Introduction
Hepatocellular carcinoma (HCC) is one of the most
common types of malignancy worldwide, leading to >500,000
mortalities every year (1).
Conventional chemotherapy and radiation treatments for HCC have
been disappointing, with an overall 5-year survival rate of <10%
(2). Although surgical resection
has been considered to be the treatment methodology with the most
curative potential, only an extremely small proportion of patients
with primary liver cancer benefit transiently from surgical
treatment, as recurrence rates are high following surgery. The
majority of patients present with advanced-stage cancer and chronic
hepatic dysfunction, limiting available surgery options (3,4). Other
therapeutic approaches, including local alcohol injection, hepatic
arterial immobilization and radiotherapy have not been found to
significantly improve prognosis. These results highlight the urgent
requirement for new therapies for HCC treatment. Gene therapy and
immunotherapy are promising methods and extremely important. Gene
therapy for malignant neoplasms has received considerable attention
within the field and extensive experience associated with gene
therapy, including toxicity, pharmacology and clinical indications,
has been gained and reported (5,6).
Human telomerase reverse transcriptase (hTERT) has
been identified as the catalytic enzyme required for telomere
elongation. hTERT is expressed in the majority of tumor cells but
is rarely expressed in human adult cells. It has been reported that
80–90% of HCCs express hTERT, and so the enzyme is a potential
target in gene therapy for HCC (7,8).
Adenovirus-mediated delivery of hTERT polypeptides into tumor cells
is a well-studied approach that facilitates the eradication of
tumors (9). hTERTC27, a 27-kDa
C-terminal polypeptide of hTERT, is capable of inducing telomere
dysfunction and anaphase chromosome end-to-end fusions in
hTERT-positive HeLa cells. Overexpression of hTERTC27 also inhibits
HeLa cell growth and tumorigenicity in nude mouse xenografts
(10). Notably, the actions of
hTERTC27 are mediated without perturbing the endogenous telomerase
activity, thereby minimizing the potential side effects on
telomerase-positive reproductive and proliferative cells of renewal
tissues in antitelomerase therapies (11,12).
In addition, the antitumor effect of hTERTC27 has been explored by
delivering this gene to human glioblastoma multiforme cells using
adeno-associated virus (AAV). It has been reported that
intratumoral injection of recombinant AAV carrying hTERTC27
(rAAV-hTERTC27) is highly potent in inhibiting the growth of human
U87-MG glioblastoma cells in athymic nude mice (13). In our previous study, it was
demonstrated that hTERTC27 carried by adenovirus is able to augment
the concentration of interleukin-2 (IL-2) and interferon-γ (IFN-γ)
and induce antigen-specific cytotoxic T lymphocytes (CTLs) against
glioma cells in vitro, indicating that adenovirus-delivered
hTERTC27 may prolong survival time and inhibit the growth of
glioma-bearing mice (14).
In the present study, hTERTC27 was delivered into
murine HCC cells by adenovirus and its efficacy was observed to
further explore the possible involvement of an immune response in
cancer regression mediated by hTERTC27.
Materials and methods
Cell culture
Mouse HCC cells, Hepa 1–6, were obtained from the
American Type Culture Collection (Rockville, MD, USA). The cell
line was cultured in DMEM supplemented with 10% heat inactivated
fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin
(Invitrogen Life Technologies, Carlsbad, CA, USA). The cells were
incubated at 37°C in 5% CO2 and passaged every three
days.
Animals
C57BL/6 mice (5–8 weeks old) were purchased from
Guangdong Medical Experimental Animal Center (Guangzhou, China) and
were housed under aseptic conditions. All experimental protocols
were performed in accordance with National Institutes of Health
Guidelines and approved by the Animal Care and Use Committee of Sun
Yat-sen University (Guangzhou, China).
Cell proliferation assay
Hepa 1–6 cells were seeded at a density of
1.0×104 cells/well in 96-well culture plates,
transfected with rAdv-hTERTC27 or rAdv-EGFP at a multiplicity of
infection of 30, and then incubated for 48 h. The presence of
viable cells was tested through CCK-8 colorimetric assays (Dojindo
Molecular Technologies, Inc., Gaithersburg, MD, USA) according to
the manufacturer’s instructions. Absorbance values (at 450 nm),
proportional to the number of living cells, were recorded for the
culture medium of each sample using a Bio-Rad model 550 microplate
reader (Hercules, CA, USA).
