Introduction
Telomerase, a specialized ribonucleoprotein, plays
an essential role in cell proliferation by protecting against the
problem of end-replication by adding TTAGGG repeats to telomeres
(1). The majority of normal human
cells have no detectable telomerase activity, however, activity is
commonly detected in cancer cells (2,3). The
inhibition of telomerase causes a progressive and critical
reduction of telomeres, leading to a potent signal for the blockage
of cell proliferation and the induction of apoptosis (4). Targeting the inhibition of telomerase
activity and the induction of apoptosis may have a selective effect
on cancer cells. Clinically, B-cell acute lymphoblastic leukemia is
curable, however, ≥50% of adults experience treatment failure as a
consequence of drug resistance and the inability of older adults to
tolerate the side-effects of therapy (5). Therefore, it is desirable to develop
novel anticancer drugs against B-cell leukemia, including those
targeting the inhibition of telomerase activity, to prevent
side-effects following chemotherapy. Our previous study reported
that treatment with caffeic acid undecyl ester (CAUE), a novel
caffeic acid derivative, reduced cell survival in human B-cell
leukemia NALM-6 cells, but exhibited no significant effect on the
survival of normal lymphocytes. In addition, the cytotoxic
induction mechanisms of CAUE were shown to be involved in the
intrinsic apoptotic pathway in a caspase-dependent manner (6). The present study focused on the
inhibitory effects of telomerase activity by CAUE in a NALM-6 cell
culture system.
Materials and methods
Materials and cell culture
CAUE was prepared as described previously (7). All other reagents, unless otherwise
stated, were of the highest grade available and purchased from
Sigma-Aldrich (St. Louis, MO, USA) or Wako Pure Chemical
Industries, Ltd. (Osaka, Japan). Antibodies against human
telomerase reverse transcriptase (hTERT; rabbit polyclonal; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA USA) and β-actin as the
loading control (rabbit polyclonal; Cell Signaling Technology,
Inc., Danvers, MA, USA) were used. Human B-cell leukemia NALM-6
cells were supplied by the Cell Resource Center for Biomedical
Research (Tohoku University, Sendai, Japan). Cell culture reagents
were obtained from Invitrogen Life Technologies (Carlsbad, CA, USA)
and the cells were routinely cultured using standard methods, as
described previously (8,9).
DNA, RNA and protein synthesis
assays
The effect of CAUE on the synthesis of DNA, RNA and
protein was determined by incorporation of the radioactive
precursors [3H]-thymidine, [3H]-uridine and
[14C]-leucine (GE Healthcare, Amersham, UK). Briefly,
4×105 cells/ml were cultured in 96-well round-bottom
plates in a total volume of 100 μl culture medium with or without
the indicated concentrations of CAUE. Following incubation for 4 h,
[3H]-thymidine (37 MBq/ml), [3H]-uridine (37
MBq/ml) or [14C]-leucine (1.85 MBq/ml) were added, each
corresponding to a total activity of 148 Bq, and incubated for an
additional 90 min. The cells were harvested on filter membranes
using a Labo Mash cell harvester (Futaba Medical Inc., Tokyo,
Japan). Subsequent to drying, the radioactivity of the material was
measured by a LS-6500 liquid scintillation β-counter (Beckman
Coulter, Miami, FL, USA).
Telomerase activity assay
Telomerase activity was measured using a stretch
PCR-based TeloChaser system (Toyobo Co., Ltd., Osaka, Japan),
according to the manufacturer’s instructions. Briefly,
4×105 cells were lysed in 50 μl lysis reagent and
incubated on ice for 20 min. Following centrifugation at 12,000 × g
for 20 min, DNA products were isolated and 26 cycles of PCR
amplification were performed at 95°C for 30 sec, 68°C for 30 sec
and 72°C for 45 sec. PCR products were electrophoresed on a 10%
polyacrylamide gel and stained with ethidium bromide. Images were
captured using the FLA-3000G image analyzer (Fujifilm Corp., Tokyo,
Japan).
