Introduction
The American Cancer Society estimated that 21,880
women in the United States would be diagnosed with ovarian cancer
and 14,621 of them would succumb to this disease in 2010 (1). The current standard treatment for
advanced-stage ovarian cancer is cytoreductive surgery and
cisplatin-based combination chemotherapy. However, drug resistance
commonly develops following a few cycles of therapy and the
mechanism of drug resistance remains unclear. Studies have
demonstrated that mammalian target of mammalian target of rapamycin
(mTOR) may contribute to this cisplatin resistance (2).
microRNAs (miRNAs/miRs) are post-transcriptional
regulators that bind to complementary sequences on target messenger
RNA (mRNA) transcripts, usually resulting in translational
repression or target degradation and gene silencing (1,3).
miR-199a is located on human chromosome 19q13.2 (3) and has been detected in human ovarian
carcinoma. The low expression of miR-199a has been previously
detected in ovarian carcinoma and is significantly correlated with
a poor prognosis (3). The purpose
of the present study was to define the role of this miRNA during
the development of cisplatin drug resistance in the human OV2008
and C13* ovarian cancer cell lines by analyzing the expression
levels of miR-199a and mTOR, a possible target of miR-199a.
Materials and methods
Cell lines and culture
The cisplatin-resistant ovarian cancer cell line
(C13*) and its sensitive variant (OV2008) were gifts from Dr Rakesh
Goel at Ottawa Regional Cancer Center, Ottawa, Canada. These cell
lines were maintained at 37ºC in RPMI-1640 complete medium
supplemented with 2 mM L-glutamine and 10% fetal bovine serum in a
humidified atmosphere of 5% CO2.
Reagents and antibodies
Cisplatin and DMSO were purchased from Sigma
Chemical Inc. (St. Louis, MO, USA). Fetal bovine serum, RPMI-1640,
Lipofectamine 2000 reagent and TRIzol™ reagent were purchased from
Life Technologies Inc. (Carlsbad, CA, USA). Cell counting kit-8
(CCK-8) was purchased from Dojindo Molecular Technologies, Inc.
(Kumamoto, Japan). Rabbit anti-human mTOR polyclonal antibody was
obtained from Cell Signaling Technology Inc. (Danvers, MA, USA) and
β-actin antibody was obtained from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). Luciferase reporter vectors were obtained
from Promega Corporation (Madison, WI, USA) and PCR primers were
obtained from Invitrogen Corporation (Carlsbad, CA, USA).
miRNA transfection
The miR-199a mimics and inhibitors were purchased
from Ambion (Life Technologies Inc.). OV2008 and C13* cells in the
exponential phase of growth were plated in six-well plates at
3.5×105 cells/well and cultured for 16 h. The cells were
then transfected with the mimics or inhibitors of miR-199a or the
negative control (NC) RNA, at a final concentration of 100 nM using
Lipofectamine 2000 (Invitrogen) and OPTI-MEM reduced serum medium
(Life Technologies Inc.), according to the manufacturer’s
instructions. To determine the expression of mTOR, at 48 h
post-transfection, the transfected cells were collected to measure
the mRNA and protein levels.
Quantitative (q)PCR for miR-199a and mTOR
mRNA detection
Total RNA was extracted from cultured OV2008 and
C13* cells according to the TRIzol-chloroform protocol and reverse
transcribed into cDNA using M-MLV reverse transcriptase (Promega)
and oligo(dT). The Bulge-Loop™ miRNA qPCR primer set for
hsa-miR-199a (MQP-0101; RiboBio, Guangzhou, China) and U6 snRNA
(MQP-0201; RiboBio) were used according to the manufacturer’s
instructions. The cDNA was used for the amplification of mature
miR-199a, mTOR, GAPDH and U6 snRNA through qPCR. The primer
sequences of the mTOR and GAPDH were as follows: mTOR forward,
5′-AGGCCGCATTGTCTCTATCAA-3′ and reverse,
5′-GCAGTAAATGCAGGTAGTCATCCA-3′; and GAPDH forward,
5′-GTCAGTGGTGGACCTGACCT-3′ and reverse, 5′-AGGGGAGATTCAGTGTGGTG-3′.
For the reverse transcription, 500 ng total RNA was transcribed
into cDNA in a 20 μl reaction volume at 42ºC for 45 min with the
GeneAmp Gold RNA PCR Reagent kit (Applied Biosystems, Foster City,
CA, USA). qPCR was performed in a 20 μl reaction volume containing
10 μl SYBR Green PCR Master Mix (Applied Biosystems). The cycle
conditions were 95ºC for 3 min, followed by 40 cycles of 95ºC for
20 sec, 60ºC for 30 sec and 70ºC for 30 sec. The relative miRNA
levels of the samples from each cell line in each group were
calculated using the 2−ΔΔCt method.