Detection of apoptosis in vitro
Following 48 h of incubation and treatment as
described, cells were stained with propidium iodide (PI) solution
and Hochest 33258, as described previously (15,16)
and observed with a fluorescence microscope (model IX70; Olympus,
Tokyo, Japan). Cellular apoptosis was also detected using a Cell
Death Detection ELISA Plus kit (Roche Diagnostics, Mannheim,
Germany) according to the manufacturer’s instructions.
rAdv-hTERTC27 transduction of dendritic
cells (DCs) and induction of immune response
To determine the DC induction of CTL cytotoxicity
against HCC cell lines, DCs and T cells were obtained as described
previously (14). T cells were
cocultured with DCs in a 24-well culture plate in 1 ml complete
RPMI-1640 medium for 72 h. CTLs were collected and used as effector
cells in CTL assays against Hepa 1–6 cells. The Hepa 1–6 cells, as
target (T) cells, were placed in 96-well culture plates at
1×104 cells/well and cocultured with effector (E) cells
(CTL) at the indicated ratios (E:T = 5:1, 20:1 and 40:1) for 72 h.
The cytotoxic activities were determined by CCK-8 colorimetric
assays.
HCC inoculation and intravenous injection
of rAdv-hTERTC27
Hepa 1–6 cells were harvested during the exponential
growth phase and washed twice in PBS. The cells were resuspended in
PBS at a density of 5×107 cells/ml and 0.1 ml
(5×106 cells/ml) of the cell suspension was injected
directly into the hepatic capsule of the C57BL/6 mice. Mice were
divided into four groups (eight mice/group) 7 days following tumor
cell inoculation, and were treated under the following conditions:
groups 1 and 2 received viral injections of 5.0×107 pfu
rAdv-hTERTC27 and an equal volume of the hTERTC27 polypeptide,
respectively, representing treatment groups; groups 3 and 4
received 5.0×107 pfu rAdv-EGFP and an equal volume of
PBS, respectively, representing control groups. All treatments were
administered via the tail vein. For the welfare of animals in
experimental neoplasia, mice with tumor burdens >10% of their
body weight were sacrificed immediately. Otherwise, 4 weeks
following treatment, the mice were sacrificed under ether
anesthesia and the total volume of the tumor (mm3) was
calculated using the following formula: V = 1/2 × ab2,
where a and b represent the long and short diameters of the tumor,
respectively.
Statistical analysis
Data are presented as the means ± SD. Statistical
significance was assessed by one-way analysis of variance and
Student’s t-test. Prism 5.0 (GraphPad Software, San Diego, CA, USA)
was used for all calculations. P<0.05 was considered to indicate
a statistically significant difference.
Results
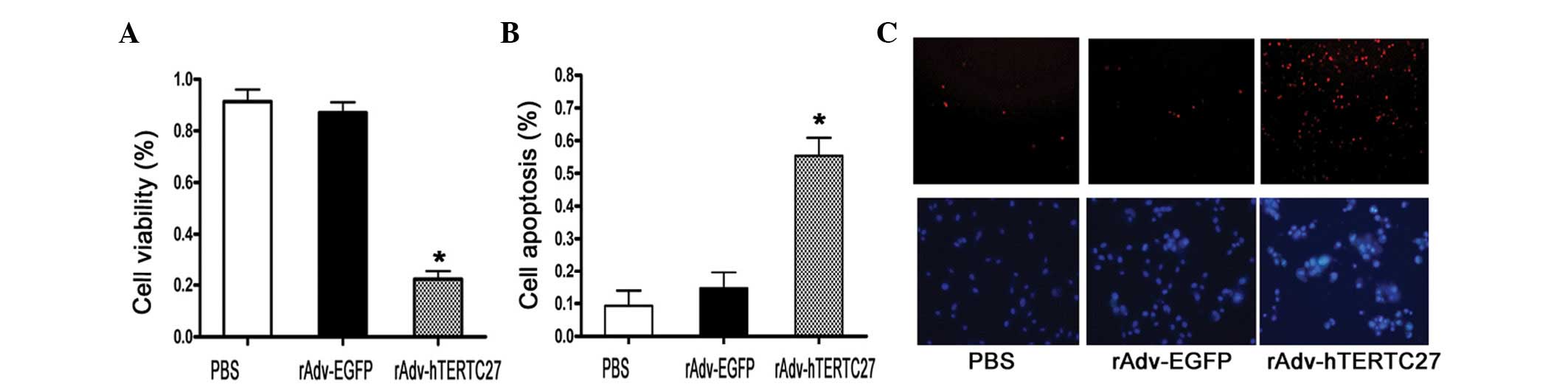
Overexpression of hTERTC27 regulates Hepa
1–6 cell viability and apoptosis
To test the effect of rAdv-hTERTC27 on Hepa 1–6
cells, cellular proliferation and apoptosis detection assays were
performed. As demonstrated in Fig.
