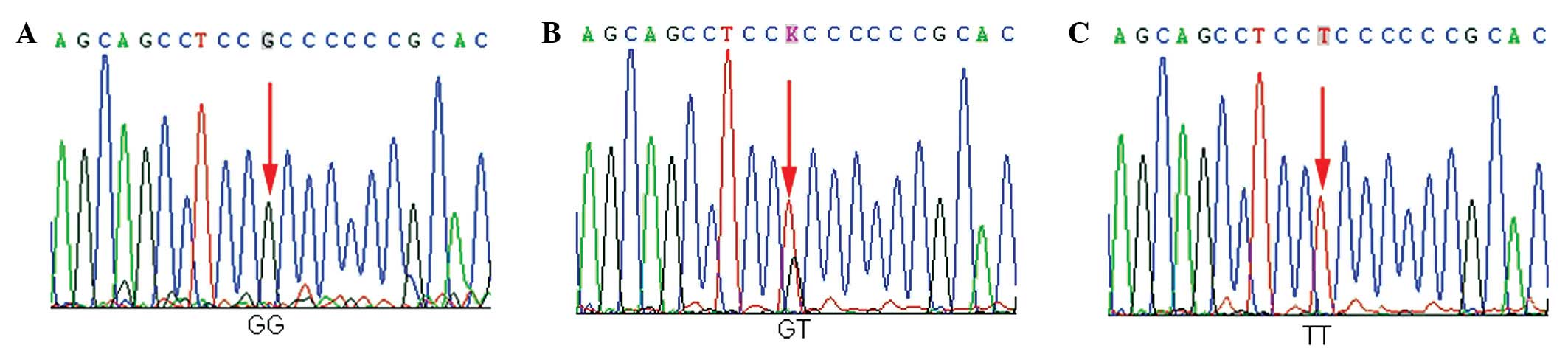
The three genotypes of -216 G/T in EGFR,
namely G/G, G/T and T/T, were detected among the subjects from the
Chinese population. Each genotype is demonstrated by a
representative sequencing wave figure in Fig. 1. The genotype and allele frequencies
of -216G/T in primary lung adenocarcinoma and pleural metastasis
are described in Table II. The
minor allele, T, was detected in 32.85% of the chromosomes in
patients with pleural metastasis, which was a significantly higher
rate than the 23.93% in patients with primary lung adenocarcinoma
(OR, 1.56; 95% CI, 1.217–1.989; P=0.000; Table II). Similarly, the genotype
frequencies of GT and TT in pleural metastasis were significantly
higher compared with those in primary lung adenocarcinoma, with ORs
of 1.39 (95% CI, 1.003–1.994) and 1.46 (95% CI, 1.015–1.963),
respectively (Table II).
Furthermore, following adjustment for the clinicopathological
variables using logistic regression analysis, the adjusted ORs were
1.46 (95% CI, 1.015–1.963) for G/T and 1.97 (95% CI, 1.051–3.152)
for T/T (Table II).
 | Table IIAssociation of -216G/T
genotype/allele frequencies in EGFR with the risk of pleural
metastasis of lung adenocarcinoma. |
Table II
Association of -216G/T
genotype/allele frequencies in EGFR with the risk of pleural
metastasis of lung adenocarcinoma.