Western blot analysis
The cells were harvested and homogenized with lysis
buffer at 48 h post-transfection. Proteins were resolved in an
SDS/PAGE gel and transferred onto PVDF membranes, then subjected to
immunoblot analysis using polyclonal antibodies against mTOR and
β-actin. All antibodies were used at 1 μg/ml working concentration
in PBS with 5% skimmed milk. The membrane was incubated with
anti-mTOR and anti-β-actin antibodies separately overnight at 4ºC.
Subsequent to washing the membrane with TBST, it was incubated with
horseradish peroxidase (HRD)-conjugated rabbit secondary antibody.
Specific proteins were visualized using enhanced chemiluminescence
following the manufacturer’s instructions (Pierce Biotechnology,
Inc., Rockford, IL, USA). Then, the blots were exposed to X-ray
film to obtain optimal bands. The bands were quantified by using
Image J software (National Institutes of Health, Bethesda, MD,
USA).
Construction of vector and luciferase
reporter assay
The 3′-untranslated region (UTR) fragments of the
mTOR gene were predicted to be complementary to the sequence of
miR-199a according to an analysis of the miRNA target gene
prediction database, TargetScan. The whole sequence of the mTOR
3′-UTR was amplified by PCR using human genomic DNA as a template.
The primers for the 3′-UTR segment were
5′-CTGGAGGCCCAGATGTGCCCATCACG-3′ (sense) and
5′-ACATATGTTTAAAATTCTGATGTCAT-3′ (antisense). The PCR product was
ligated into the PGM-T vector (Tiangen Biotech, Beijing, China).
The mTOR 3′-UTR inserts were removed from the PGM-T plasmid and
cloned downstream of the Renilla luciferase reporter gene
(psiCHECK-2™; Promega). The accuracy of the inserted gene was
confirmed by sequencing. At 24 h prior to transfection, the cells
were plated at 5×105 cells/well into 96-well plates.
Luciferase 3′-UTR-reporter vectors (100 ng) and 100 nmol miR-199a
mimics were co-transfected into C13* cells using Lipofectamine 2000
reagent according to the manufacturer’s instructions (Invitrogen).
At 24 h post-transfection, the cells were harvested and lysed with
passive lysis buffer (Promega). Luciferase activity assays were
performed using the Dual Luciferase Reporter Assay System (Promega)
following the manufacturer’s instructions. Three independent
experiments were performed in triplicate.
Cell viability measured by CCK-8
assay
The cytotoxic effects of cisplatin were determined
with the CCK-8 assay. The cells were seeded in triplicate in
96-well plates the day prior to the experiment at a density of
5×105 cells/well. Subsequent to 24 h, the OV2008 and
C13* cells were transfected with the inhibitors or mimics of
miR-199a for 24 h. Following 12 h of incubation, the cells were
then treated with various concentrations of cisplatin for 48 h. The
absorbance at 450 nm was measured using a multilabel plate reader
(Perkin-Elmer, Waltham, MA, USA). The results are presented as the
mean ± SD of three separate experiments, with six determinations
per experiment.
Apoptosis assay
At 24 h post-transfection, as described previously,
the OV2008 and C13* cells were treated with cisplatin at a
concentration of 40 μM for 48 h. The cells were washed twice with
cold 10 mM PBS and resuspended in 1X binding buffer (BD
Biosciences, San Jose, CA, USA) at a concentration of
1×106 cells/ml. The cells were stained with 5 μl Annexin
V and 10 μl propidium iodide (PI), using the Annexin V apoptosis
detection kit (KeyGen Biotech, Nanjing, China) for 20 min at room
temperature in the dark. The analysis of the apoptotic cells was
performed with a FACScan (BD Biosciences) and the data were
analyzed using CellQuest version 3.3 software (BD Biosciences). The
experiment was repeated three times.
Statistical analysis
All experiments were repeated at least three times.