1, rAdv-hTERTC27 significantly inhibited the proliferation of
the Hepa 1–6 cells (Fig. 1A).
Cellular apoptosis was induced by rAdv-hTERTC27 and determined by
PI and Hochest staining, as well as cell death ELISA detection
(Fig. 1B and C). These results
indicated that hTERTC27 may induce hepatocellular carcinoma cell
apoptosis effectively in vitro.
rAdv-hTERTC27-DCs induce T lymphocyte
proliferation and prime CTLs against Hepa 1–6 HCC cells in
vitro
rAdv-hTERTC27-DCs, rAdv-EGFP-DCs and PBS-DCs were
cocultured with lymphocytes for 72 h at ratios of DC:T of 1:5,
1:10, 1:20 and 1:40. The results demonstrated that the effect of
rAdv-hTERTC27-DC induction of T lymphocytes is markedly stronger
than that by rAdv-EGFP-DCs and PBS-DCs when the DC:T ratio was
≥1:10 (P<0.05). However, no significant differences were
identified between rAdv-EGFP and PBS (Fig. 2A). To test the hypothesis that the
immune response is one of the predominant mechanisms responsible
for the inhibition of tumor growth by rAdv-hTERTC27, mouse splenic
T cells were further examined and primed in vitro with
rAdv-hTERTC27-DCs to elicit cytotoxic reactivity against tumor
cells. The results revealed that at E:T ratios of 5:1, 20:1 and
40:1, the lytic activity of CTLs was 16.16±2.75, 44.44±3.11 and
65.21±2.98%, respectively. However, the lytic activity of CTLs in
rAdv-EGFP and PBS was 17.79±2.95, 33.65±3.16 and 40.54±3.18%, and
16.99±2.97, 30.57±2.64 and 48.72±3.45%, respectively. These results
demonstrated that T cells primed with rAdv-hTERTC27-DCs in
vitro were able to lyse tumor cells effectively as compared
with the rAdv-EGFP-DCs and PBS-DCs, when the E:T ratio was ≥20:1
(P<0.05). However, no significant differences were identified
between rAdv-EGFP and PBS (Fig.
2B).