| Primary lung
adenocarcinoma (n=326) | Pleural metastasis
(n=312) | | |
|---|
|
|
| | |
|---|
|
Genotype/allele | na | % | na | % | OR (95% CI) | Adjusted OR (95%
CI)b |
|---|
| Genotype |
| GG | 194 | 59.51 | 146 | 46.79 | | |
| GT | 108 | 33.13 | 127 | 40.71 | 1.39
(1.003–1.914) | 1.46
(1.015–1.963) |
| TT | 24 | 7.36 | 39 | 12.50 | 1.80
(1.054–3.067) | 1.97
(1.051–3.152) |
| Allele |
| G | 496 | 76.07 | 419 | 67.15 | | |
| T | 156 | 23.93 | 205 | 32.85 | 1.56
(1.217–1.989) | |
EGFR expression
It has been demonstrated experimentally that -216G/T
variants result in EGFR activation and thereby increased
EGFR expression, suggesting that there may be potential differences
in EGFR expression among individuals with the various -216G/T
genotypes. In line with such a concept, EGFR expression was
assessed in the present study in the primary lung adenocarcinoma
tissues of various -216G/T genotypes by immunohistochemical
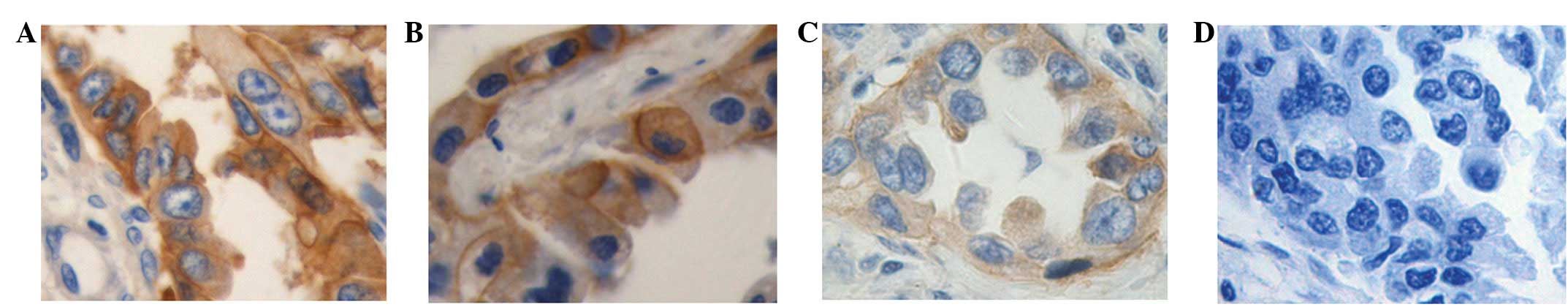
staining. The immunohistochemical staining was performed in the
tumor tissues of primary lung adenocarcinoma, which included 21
cases with the G/G genotype, 22 cases with the G/T genotype and 19
cases with the T/T genotype. As shown in Fig. 2, the diffuse/intense brown staining
represented the positive expression of the EGFR protein. The EGFR
expression scores were 10 for the G/T genotype and 7 for the T/T
genotype, each being significantly higher than the score of 3 for
the G/G genotype (P<0.05).
Discussion
Although a number of mutations/variants in the
EGFR gene have been demonstrated to be associated with the
development and metastasis of lung cancer (9,10), it
remains largely unclear whether -216G/T, a functional variant in
the EGFR promoter, has any critical role in the pleural
metastasis of lung adenocarcinoma. In the present study, the
-216G/T genotypes of G/T and T/T were detected in patients with
pleural metastasis at higher frequencies compared with cases of
primary lung adenocarcinoma, and the expression of the EGFR protein
was also increased significantly in the former group compared with
the latter. All these results collectively indicate that -216G/T is
associated with the pleural metastasis of lung adenocarcinoma,
possibly by affecting EGFR overexpression.
To the best of our knowledge, the present study
demonstrates for the first time that 216G/T and T/T are associated
with an increased risk for the pleural metastasis of lung
adenocarcinoma. Several other studies, although different in
certain aspects from the present study of germline -216G/T
variants, have also documented the associations of somatic
mutations in EGFR with pleural metastasis of lung cancer.
One study reported that the rate of somatic mutations in
EGFR was significantly higher in lung cancer patients with
pleural metastasis compared with patients without metastasis (68.4
vs. 50.5%) (34). Another study
observed that somatic mutations of EGFR were discordant
between primary tumors and corresponding pleural metastases in a
significant portion of lung adenocarcinomas, although the mutation
frequency was higher in primary lesions compared with pleural
metastases (35). The reasons for
such contrary results remain unknown at present. More recently, a
study noted that de4 EGFR, a novel EGFR variant with
aberrant splicing of exon 4, exhibited a higher level of metastasis
promoting activity in comparison with the wild-type (36). Therefore, together, the evidence
from the present and previous studies suggests that EGFR
mutations/variants may be involved in the process of the pleural
metastasis of lung cancer, although with certain inconsistencies
between various studies.