Numerical data are presented as the mean ± SD. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression levels of miR-199a in OV2008
and C13* ovarian cancer cells
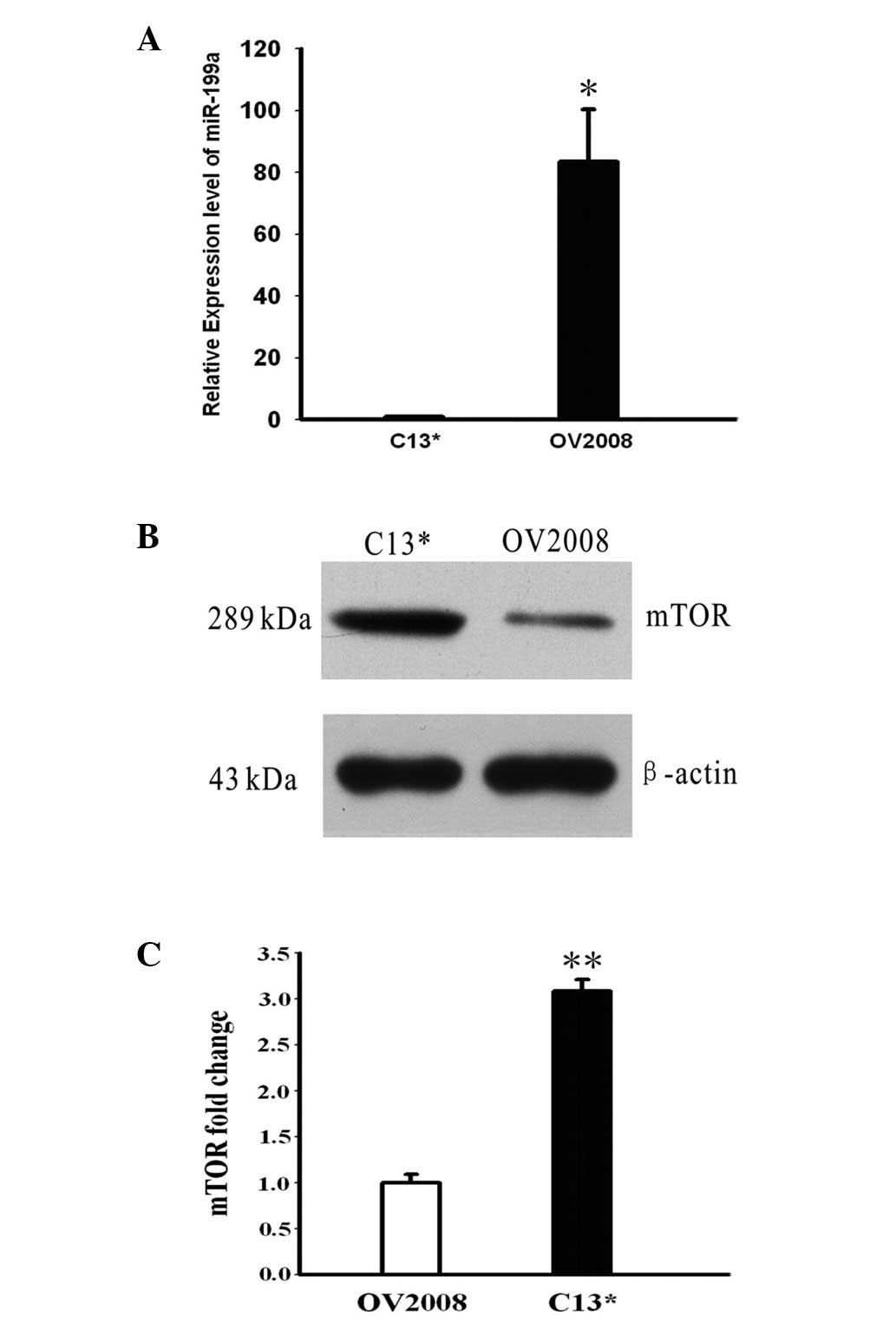
The levels of miR-199a and mTOR were detected by
qPCR and western blotting in the OV2008 and C13* cells. The
expression levels of miR-199a were, on average, 83.4-fold higher in
the OV2008 cells compared with the C13* cells (P<0.05, Fig. 1A). As shown in Fig. 1B, the expression of mTOR was
noticeably higher in the C13* cells compared with OV2008 cells, as
demonstrated by western blotting.
Effect of miR-199a on sensitivity to
cisplatin treatment in C13* and OV2008 cells
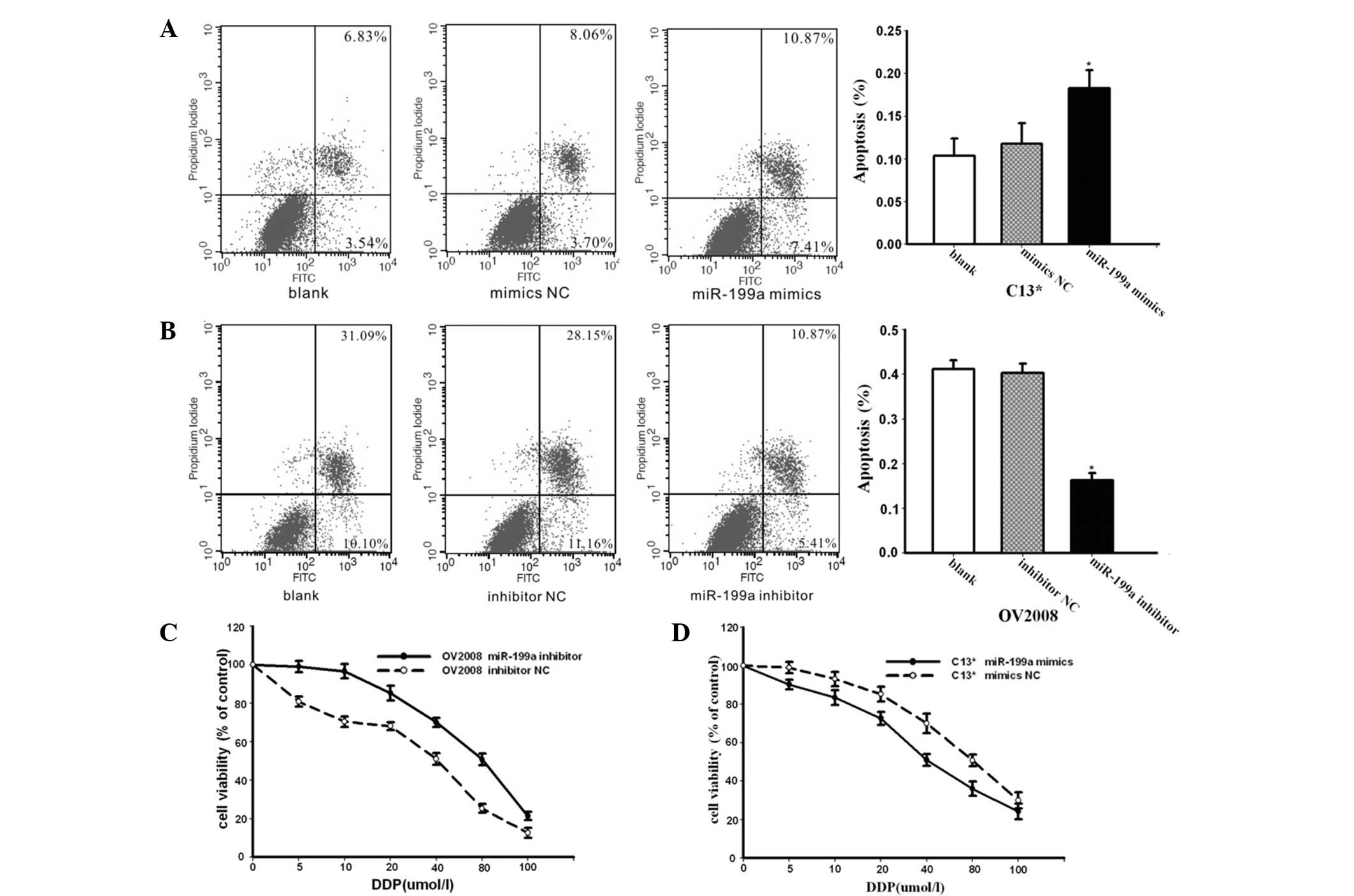
The effect of miR-199a was further investigated in
the cisplatin-resistant ovarian cancer cell line C13* through
treatment with cisplatin. The C13* cells were transfected with
miR-199a mimics and miR-mimic negative controls (NCs) and treated
with of 40 μM cisplatin for 24 h. Apoptosis assays using annexin V
staining indicated that the mimics of endogenous miR-199a enhanced
cisplatin-induced apoptosis compared with the NC group (Fig. 2A). As shown in Fig. 2B, the OV2008 cells were transfected
with miR-199a inhibitor or inhibitor NC, followed by treatment with
40 μM cisplatin for 24 h. Apoptosis assays using annexin-V staining
showed that significantly lower apoptosis ratios were detected in
the OV2008 cells transfected with miR-199a inhibitor compared with
the NC group (Fig. 2B). These
results indicated that miR-199a is able to reverse
cisplatin-resistance in ovarian cancer cells by promoting
cisplatin-induced apoptosis in vitro.
To further demonstrate whether miR-199a was able to
regulate the sensitivity of OV2008 and C13* cells to cisplatin, the
OV2008 and C13* cells were transfected with inhibitors of miR-199a
or mimics of miR-199a, respectively. The cells were then incubated
with cisplatin at various concentrations and the viability of cells
was evaluated using the CCK-8 assay. As shown in Fig. 2C, transfection with inhibitors of
miR-199a markedly decreased the sensitivity of the OV2008 cells to
cisplatin compared with the cells treated with NC. The C13* cells
transfected with mimics of miR-199a exhibited increased sensitivity
to cisplatin compared with the cells treated with NC (Fig. 2D). These results clearly indicate
that miR-199a is significant in the cisplatin resistance mechanism
of ovarian cancer cells.