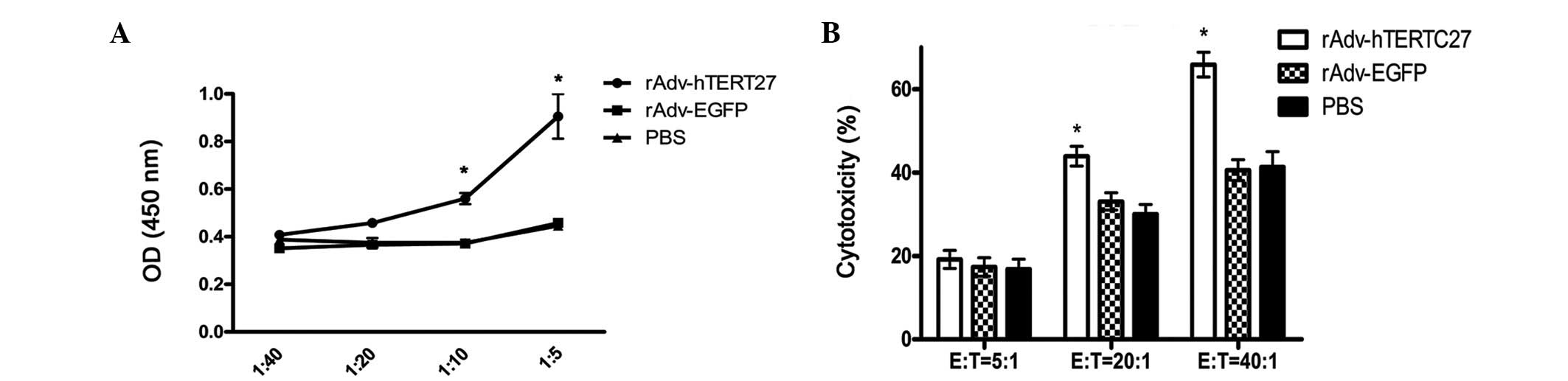 | Figure 2DCs transfected with rAdv-hTERTC27
induce T lymphocyte proliferation and prime cytotoxic activity of
CTLs. (A) Following transfection with or without recombinant
adenovirus for 24 h, DCs were cocultured with lymphocytes for 72 h
at ratios of DC:T of 1:5, 1:10, 1:20 and 1:40. Following
stimulation, the lymphocyte proliferation activity was analyzed
using CCK-8 colorimetric assays. Experiments were repeated three
times and representative results are presented.
*P<0.05, vs. PBS and rAdv-EGFP. (B) Allogeneic T
cells were cocultured with rAdv-hTERTC27-DCs, rAdv-EGFP-DCs and
PBS-DCs for 72 h. Hepa 1–6 cells, as target cells, were cocultured
with effector cells (CTLs) at the indicated ratios (E:T = 5:1, 20:1
and 40:1) for 72 h. Cytotoxicity assay assessed by CCK-8
colorimetric assays. Experiments were repeated three times and
results are presented. *P<0.05, vs. PBS and
rAdv-EGFP. DCs, dendritic cells; rAdv, recombinant adenovirus;
hTERTC27, 27-kDa C-terminal fragment of human telomerase reverse
transcriptase; CTL, cytotoxic T lymphocyte; E, effector; T,
target. |
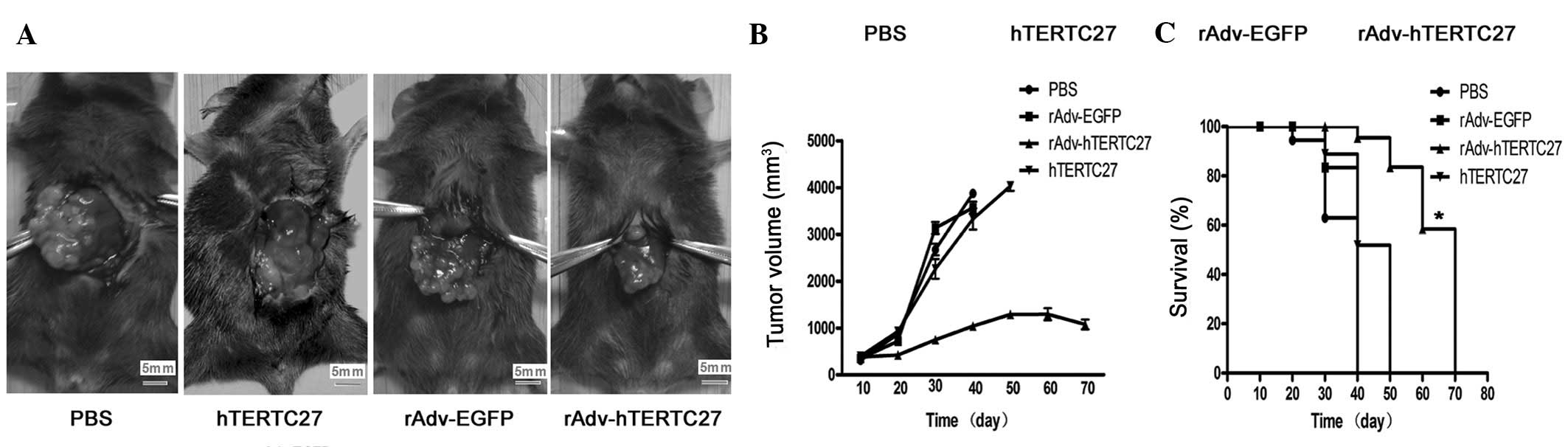
Intravenous injection of rAdv-hTERTC27
significantly inhibits the growth of HCC in mice
To demonstrate the antitumor effect of hTERTC27
in vivo, an HCC model was established by implanting mouse
Hepa 1–6 HCC cells into the hepatic capsule of C57BL/6 mice. PBS,
rAdv-EGFP, rAdv-hTERTC27 and hTERTC27 polypeptides were injected 7
days later via the tail vein. As demonstrated in Fig. 3A, tumor volumes of
rAdv-hTERTC27-treated mice were markedly smaller than those of
rAdv-EGFP-, PBS- and hTERTC27-treated mice 4 weeks following
injection. The mean tumor volume of the rAdv-hTERTC27, rAdv-EGFP,
PBS and hTERTC27-treated mice was 1,012±51.63, 2,567±32.21,
2,789±29.12 and 2,412±34.51 mm3, respectively (Fig. 3B). In addition, the average life
span of rAdv-EGFP-, PBS-and hTERTC27-treated mice bearing
hepatocellular tumors were 34.5, 31 and 36 days, respectively.