-216G/T is located in the essential region of the
EGFR promoter and the G to T allele transition at this loci
leads to increased EGFR transcription by causing the binding
of Sp1 and promoter activity (22,24).
Therefore, EGFR expression was examined in lung adenocarcinoma
patients with various genotypes in order to further clarify the
potential molecular mechanism underlying the pleural metastasis
associated with the -216G/T variants. The present study showed that
T/T and G/T were associated with an increase in EGFR expression
compared with G/G, indicating that -216G/T variants may contribute,
at least partially, to the promoter activity and thereby the
variability of EGFR expression in lung tumor cells. Thus, it is
rational to deduce that EGFR overexpression due to -216G/T variants
is likely to promote the pleural metastasis of lung cancer. Several
other studies have also indicated a critical role for EGFR
overexpression in the metastasis of lung adenocarcinoma. Clinical
studies have shown that the elevated serum levels or overexpression
of the EGFR protein were associated with the aggressiveness and
metastasis of NSCLC (37).
Moreover, EGFR overexpression due to polymorphisms has been
observed in several other types of tumors, including breast and
gastrointestinal cancer (29,34–36),
and EGFR inhibitors were able to inhibit the metastasis and
invasiveness of tumor cells, including lung cancers, even at a low
dose that had no significant effect on primary tumor growth
(38,39). Therefore, the majority of clinical
studies have demonstrated that the pleural metastasis of lung
cancer was closely associated with the overexpression of EGFR,
although certain others have reported contrary results (40). Consistent with the majority of
clinical studies, experimental studies have also shown that the
activation of the EGFR pathway was likely to be involved in the
process of cancer metastasis (41),
while EGFR overexpression promoted the metastasis of several types
of tumor cells (42–44).
Ethnic differences in the distributions of
EGFR mutations and polymorphisms have been identified
between Asian and Caucasian individuals and are considered to be
responsible for the ethnic differences in clinical responses to
EGFR inhibitor treatment (45–48).
Asian ethnicity is known to be a predictor of a good clinical
response to EGFR inhibitors and is associated with a high incidence
of EGFR mutations (45,46).
Similarly, ethnic differences are also evident in the frequency of
-216G/T variants. Previous studies have reported that the
heterogeneous -216G/T and the minor allele, T, were common in
African American and Caucasian populations, but less frequent in
Asian individuals (18,22). In the present study, a low frequency
of T/T was observed in a Chinese population, similar to studies
reported in Asian populations, which included Chinese individuals
(18,49).
The present findings may have certain clinical
implications. EGFR mutations are now attractive targets for
the treatment and prevention of lung cancer. Studies have shown
that somatic mutations in the EGFR tyrosine kinase domain are
associated with an advanced stage, poor prognosis, survival outcome
and clinical response of NSCLC to EGFR inhibitors (50–52).
Thus, -216G/T, a germline variant loci, may also contribute to the
variability in biological characteristics and treatment response to
EGFR inhibitors and could be used as a predictive biomarker.
However, it should be noted that in contrast to the majority of
somatic mutations reported previously, the clinical implications of
this less frequent variant of -216 G/T remain largely unknown and
require further investigation.
In conclusion, the present study demonstrated for
the first time that the -216G/T polymorphism in the EGFR
promoter is a genetic susceptibility factor for the pleural
metastasis of lung adenocarcinoma in a Chinese population, with the
T allele and G/T and T/T genotypes being associated with increased
metastatic risk. Additional studies are required to confirm these
conclusions in other populations due to the evident ethnic
differences with regard to EGFR mutations/variants.