Regulation of mTOR expression by
miR-199a
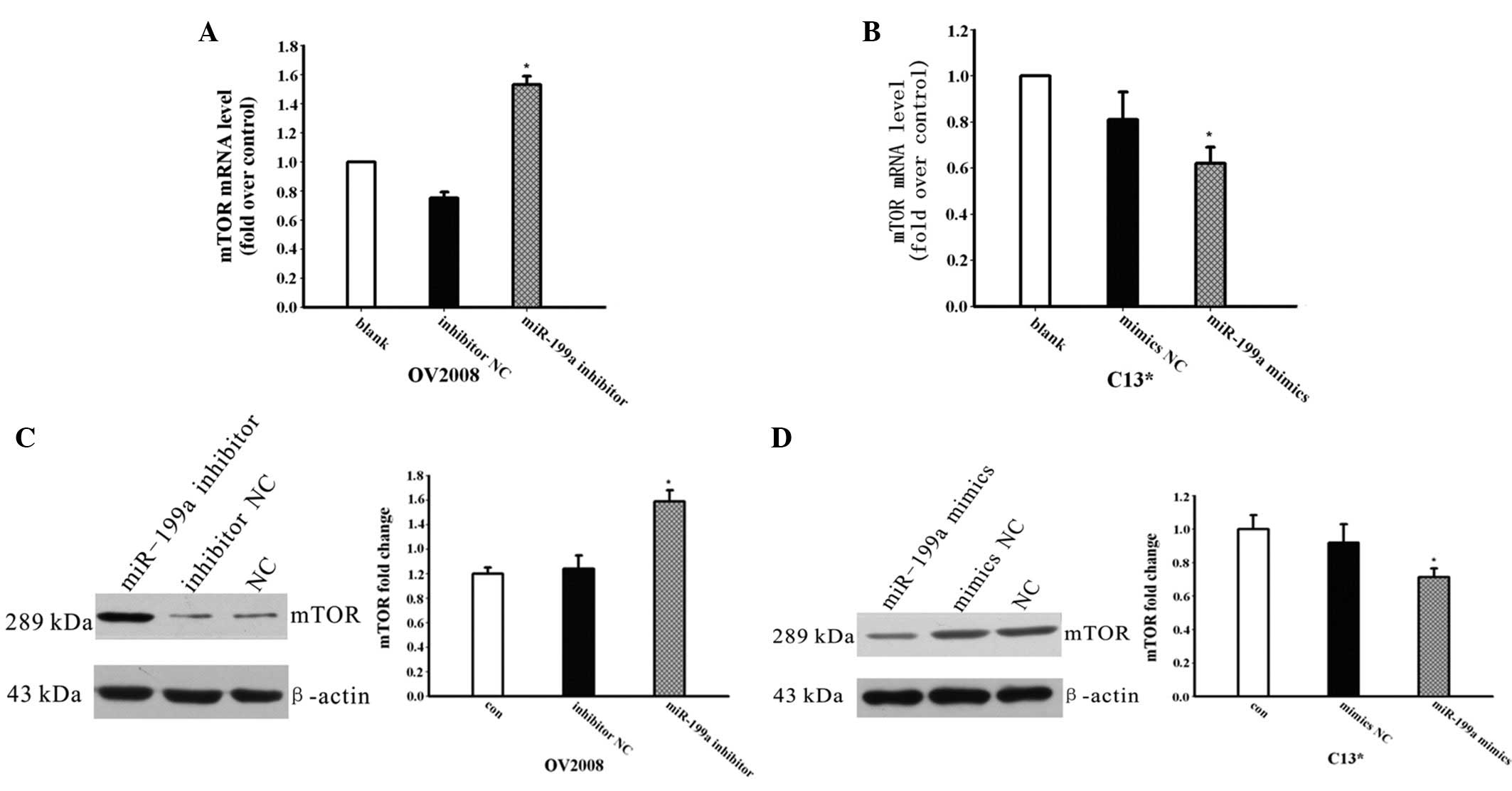
To investigate whether miR-199a is involved in the
regulation of the expression of mTOR, the mimics or inhibitors of
miR-199a were transfected into the C13* and OV2008 cells,
respectively, and the mRNA and protein expression levels of mTOR
were detected by qPCR and western blotting. As shown in Fig. 3A, the expression of mTOR mRNA was
increased following miR-199a inhibitor transfection in the OV2008
cells. The mTOR protein expression level was also increased
(Fig. 3C), while the mRNA and
protein levels of mTOR were decreased in the C13* cells transfected
with miR-199a mimics compared with the NC groups (Fig. 3D).