However, life span increased to 68 days in rAdv-hTERTC27-treated
mice, although tumor-bearing mice died from progressive tumors. The
prolonged survival rate of tumor-bearing mice was observed in the
majority of rAdv-hTERTC27 groups compared with in rAdv-EGFP, PBS
and hTERTC27 groups (Fig. 3C;
P<0.05). The results demonstrated that the anti-tumor effects of
rAdv-hTERTC27 are achieved effectively in vivo.
Discussion
In the current study, the therapeutic effect of
rAdv-hTERTC27 on HCC, in vivo and in vitro, was
demonstrated. Results indicated that rAdv-hTERTC27 produces a
longer-lasting effect on the generation of effective antitumor
immunity as a reagent via intravenous administration.
The gene delivery vector of choice was the
adenovirus, as it has numerous advantages, including a high gene
transduction efficiency, stability in the bloodstream and an
acceptable safety profile (17,18,19).
However, systemic administration of conventional adenovirus is
capable of leading to the acute accumulation of virus particles and
transgene expression in the liver, which may cause severe
hepatotoxicity. Therefore, the clinical application of the
adenovirus for systemic administration has been limited. To
determine the application of the adenovirus in systemic cancer gene
therapy in clinical patients, the accumulation must be enhanced in
target tumors and hepatic distribution must be minimized. As a
universal tumor-associated antigen, hTERT is an ideal target for
cancer therapy. It is well-known that hTERT is expressed by the
majority of human forms of cancer but rarely in normal cells
(20). The widespread expression of
telomerase in tumors indicates that peptide fragments of hTERT may
serve as tumor-specific antigens and this hypothesis has been
confirmed in several studies (21,22).
Therefore, in the present study, rAdv-hTERTC27 was developed as a
TERT-targeting gene therapy.
Previously, it was demonstrated that overexpression
of hTERTC27 inhibits HeLa cell growth and tumorigenicity in nude
mouse xenografts (10). The
antitumor actions of hTERTC27 are likely to be effected by
increased apoptosis, necrosis and infiltration of polymorphonuclear
leukocytes, reducing angiogenesis in glioblastoma (13). The results of the present study
demonstrated that rAdv-hTERTC27 effectively reduces growth and
increases apoptosis of Hepa 1–6 HCC cells in vitro. It also
inhibited the tumor volume in HCC mouse models intravenously
injected with rAdv-hTERTC27. The indicated statistically
significant difference between the results for rAdv-hTERTC27 and
hTERTC27 polypeptides may be accounted for by two hypotheses: The
high efficiency of recombinant adenovirus as a gene delivery vector
or, more importantly, rAdv-hTERTC27 may promote specific mouse
mechanisms in the bloodstream that markedly augment the antitumor
ability of hTERTC27.