Acknowledgements
The authors would like to thank Dr Weixia Ma and
others (Department of Cardiothoracic Surgery and the Thoracoscopy
Division and Bronchoscopy Room, Shandong Provincial Hospital) for
their help in collecting the samples. The present study was
supported by the National Natural Science Foundation of China
(30472203), the Science and Technology Planning Project of Shandong
Province (2004BS02006) and a grant from the Department of Health of
Shandong Province (2005HW098).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Govindan R, Page N, Morgensztern D, Read
W, Tierney R, Vlahiotis A, et al: Changing epidemiology of
small-cell lung cancer in the United States over the last 30 years:
analysis of the surveillance, epidemiologic, and end results
database. J Clin Oncol. 24:4539–4544. 2006.PubMed/NCBI
|
|
3
|
Wahbah M, Boroumand N, Castro C, El-Zeky F
and Eltorky M: Changing trends in the distribution of the
histologic types of lung cancer: a review of 4,439 cases. Ann Diagn
Pathol. 11:89–96. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kerr KM: Pulmonary adenocarcinomas:
classification and reporting. Histopathology. 54:12–27. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharma SV, Bell DW, Settleman J and Haber
DA: Epidermal growth factor receptor mutations in lung cancer. Nat
Rev Cancer. 7:169–181. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Selvaggi G, Novello S, Torri V, Leonardo
E, De Giuli P, Borasio P, et al: Epidermal growth factor receptor
overexpression correlates with a poor prognosis in completely
resected non-small-cell lung cancer. Ann Oncol. 15:28–32. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fontanini G, Vignati S, Bigini D, Mussi A,
Lucchi H, Angeletti CA, et al: Epidermal growth factor receptor
(EGFr) expression in non-small cell lung carcinomas correlates with
metastatic involvement of hilar and mediastinal lymph nodes in the
squamous subtype. Eur J Cancer. 31A:178–183. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rao C, Hu Q, Ma J, Li J, Zhang C, Shen L
and Wei Q: Comparison of the epidermal growth factor receptor
protein expression between primary non-small cell lung cancer and
paired lymph node metastases: implications for targeted nuclide
radiotherapy. J Exp Clin Cancer Res. 29:72010. View Article : Google Scholar
|
|
10
|
Veale D, Kerr N, Gibson GJ, Kelly PJ and
Harris AL: The relationship of quantitative epidermal growth factor
receptor expression in non-small cell lung cancer to long term
survival. Br J Cancer. 68:162–165. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scagliotti GV, Selvaggi G, Novello S and
Hirsch FR: The biology of epidermal growth factor receptor in lung
cancer. Clin Cancer Res. 10:4227s–4232s. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jänne PA, Engelman JA and Johnson BE:
Epidermal growth factor receptor mutations in non-small-cell lung
cancer: implications for treatment and tumor biology. J Clin Oncol.
23:3227–3234. 2005.PubMed/NCBI
|
|
13
|
Eberhard DA, Giaccone G and Johnson BE;
Non-Small-Cell Lung Cancer Working Group. Biomarkers of response to
epidermal growth factor receptor inhibitors in Non-Small-Cell Lung
Cancer Working Group: standardization for use in the clinical trial
setting. J Clin Oncol. 26:983–994. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pao W and Chmielecki J: Rational,
biologically based treatment of EGFR-mutant non-small-cell lung
cancer. Nat Rev Cancer. 10:760–774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rowinsky EK: The erbB family: targets for
therapeutic development against cancer and therapeutic strategies
using monoclonal antibodies and tyrosine kinase inhibitors. Annu
Rev Med. 55:433–457. 2004. View Article : Google Scholar
|
|
16
|
Araújo A, Ribeiro R, Azevedo I, Coelho A,
Soares M, Sousa B, et al: Genetic polymorphisms of the epidermal
growth factor and related receptor in non-small cell lung cancer -
a review of the literature. Oncologist. 12:201–210. 2007.PubMed/NCBI
|
|
17
|
Choi JE, Park SH, Kim KM, Lee WK, Kam S,
Cha SI, et al: Polymorphisms in the epidermal growth factor
receptor gene and the risk of primary lung cancer: a case-control
study. BMC Cancer. 7:1992007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nomura M, Shigematsu H, Li L, Suzuki M,
Takahashi T, Estess P, et al: Polymorphisms, mutations, and
amplification of the EGFR gene in non-small cell lung cancers. PLoS
Med. 4:e1252007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kosaka T, Yatabe Y, Endoh H, Kuwano H,
Takahashi T and Mitsudomi T: Mutations of the epidermal growth
factor receptor gene in lung cancer: biological and clinical
implications. Cancer Res. 64:8919–8923. 2004. View Article : Google Scholar
|
|
20
|
Kageyama R, Merlino GT and Pastan I: A
transcription factor active on the epidermal growth factor receptor
gene. Proc Natl Acad Sci USA. 85:5016–5020. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen LL, Clawson ML, Bilgrami S and
Carmichael G: A sequence-specific single-stranded DNA-binding
protein that is responsive to epidermal growth factor recognizes an
S1 nuclease-sensitive region in the epidermal growth factor
receptor promoter. Cell Growth Differ. 4:975–983. 1993.