Western blotting
The effects of cellular signal transduction on hTERT
protein expression by CAUE were determined by western blotting
(10). Briefly, the cells were
incubated with the indicated concentrations of CAUE, washed with
phosphate-buffered saline (PBS) and lysed. Protein concentrations
were measured using the BCA™ protein assay kit (Thermo Fisher
Scientific Inc., Rockford, IL, USA), according to the
manufacturer’s instructions. Samples of each protein (30 μg) were
loaded onto 7.5% sodium dodecyl sulfate-polyacrylamide gels.
Following electrophoresis, the protein was transferred to
polyvinylidene difluoride membranes and blocked with Blocking
One® (Nacalai Tesque, Inc., Kyoto, Japan) for 1 h, prior
to incubation with antibody overnight at 4°C. The membranes were
then washed with wash buffer (PBS containing 0.05% Tween 20) and
incubated with horseradish peroxidase-linked secondary antibody for
1 h. Subsequent to being washed with wash buffer, the protein
levels were analyzed by enhanced chemiluminescence using
Pierce® western blotting substrate (Thermo Fisher
Scientific Inc.).
Statistical analysis
Statistical analysis was performed using a one-way
analysis of variance, followed by Williams’ multiple comparison
test. P<0.01 was considered to indicate a statistically
significant difference.
Results
Effects of CAUE on DNA, RNA and protein
synthesis
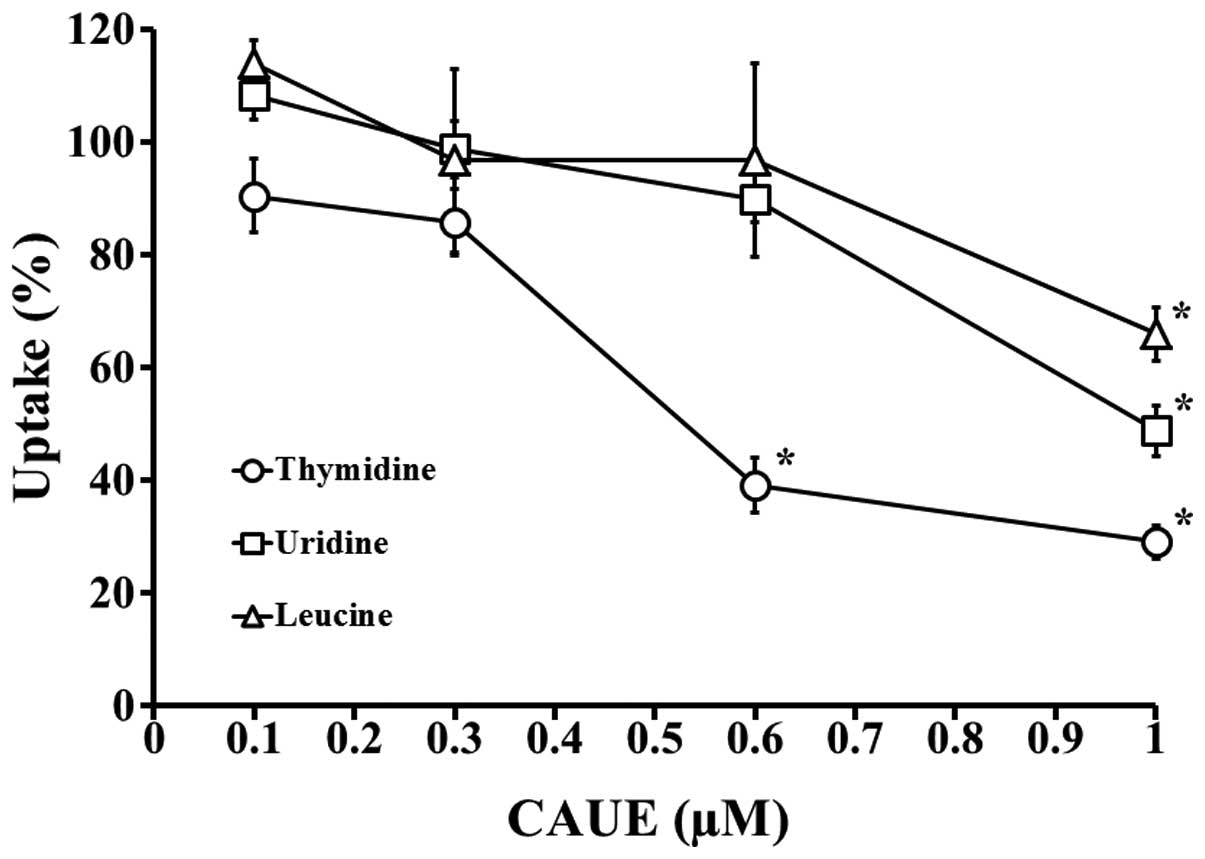
To investigate the cytotoxic mechanisms of CAUE, the
kinetics of macromolecule synthesis were examined (Fig. 1) and the incorporation of
radiolabeled substrates into DNA, RNA and protein was monitored. No
effect was identified on CAUE at concentrations of <0.3 μM,
however, CAUE showed significant inhibition of DNA replication at
0.6 μM (39.1% vs. CAUE vehicle group). In addition, no effects were
identified on RNA and protein synthesis. Following treatment with
higher concentrations of CAUE (1 μM), the DNA, RNA and protein
levels significantly decreased to 29.0, 48.8 and 65.9%,
respectively.