In our previous study, the potential mechanisms
underlying the effects of rAdv-hTERTC27 were explored and
rAdv-hTERTC27 was demonstrated to increase T cell proliferation and
augment the concentration of IL-2 and IFN-γ in the supernatant of T
cells. In the current study, DCs infected with rAdv-hTERTC27
markedly increased the activated cytotoxicity of T cells against
Hepa 1–6 cells. It is well-known that IFN-γ and IL-2 are critical
for T cell responses. IFN-γ is primarily responsible for activating
and regulating the development and persistence of CTLs (23,24).
IL-2 is capable of inducing a distinct CTL effector function
(25) and the administration of
adenovirus vector-encoding mouse IL-2 (AdmIL-2) may augment the
antitumor effect of TRAIL on tumors in mice (26). Activated T cells are able to produce
IFN-γ and induce cytolysis of autologous tumor or semi-allogeneic
targets by an MHC class I-restricted mechanism (27). DCs transduced with the recombinant
adenovirus-encoding peptide may effectively induce antigen-specific
T cell proliferation, augment the number of IFN-γ-secreting T-cells
and induce antigen-specific CTLs capable of lysing target cells
pulsed with the peptide (28,29).
The immunoregulatory function of DCs induced by rAdv-hTERTC27 is
capable of potentiating the initiation, expansion and effect or
phases of an evolving adaptive T cell-mediated immune response that
ultimately leads to the inhibition of tumor cells. By contrast,
increased IFN-γ secretion by T cells may enhance the activation of
CTLs again. Therefore, hTERTC27 gene-modified DCs are capable of
generating a specific CTL response against various hTERT-expressing
cancer cell lines. The susceptibility of tumor cell lines of
various origins to lysis by telomerase-specific CTLs indicates that
hTERTC27 may be used alone or in combination with other tumor
antigens in immunotherapy against a wide range of cancer types.
Adoptive immunotherapy with expanded antigen-specific CTLs may be
an effective approach to treat cancer. However, the efficacy of the
adenovirus to generate hTERTC27-specific CTLs indicates that this
form of vaccination must also be considered in cases where
potential adenovirus toxicity or high levels of neutralizing
antibodies is a concern. In addition, DC-hTERTC27-based cancer
vaccines that have the potential to maximize the protection against
various hTERT+ tumor cells must be investigated, and the
dose of recombinant adenovirus ought to be minimized to decrease
the side effects of gene therapy and immunotherapy.
In the current study, an original HCC model in
C57BL/6 mice was established. C57BL/6 mice were used in this model
as Hepa 1–6 cell lines are derived from a chemically induced
hepatoma from the C57BL/6 mouse (30). In addition, immunocompetent animal
models are suitable for the assessment of immune responses elicited
by hTERTC27. Tumor growth was inhibited significantly, compared
with the control groups, when mice were administered with
intravenous injection of a single dose of 5×107 pfu
rAdv-hTERTC27. Through the immunoregulatory function added to gene
regulation, the dose of rAdv-hTERTC27 was decreased to a higher
degree than was previously reported (31). Thus, the potential side effects of
the adenovirus were lower, which was a concern with regards to gene
therapy and immunotherapy.
In conclusion, the results of the current study
demonstrate that rAdv-hTERTC27 induces tumor cell apoptosis in
murine HCC models. We hypothesize that recombinant adenoviral
constructs containing the hTERTC27 polypeptide represent promising
intravenous drugs for use in clinical practice against HCC.
Acknowledgements
This study was supported by the ‘863’ Programs of
China (2007AA021101), National Natural Science Foundation of China
(30672411 and 30973479), the Science and Technology Planning
Project of Guangdong Province (2009B060700040) and the Science and
Technology Planning Project of Guangdong Province (2011B031800141).
The plasmid, plEGFP-hTERTC27, containing the hTERT COOH-terminal
polypeptide was kindly provided by Professor JJ Huang (Laboratory
of Tumor and Molecular Biology, Beijing Institute of
Biotechnology).
References
|
1
|
Fung SK and Lok AS: Management of patients
with hepatitis B virus-induced cirrhosis. J Hepatol. 42(Suppl 1):
S54–S64. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang YH, Wu JC, Chen SC, Chen CH, Chiang
JH, Huo TI, Lee PC, Chang FY and Lee SD: Survival benefit of
transcatheter arterial chemoembolization in patients with
hepatocellular carcinoma larger than 10 cm in diameter. Aliment
Pharmacol Ther. 23:129–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rampone B, Schiavone B, Martino A, Viviano
C and Confuorto G: Current management strategy of hepatocellular
carcinoma. World J Gastroenterol. 15:3210–3216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ng KK and Poon RT: Current treatment
strategy for hepatocellular carcinoma. Saudi Med J. 28:1330–1338.