mTOR may be a target gene of
miR-199a
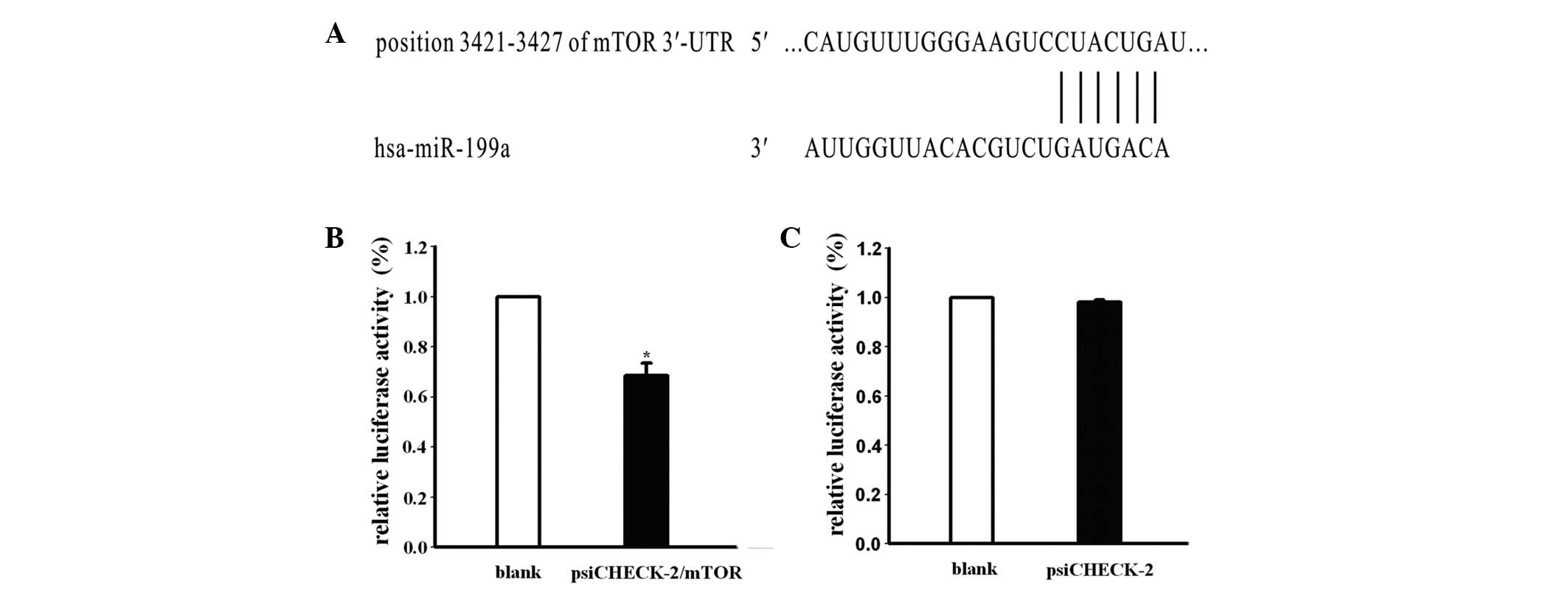
Based on the present data, we attempted to identify
whether mTOR is the target gene of miR-199a, which may explain
miR-199a-related cisplatin resistance in ovarian cancer cells.
Subsequent to analyzing miRNA target prediction public databases
(TargetScan, Pictar), it was observed that the 3′-UTR mRNA of mTOR
included a highly-conserved binding site for miR-199a (Fig. 4A). To investigate the association
between mTOR and miRNA, the C13* cells were co-transfected with
mimics of miR-199a and vector containing a psiCHECK-2 Renilla
luciferase reporter gene and the 3′-UTR mRNA of mTOR or empty
vector psiCHECK-2. mTOR fluorescence intensity was detected at 24 h
post-transfection. The results showed that the luciferase activity
of the psiCHECK-2 Renilla luciferase reporter gene with the 3′-UTR
mRNA of mTOR was significantly decreased by 68.4%, but that there
was no difference in luciferase activity between the empty vector
psiCHECK-2 and controls (Fig. 4B and
C). These results indicated that mTOR expression was
significantly blocked by miR-199a. Consequently, mTOR may be the
target gene of miR-199a.
Discussion
Aberrant miRNAs are capable of affecting the
expression of target proteins, which may affect cell death
signaling pathways, drug targets or cell cycle-related proteins.
This may lead to altered resistance to cytotoxic therapy (4). Academic conferences have paid close
attention to miRNAs (5). Currently,
the main obstacle to successful chemotherapy is the development of
drug resistance to chemotherapeutics. The defective apoptosis
pathway is a major mechanism of drug resistance in ovarian cancer
cells. Increasing evidence indicates that miRNAs are involved, at
least partially, in the drug resistance of ovarian cancer cells,
through this mechanism (6).
Restoring attenuated levels of miR-199a in human
hepatocarcinoma cells results in G1-phase cell cycle
arrest, leading to reduced invasive ability, increased
susceptibility to hypoxia and enhanced sensitivity to
doxorubicin-induced apoptosis (7).
These characteristics make miR-199a a biomarker of hepatocarcinoma
(8). According to Sorrentino et
al, miRNAs may play a significant role in drug resistance by
targeting different genes in different cancer cell lines (6). Based on previous research, miR-199a
may be a potential cancer suppresser and could act as a new
therapeutic target for ovarian cancer patients with a risk for
cisplatin resistance.