Effects of CAUE on telomerase activity
and expression of hTERT
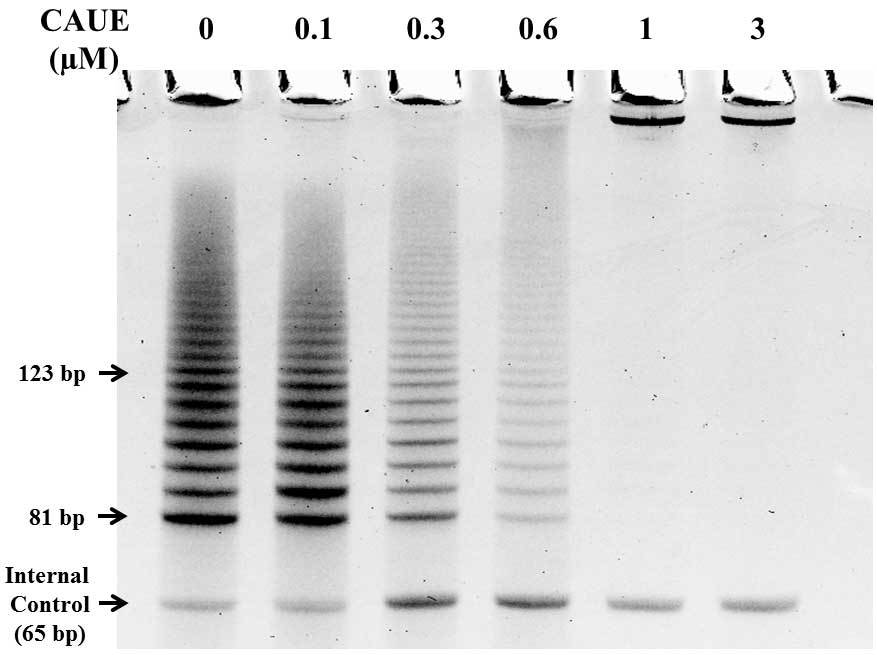
To examine the effects of CAUE on telomerase
activity, the NALM-6 cells were incubated in the absence (CAUE
vehicle) or presence of CAUE. Telomerase activity was measured by
stretch PCR (Fig. 2) and expressed
as a ladder of 6-bp bands or multiples of 6-bp intervals.