2007.PubMed/NCBI
|
|
5
|
Makower D, Rozenblit A, Kaufman H, Edelman
M, Lane ME, Zwiebel J, Haynes H and Wadler S: Phase II clinical
trial of intralesional administration of the oncolytic adenovirus
ONYX-015 in patients with hepatobiliary tumors with correlative p53
studies. Clin Cancer Res. 9:693–702. 2003.PubMed/NCBI
|
|
6
|
Reid T, Galanis E, Abbruzzese J, Sze D,
Wein LM, Andrews J, Randlev B, Heise C, Uprichard M, Hatfield M, et
al: Hepatic arterial infusion of a replication-selective oncolytic
adenovirus (dl1520): phase II viral, immunologic, and clinical
endpoints. Cancer Res. 62:6070–6079. 2002.PubMed/NCBI
|
|
7
|
Abdul-Ghani R, Ohana P, Matouk I, Ayesh S,
Ayesh B, Laster M, Bibi O, Giladi H, Molnar-Kimber K, Sughayer MA,
et al: Use of transcriptional regulatory sequences of telomerase
(hTER and hTERT) for selective killing of cancer cells. Mol Ther.
2:539–544. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mizukoshi E, Nakamoto Y, Marukawa Y, Arai
K, Yamashita T, Tsuji H, Kuzushima K, Takiguchi M and Kaneko S:
Cytotoxic T cell responses to human telomerase reverse
transcriptase in patients with hepatocellular carcinoma.
Hepatology. 43:1284–1294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zou W, Luo C, Zhang Z, Liu J, Gu J, Pei Z,
Qian C and Liu X: A novel oncolytic adenovirus targeting to
telomerase activity in tumor cells with potent. Oncogene.
23:457–464. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang JJ, Lin MC, Bai YX, Jing DD, Wong
BC, Han SW, Lin J, Xu B, Huang CF and Kung HF: Ectopic expression
of a COOH-terminal fragment of the human telomerase reverse
transcriptase leads to telomere dysfunction and reduction of growth
and tumorigenicity in HeLa cells. Cancer Res. 62:3226–3232.
2002.PubMed/NCBI
|
|
11
|
Shay JW, Zou Y, Hiyama E and Wright WE:
Telomerase and cancer. Hum Mol Genet. 10:677–685. 2001. View Article : Google Scholar
|
|
12
|
Huo LF, Tang JW, Huang JJ, Huang PT, Huang
CF, Kung HF and Lin MC: Cancer immunotherapy targeting the
telomerase reverse transcriptase. Cell Mol Immunol. 3:1–11.
2006.PubMed/NCBI
|
|
13
|
Ng SS, Gao Y, Chau DH, Li GH, Lai LH,
Huang PT, Huang CF, Huang JJ, Chen YC, Kung HF and Lin MC: A novel
glioblastoma cancer gene therapy using AAV-mediated long-term
expression of human TERT C-terminal polypeptide. Cancer Gene Ther.
14:561–572. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gong HX, He L, Li XP, Wang YD, Li Y, Huang
JJ, Wang Z, Xie D, Kung HF and Peng Y: Effective antitumor immunity
against murine gliomas using dendritic cells transduced with
hTERTC27 recombinant adenovirus. Oncol Rep. 27:1163–1169.