|
|
22
|
Liu W, Innocenti F, Wu MH, Desai AA, Dolan
ME, Cook EH Jr and Ratain MJ: A functional common polymorphism in a
Sp1 recognition site of the epidermal growth factor receptor gene
promoter. Cancer Res. 65:46–53. 2005.PubMed/NCBI
|
|
23
|
Johnson AC, Ishii S, Jinno Y, Pastan I and
Merlino GT: Epidermal growth factor receptor gene promoter.
Deletion analysis and identification of nuclear protein binding
sites. J Biol Chem. 263:5693–5699. 1988.PubMed/NCBI
|
|
24
|
Kageyama R, Merlino GT and Pastan I:
Epidermal growth factor (EGF) receptor gene transcription.
Requirement for Sp1 and an EGF receptor-specific factor. J Biol
Chem. 263:6329–6336. 1988.PubMed/NCBI
|
|
25
|
Grinstein E, Jundt F, Weinert I, Wernet P
and Royer HD: Sp1 as G1 cell cycle phase specific transcription
factor in epithelial cells. Oncogene. 21:1485–1492. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McKibbin T, Zhao W, Tagen M, Daw NC,
Furman WL, McGregor LM, et al: Epidermal growth factor receptor
polymorphisms and risk for toxicity in paediatric patients treated
with gefitinib. Eur J Cancer. 46:2045–2051. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bandrés E, Barricarte R, Cantero C,
Honorato B, Malumbres R, Zárate R, et al: Epidermal growth factor
receptor (EGFR) polymorphisms and survival in head and neck cancer
patients. Oral Oncol. 43:713–719. 2007.
|
|
28
|
Liu G, Gurubhagavatula S, Zhou W, Wang Z,
Yeap BY, Asomaning K, et al: Epidermal growth factor receptor
polymorphisms and clinical outcomes in non-small-cell lung cancer
patients treated with gefitinib. Pharmacogenomics J. 8:129–138.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gregorc V, Hidalgo M, Spreafico A, Cusatis
G, Ludovini V, Ingersoll RG, et al: Germline polymorphisms in EGFR
and survival in patients with lung cancer receiving gefitinib. Clin
Pharmacol Ther. 83:477–484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shepherd FA, Crowley J, Van Houtte P, et
al: The international Association For the Study of Lung Cancer
Staging Project: proposals regarding the clinical staging of small
cell lung cancer in the forthcoming (seventh) edition of the tumor
node, metastasis classification for lung cancer. J Thorac Oncol.
2:1067–1077. 2007. View Article : Google Scholar
|
|
31
|
Nagashio R, Sato Y, Matsumoto T, Kageyama
T, Satoh Y, Shinichiro R, et al: Expression of RACK1 is a novel
biomarker in pulmonary adenocarcinomas. Lung Cancer. 69:54–59.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kato T, Daigo Y, Aragaki M, Ishikawa K,
Sato M, Kondo S and Kaji M: Overexpression of MAD2 predicts
clinical outcome in primary lung cancer patients. Lung Cancer.
74:124–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu SG, Chang YL, Lin JW, et al: Including
total EGFR staining in scoring improves EGFR mutations detection by
mutation-specific antibodies and EGFR TKIs response prediction.
Plos One. 6:e233032011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu SG, Gow CH, Yu CJ, Chang YL, Yang CH,
Hsu YC, et al: Frequent epidermal growth factor receptor gene
mutations in malignant pleural effusion of lung adenocarcinoma. Eur
Respir J. 32:924–930. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han HS, Eom DW, Kim JH, Kim KH, Shin HM,
An JY, et al: EGFR mutation status in primary lung adenocarcinomas
and corresponding metastatic lesions: discordance in pleural
metastases. Clin Lung Cancer. 12:380–386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang H, Zhou M, Shi B, Zhang Q, Jiang H,
Sun Y, et al: Identification of an exon 4-deletion variant of
epidermal growth factor receptor with increased
metastasis-promoting capacity. Neoplasia. 13:461–471.