Telomerase activity was significantly suppressed following
treatment with CAUE in a concentration-dependent manner when
compared with the untreated cells. The percentage inhibition of
telomerase was calculated using the band intensity, and the results
revealed that when compared with that of the CAUE vehicle group
(100%) telomerase activity decreased to 92, 64, 19 and 0% following
treatment with 0.1, 0.3, 0.6 and 1 μM CAUE, respectively. To verify
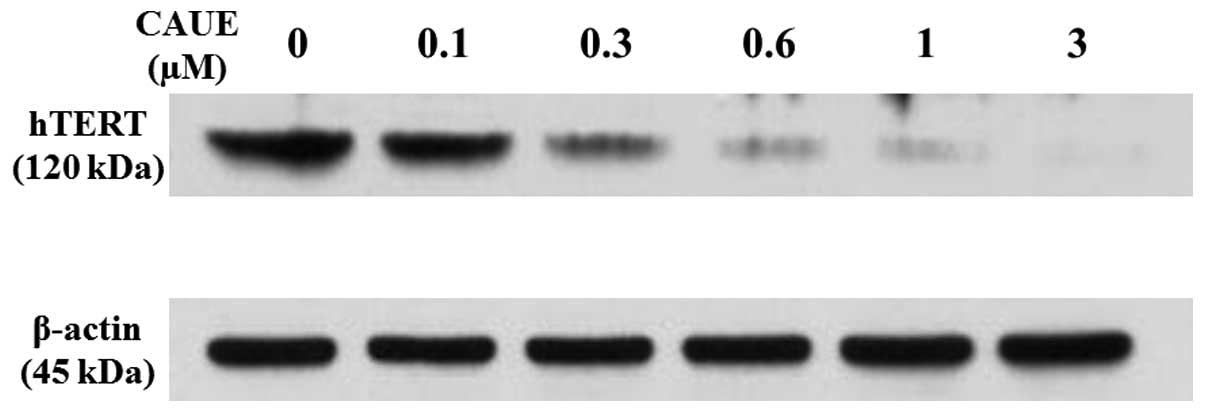
the mechanisms for the inhibitory effect of CAUE on telomerase
activity, the telomerase-component gene was investigated in the
NALM-6 cells to determine if CAUE was able to modulate its
expression. The hTERT subunit of telomerase functions as a critical
determinant of enzyme activity (11), therefore, changes in hTERT protein
expression due to CAUE treatment were examined by western blotting.
As presented in Fig. 3, CAUE
induced a concentration-dependent decrease in hTERT expression
compared with the CAUE vehicle group (100%). At concentrations of
0.1, 0.3, 0.6 and 1 μM CAUE, hTERT expression was 96, 48, 11 and
7%, respectively, as determined by densitometry analysis. The
highest concentration of CAUE (3 μM) showed complete inhibition of
telomerase activity (Fig. 2) and
hTERT expression (Fig. 3).
Discussion
Our previous study demonstrated that CAUE exhibited
potent cytotoxic effects on human B-cell leukemia NALM-6 cells, but
not on normal human lymphocytes (6). Activated B cells exhibit significantly
longer telomeres and increased telomerase activity (12). The present study aimed to
investigate the cytotoxic mechanisms of CAUE in NALM-6 cells and,
as shown in Fig. 1, CAUE exhibited
preferential damage to DNA synthesis compared with RNA and protein
synthesis. This indicated that CAUE directly affects the nucleus
and impairs DNA synthesis, resulting in the induction of
apoptosis.
Caffeic acid phenethyl ester is a parent compound of
CAUE and one of its pharmacological mechanisms of DNA damage
involves the inhibition of nuclear factor κB (NF-κB) (13). Caffeic acid derivatives block NF-κB
activation (7), and it has been
hypothesized that NF-κB inhibitory molecules are clinically
beneficial as single therapeutic agents or in combination with
classical chemotherapeutic agents for the treatment of
hematological malignancies (14).
Therefore, CAUE may inhibit NF-κB in leukemia cells and damage DNA
to trigger the induction of apoptosis. NF-κB regulates hTERT
expression by binding to a site 350-bp upstream of the
translational initiation site (15). In addition, it has been reported
that telomerase directly regulates NF-κB-dependent genes in cancer
cells (16). Thus, there is a close
correlation between NF-κB and telomerase activity. The results of
the present study indicate that CAUE inhibits telomerase activation
via mediation of hTERT protein expression, therefore, we
hypothesize that the inhibition by CAUE is dependent on the
inhibition of NF-κB activation.
In conclusion, CAUE inhibits DNA synthesis and
suppresses telomerase activity. Targeting the inhibition of
telomerase has been hypothesized to be beneficial for cancer
chemotherapy due to its selectivity against malignant cells,
thereby reducing side-effects. Telomerase inhibition is likely to
be tested on humans in the future, in order to treat lymphoid
cancers, including B-cell leukemia (17). The observations of the present study
may therefore aid the development of therapeutic strategies for
leukemia patients.