In the present study, the expression levels of
miR-199a were analyzed in OV2008 and C13* cells and attempts were
made to identify the molecular mechanism between cisplatin
resistance and miR-199a. First, it was demonstrated that the
miR-199a level was lower in the C13* cells compared with the
parental OV2008 cells, while the expression of mTOR was noticeably
higher. Subsequently, inhibitors of miR-199a were transfected into
the OV2008 cells and it was observed that the miR-199a reduction
increased the mTOR expression level, decreasing the sensitivity of
the OV2008 cells to cisplatin. Transfecting mimics of miR-199a into
the C13* cells caused the expression level of mTOR to be reduced,
while the sensitivity to cisplatin was increased. These results
show that miR-199a is able to reverse cisplatin chemoresistance by
the negative regulation of mTOR expression. It is known that the
effect of miRNAs depends on the process of post-transcriptional
gene silencing. Consequently, miRNAs may inhibit certain
transcription factors that are associated with the translation of
mTOR during this process.
To identify specific mRNA functional fragments in
miRNA, various algorithms for the target prediction were tested,
including those used in the TargetScan, PicTar and miRanda web
sites. The intersection of algorithms indicated that mTOR was a
potential target gene of the mature miR-199a. mTOR has been
demonstrated to be a crucial kinase acting downstream of the
activation of the PI3K signaling pathway. Evidence indicates that
mTOR functions as a master switch of cellular catabolism and
anabolism, thus determining whether cancer cells grow and
proliferate (7). Moreover, mTOR has
been demonstrated to have marked effects on the modulation of
apoptotic cell death, which primarily depends on the cellular
context and downstream signaling proteins, including p53, B-cell
lymphoma (BCL2), p21, p27 and c-MYC (9). mTOR inhibition restores sensitivity to
certain existing chemotherapeutic agents such as cisplatin,
trastuzumab and gefitinib. The molecular mechanisms leading to
apoptosis in tumor cells have not been fully understood. One
possible association between mTOR inhibition and apoptosis
induction may be provided by the downstream target S6K1, which
phosphorylates the pro-apoptotic molecule BCL2-antagonist of cell
death (BAD) on Ser136, a reaction that disturbs the binding of BAD
to the mitochondrial death inhibitors BCL-XL and BCL2, thus
inactivating BAD (10). In this
case, rapamycin-mediated S6K1 inactivation would indirectly cause
BAD activation. Moreover, several growth factors that activate the
PI3K and S6K1 signaling pathway were recently shown to increase the
expression of BCL2, thus promoting cell survival in myeloid
progenitor cells (11). Studies in
cancer cells have indicated that BCL2 contributes to chemotherapy
resistance, and the aberrant expression of BCL2 has been associated
with drug resistance to commonly used anticancer agents (12).
Tsurutani et al(13) demonstrated that LY294002 and
imatinib caused greater than additive increases in apoptosis
compared with apoptosis caused by the inhibitor or imatinib alone.
This result indicated that laminin-mediated activation of the
PI3K/AKT/mTOR signaling pathway was a mechanism of cellular
survival and therapeutic resistance in small cell lung cancer
cells. CCI-779, a known mTOR inhibitor, is able to reverse
cisplatin resistance (2) and mTOR
may be involved through the following possible mechanism: Active
AKT results in the activation of multiple downstream effectors that
combine with mTOR to increase the translation of proteins essential
for survival. The inhibition of mTOR enhances the sensitivity of a
broad range of chemocytotoxic agents, including cisplatin,
carboplatin, doxorubicin, mitoxantrone and doxcetaxel in numerous
types of human cancers. RAD001 is an mTOR inhibitor. RAD001 in
combination with cisplatin was shown to induce a distinct increase
in the number of apoptotic cells by downregulating the pro-survival
molecules, BCL2, survivin and cyclinD1, compared with RAD001 or
cisplatin alone. RAD001 enhanced the sensitivity of hepatocellular
carcinoma cells to cisplatin in the p53-dependent and -independent
pathways (14). Certain inhibitors
of PI3K/mTOR have been involved in clinical trials (15).
The AKT/mTOR signaling pathway has a major role in
cisplatin resistance in ovarian cancer cells; LY294002, the
inhibitor of PI3K/mTOR, has been shown to sensitize ovarian cancer
cells to cisplatin (16). Another
study indicated that the expression of the PI3K-p85 subunit was
higher in epithelial ovarian cancer specimens at the protein level,
but that it was not detected in the normal ovarian epithelium
(17). In the chemoresistant
ovarian cancer cells, SKOV3/DDP and SKOV3/MCA, elevated activation
of the AKT/mTOR/survivin signaling was observed. Downregulation of
the mTOR/survivin signaling pathway attenuated cisplatin resistance
(17). The mTOR signaling pathway
may be involved in regulating the phosphorylation status of p70S6K
at Ser371 in the mediation of chemoresistance in ovarian cancer
(18).