2011.PubMed/NCBI
|
|
37
|
Sasaki H, Yukiue H, Mizuno K, Sekimura A,
Konishi A, Yano M, et al: Elevated serum epidermal growth factor
receptor level is correlated with lymph node metastasis in lung
cancer. Int J Clin Oncol. 8:79–82. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang Z, Bagheri-Yarmand R, Wang RA, Adam
L, Papadimitrakopoulou VV, Clayman GL, et al: The epidermal growth
factor receptor tyrosine kinase inhibitor ZD1839 (Iressa)
suppresses c-Src and Pak1 pathways and invasiveness of human cancer
cells. Clin Cancer Res. 10:658–667. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang D, LaFortune TA, Krishnamurthy S,
Esteva FJ, Cristofanilli M, Liu P, et al: Epidermal growth factor
receptor tyrosine kinase inhibitor reverses mesenchymal to
epithelial phenotype and inhibits metastasis in inflammatory breast
cancer. Clin Cancer Res. 15:6639–6648. 2009. View Article : Google Scholar
|
|
40
|
Moutinho C, Mateus AR, Milanezi F,
Carneiro F, Seruca R and Suriano G: Epidermal growth factor
receptor structural alterations in gastric cancer. BMC Cancer.
8:102008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ueno S, Mojic M, Ohashi Y, Higashi N,
Hayakawa Y and Irimura T: Asialoglycoprotein receptor promotes
cancer metastasis by activating the EGFR-ERK pathway. Cancer Res.
71:6419–6427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xue C, Wyckoff J, Liang F, Sidani M,
Violini S, Tsai KL, et al: Epidermal growth factor receptor
overexpression results in increased tumor cell motility in vivo
coordinately with enhanced intravasation and metastasis. Cancer
Res. 66:192–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Price JT, Wilson HM and Haites NE:
Epidermal growth factor (EGF) increases the in vitro invasion,
motility and adhesion interactions of the primary renal carcinoma
cell line, A704. Eur J Cancer. 32A:1977–1982. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Turner T, Chen P, Goodly LJ and Wells A:
EGF receptor signaling enhances in vivo invasiveness of DU-145
human prostate carcinoma cells. Clin Exp Metastasis. 14:409–418.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shigematsu H, Lin L, Takahashi T, Nomura
M, Suzuki M, Wistuba II, et al: Clinical and biological features
associated with epidermal growth factor receptor gene mutations in
lung cancers. J Natl Cancer Inst. 97:339–346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kobayashi S, Boggon TJ, Dayaram T, Jänne
PA, Kocher O, Meyerson M, et al: EGFR mutation and resistance of
non-small-cell lung cancer to gefitinib. N Engl J Med. 352:786–792.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Amador ML, Oppenheimer D, Perea S, Maitra
A, Cusatis G, Iacobuzio-Donahue C, et al: An epidermal growth
factor receptor intron 1 polymorphism mediates response to
epidermal growth factor receptor inhibitors. Cancer Res.
64:9139–9143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dubey S, Stephenson P, Levy DE, Miller JA,
Keller SM, Schiller JH, et al: EGFR dinucleotide repeat
polymorphism as a prognostic indicator in non-small cell lung
cancer. J Thorac Oncol. 1:406–412. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dong J, Dai J, Shu Y, Pan S, Xu L, Chen W,
et al: Polymorphisms in EGFR and VEGF contribute to non-small-cell
lung cancer survival in a Chinese population. Carcinogenesis.
31:1080–1086. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, et al: EGFR mutations in lung cancer:
correlation with clinical response to gefitinib therapy. Science.
304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, et al: Activating
mutations in the epidermal growth factor receptor underlying
responsiveness of non-small-cell lung cancer to gefitinib. N Engl J
Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pao W, Miller V, Zakowski M, Doherty J,
Politi K, Sarkaria I, et al: EGF receptor gene mutations are common
in lung cancers from ‘never smokers’ and are associated with
sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad
Sci USA. 101:13306–13311. 2004.
|