References
|
1
|
Counter CM, Avilion AA, LeFeuvre CE, et
al: Telomere shortening associated with chromosome instability is
arrested in immortal cells which express telomerase activity. EMBO
J. 11:1921–1929. 1992.PubMed/NCBI
|
|
2
|
Kim NW, Piatyszek MA, Prowse KR, et al:
Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shay JW and Wright WE: Telomerase activity
in human cancer. Curr Opin Oncol. 8:66–71. 1996. View Article : Google Scholar
|
|
4
|
Colangelo D and Osella D: Telomerase
inhibition and cancer: might platinum based drugs have a future as
anti-telomerase pharmacological approach? Curr Med Chem.
12:3091–3102. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schiffer CA: Treatment of high-grade
lymphoid malignancies in adults. Semin Hematol. 38(4 Suppl 10):
22–26. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tomizawa A, Kanno S, Osanai Y, et al:
Induction of apoptosis by a potent caffeic acid derivative, caffeic
acid undecyl ester, is mediated by mitochondrial damage in NALM-6
human B cell leukemia cells. Oncol Rep. 29:425–429. 2013.PubMed/NCBI
|
|
7
|
Uwai K, Osanai Y, Imaizumi T, Kanno S,
Takeshita M and Ishikawa M: Inhibitory effect of the alkyl side
chain of caffeic acid analogues on lipopolysaccharide-induced
nitric oxide production in RAW264.7 macrophages. Bioorg Med Chem.
16:7795–7803. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanno S, Maeda N, Tomizawa A, Yomogida S,
Katoh T and Ishikawa M: Involvement of p21waf1/cip1 expression in
the cytotoxicity of the potent histone deacetylase inhibitor
spiruchostatin B towards susceptible NALM-6 human B cell leukemia
cells. Int J Oncol. 40:1391–1396. 2012.PubMed/NCBI
|
|
9
|
Watanabe K, Kanno S, Tomizawa A, Yomogida
S and Ishikawa M: Acacetin induces apoptosis in human T cell
leukemia Jurkat cells via activation of a caspase cascade. Oncol
Rep. 27:204–209. 2012.PubMed/NCBI
|
|
10
|
Kanno S, Higurashi A, Watanabe Y, Shouji
A, Asou K and Ishikawa M: Susceptibility to cytosine arabinoside
(Ara-C)-induced cytotoxicity in human leukemia cell lines. Toxicol
Lett. 152:149–158. 2004.PubMed/NCBI
|
|
11
|
Greider CW: Telomerase activation. One
step on the road to cancer? Trends Genet. 15:109–112. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martens UM, Brass V, Sedlacek L, et al:
Telomere maintenance in human B lymphocytes. Br J Haematol.
119:810–818. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ozturk G, Ginis Z, Akyol S, Erden G, Gurel
A and Akyol O: The anticancer mechanism of caffeic acid phenethyl
ester (CAPE): review of melanomas, lung and prostate cancers. Eur
Rev Med Pharmacol Sci. 16:2064–2068. 2012.PubMed/NCBI
|
|
14
|
Braun T, Carvalho G, Fabre C, Grosjean J,
Fenaux P and Kroemer G: Targeting NF-kappaB in hematologic
malignancies. Cell Death Differ. 13:748–758. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yin L, Hubbard AK and Giardina C: NF-kappa
B regulates transcription of the mouse telomerase catalytic
subunit. J Biol Chem. 275:36671–36675. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghosh A, Saginc G, Leow SC, et al:
Telomerase directly regulates NF-κB-dependent transcription. Nat
Cell Biol. 14:1270–1281. 2012.
|
|
17
|
Lobetti-Bodoni C, Bernocco E, Genuardi E,
Boccadoro M and Ladetto M: Telomeres and telomerase in normal and
malignant B-cells. Hematol Oncol. 28:157–167. 2010. View Article : Google Scholar : PubMed/NCBI
|