In summary, the present study indicates that
miR-199a contributes to the reversal of cisplatin resistance by
blocking the expression of mTOR in cisplatin-resistant ovarian
cancer cells. During this process, mTOR is at least an indirect
target gene of miR-199a. Future studies are required to further
demonstrate whether mTOR is a direct target of miR-199a and whether
additional molecular mechanisms exist (19). According to the present study
results, we predict that miR-199a may be a potential therapeutic
target for cisplatin-resistant ovarian cancer.
Acknowledgements
The present study was supported by grants from the
Joint Research Fund for Young Scholars Abroad (no. 30528012), the
Natural Science Foundation of Yichang City (A12301-08) and the
Natural Science Foundation of Hubei Province (2012FFC125).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Wangpaichitr M, Wu C, You M, et al:
Inhibition of mTOR restores cisplatin sensitivity through
down-regulation of growth and anti-apoptotic proteins. Eur J
Pharmacol. 591:124–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nam EJ, Yoon H, Kim SW, et al: MicroRNA
expression profiles in serous ovarian carcinoma. Clin Cancer Res.
14:2690–2695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Torres A, Torres K, Maciejewski R and
Harvey WH: MicroRNAs and their role in gynecological tumors. Med
Res Rev. 31:895–923. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cho WC: Updates in cancer research:
insights from the AACR 100th Annual Meeting. Expert Rev Mol Diagn.
9:411–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sorrentino A, Liu CG, Addario A, Peschle
C, Scambia G and Ferlini C: Role of microRNAs in drug-resistant
ovarian cancer cells. Gynecol Oncol. 111:478–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fornari F, Milazzo M, Chieco P, et al:
MiR-199a-3p regulates mTOR and c-Met to influence the doxorubicin
sensitivity of human hepatocarcinoma cells. Cancer Res.
70:5184–5193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hou J, Lin L, Zhou W, et al:
Identification of miRNomes in human liver and hepatocellular
carcinoma reveals miR-199a/b-3p as therapeutic target for
hepatocellular carcinoma. Cancer Cell. 19:232–243. 2011. View Article : Google Scholar
|
|
9
|
Castedo M, Ferri KF and Kroemer G:
Mammalian target of rapamycin (mTOR): pro- and anti-apoptotic. Cell
Death Differ. 9:99–100. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Castedo M, Roumier T, Blanco J, et al:
Sequential involvement of Cdk1, mTOR and p53 in apoptosis induced
by the HIV-1 envelope. EMBO J. 21:4070–4080. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Alafuzoff I, Soininen H, Winblad B
and Pei JJ: Levels of mTOR and its downstream targets 4E-BP1, eEF2,
and eEF2 kinase in relationships with tau in Alzheimer’s disease
brain. FEBS J. 272:4211–4220. 2005.PubMed/NCBI
|
|
12
|
Zivny J, Klener P Jr, Pytlik R and Andera
L: The role of apoptosis in cancer development and treatment:
focusing on the development and treatment of hematologic
malignancies. Curr Pharm Des. 16:11–33. 2010. View Article : Google Scholar
|
|
13
|
Tsurutani J, West KA, Sayyah J, Gills JJ
and Dennis PA: Inhibition of the phosphatidylinositol
3-kinase/Akt/mammalian target of rapamycin pathway but not the
MEK/ERK pathway attenuates laminin-mediated small cell lung cancer
cellular survival and resistance to imatinib mesylate or
chemotherapy. Cancer Res. 65:8423–8432. 2005. View Article : Google Scholar
|
|
14
|
Tam KH, Yang ZF, Lau CK, Lam CT, Pang RW
and Poon RT: Inhibition of mTOR enhances chemosensitivity in
hepatocellular carcinoma. Cancer Lett. 273:201–209. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mazzoletti M and Broggini M: PI3K/AKT/mTOR
inhibitors in ovarian cancer. Curr Med Chem. 17:4433–4447. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng DJ, Wang J, Zhou JY and Wu GS: Role
of the Akt/mTOR survival pathway in cisplatin resistance in ovarian
cancer cells. Biochem Biophys Res Commun. 394:600–605. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang HY, Zhang PN and Sun H: Aberration
of the PI3K/AKT/mTOR signaling in epithelial ovarian cancer and its
implication in cisplatin-based chemotherapy. Eur J Obstet Gynecol
Reprod Biol. 146:81–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Foster H, Coley HM, Goumenou A, Pados G,
Harvey A and Karteris E: Differential expression of mTOR signalling
components in drug resistance in ovarian cancer. Anticancer Res.
30:3529–3534. 2010.PubMed/NCBI
|
|
19
|
Wahid F, Shehzad A, Khan T and Kim YY:
MicroRNAs: synthesis, mechanism, function, and recent clinical
trials. Biochim Biophys Acta. 1803:1231–1243. 2010. View Article : Google Scholar : PubMed/NCBI
|