2012.PubMed/NCBI
|
|
15
|
Yin D, Woodruff M, Zhang Y, Whaley S, Miao
J, Ferslew K, Zhao J and Stuart C: Morphine promotes Jurkat cell
apoptosis through pro-apoptotic FADD/P53 and anti-apoptotic
PI3K/Akt/NF-kappaB pathways. J Neuroimmunol. 174:101–107. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cowell JK and Franks LM: A rapid method
for accurate DNA measurements in single cells in situ using a
simple microfluorimeter and Hoechst 33258 as a quantitative
fluorochrome. J Histochem Cytochem. 28:206–210. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Osada S, Ebihara I, Setoguchi Y, Takahashi
H, Tomino Y and Koide H: Gene therapy for renal anemia in mice with
ploycystic kidney using an adenovirus vector encoding the human
erythropoietin gene. Kidney Int. 55:1234–1240. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mohr A, Lyons M, Deedigan L, Harte T, Shaw
G, Howard L, Barry F, O’Brien T and Zwacka R: Mesenchymal stem
cells expressing TRAIL lead to tumour growth inhibition in an
experimental lung cancer model. J Cell Mol Med. 12:2628–2643. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reid T, Warren R and Kirn D: Intravascular
adenoviral agents in cancer patients: lessons from clinical trial.
Cancer Gene Ther. 9:979–986. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim NW, Piatyszek MA, Prowse KR, Harley
CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL and Shay
JW: Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liao ZL, Luo G, Xie X, Tang XD, Bai JY,
Guo H and Yang SM: Diepitope multiple antigen peptide of hTERT
trigger stronger anti-tumor immune responses in vitro. Int
Immunopharmacol. 16:444–450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hashimoto Y, Tazawa H, Teraishi F, Kojima
T, Watanabe Y, Uno F, Yano S, Urata Y, Kagawa S and Fujiwara T: The
hTERT promoter enhances the antitumor activity of an oncolytic
adenovirus under a hypoxic microenvironment. PLoS One.
7:e392922012. View Article : Google Scholar
|
|
23
|
Iezzi G, Boni A, Degl’Innocenti E, Grioni
M, Bertilaccio MT and Bellone M: Type 2 cytotoxic T lymphocytes
modulate the activity of dendritic cells toward type 2 immune
responses. J Immunol. 177:2131–2137. 2006. View Article : Google Scholar
|
|
24
|
Knutson KL and Disis ML: Tumor
antigen-specific T helper cells in cancer immunity and
immunotherapy. Cancer Immunol Immunother. 54:721–728. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pipkin ME, Sacks JA, Cruz-Guilloty F,
Lichtenheld MG, Bevan MJ and Rao A: Interleukin-2 and inflammation
induce distinct transcriptional programs that promote the
differentiation of effector cytolytic T cells. Immunity. 32:79–90.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nishihori Y, Kato K, Tanaka M, Okamoto T,
Hagiwara S, Araki N, Kogawa K, Kuribayashi K, Nakamura K and Niitsu
Y: Interleukin-2 gene transfer potentiates the
alpha-galactosylceramide-stimulated antitumor effect by the
induction of TRAIL in NKT and NK cells in mouse models of
subcutaneous and metastatic carcinoma. Cancer Biol Ther.
8:1763–1770. 2009. View Article : Google Scholar
|
|
27
|
Koido S, Homma S, Hara E, et al: In vitro
generation of cytotoxic and regulatory T cells by fusions of human
dendritic cells and hepatocellular carcinoma cells. J Transl Med.
6:512008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oh ST, Kim CH, Park MY, Won EH, Sohn HJ,
Cho HI, Kang WK, Hong YK and Kim TG: Dendritic cells transduced
with recombinant adenoviruses induce more efficient anti-tumor
immunity than dendritic cells pulsed with peptide. Vaccine.
24:2860–2868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen L, Tang XD, Yu ST, Ai ZH, Fang DC,
Cai YG, Luo YH, Liang GP and Yang SM: Induction of anti-tumour
immunity by dendritic cells transduced with hTERT recombinant
adenovirus in mice. J Pathol. 217:685–692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Darlington GJ, Bernhard HP, Miller RA and
Ruddle FH: Expression of liver phenotypes in cultured mouse
hepatoma cells. J Natl Cancer Inst. 64:809–819. 1980.PubMed/NCBI
|
|
31
|
Gao Y, Ng SS, Chau DH, Yao H, Yang C, Man
K, Huang PT, Huang C, Huang JJ, Kung HF and Lin MC: Development of
recombinant adeno-associated virus and adenovirus cocktail system
for efficient hTERTC27 polypeptide-mediated cancer gene therapy.
Cancer Gene Ther. 15:723–732. 2008. View Article : Google Scholar : PubMed/NCBI
|