Introduction
Breast cancer is the most common type of cancer
diagnosed in women in the United States (US) (1). Cancer of the endometrium is the fourth
most common cancer diagnosis in US women, following cancers of the
lung and bronchus and the colon and rectum (1). Breast cancer is the second most common
cause of cancer mortality in US women, following mortalities due to
lung and bronchial cancers; endometrial cancers are eighth on the
list of mortalities due to cancer in US women (1).
The American Cancer Society has estimated that there
were 226,870 new cases of invasive breast cancer and 63,300 new
cases of in situ breast cancer in the year 2012 (1). The lifetime risk for a diagnosis of
breast cancer based on the 2006–2008 rates was reported at 12.29%
(2). The Surveillance, Epidemiology
and End Results (SEER) database reported the median age of
diagnosis of breast cancer during 2004–2008 as 61 years old, while
the median age of mortality due to breast cancer was 68 years old
(2). The age-adjusted incidence
rate during the same time frame was 124.0/100,000 women/year, while
the age adjusted mortality rate was 23.5/100,000 women/year
(2). The five-year relative
survival for 2001–2007 in the SEER group was 89.1%. When adjusted
by stage, the SEER reported a five-year relative survival of 98.6%
for those with locally confined disease, 83.8% for those with
regional lymph node disease and 23.3% for those with metastatic
disease (2).
The American Cancer Society has estimated that there
were 47,130 new cases of endometrial cancer in the year 2012
(1). The lifetime risk for a
diagnosis of endometrial cancer based on the 2006–2008 rates was
reported at 2.61% (3). The SEER
database reported the median age of diagnosis of endometrial cancer
during 2004–2008 as 61 years old, while the median age of mortality
due to endometrial cancer was 72 years old (3). The age adjusted incidence rate during
the same time frame was 23.9/100,000 women/year, while the age
adjusted mortality rate was 4.2/100,000 women/year (3). The five-year relative survival for
2001–2007 in the SEER group was 81.8%. When adjusted by stage, the
SEER reported a five-year relative survival of 95.8% for those with
locally confined disease, 67.0% for those with regional lymph node
disease and 15.9% for those with metastatic disease (3).
The overall survival outcomes of women who have been
diagnosed with breast and endometrial cancer have not previously
been reported in the literature. To that end, the present study
investigated the survival data with regard to patients diagnosed
with synchronous or metachronous breast and endometrial cancer,
utilizing SEER data.
Materials and methods
The present study was a retrospective,
population-based cohort study of women with a primary diagnosis of
invasive breast cancer plus a primary diagnosis of endometrial
adenocarcinoma. The SEER program database was utilized to gather
the study patients. The patients included in the study were
diagnosed between January 1, 1988 and December 31, 2007. All study
patients were recorded in the SEER database as not having evidence
of distant metastases at the time of diagnosis. Additionally, all
study patients had been followed up for at least one after the
second cancer diagnosis was recorded.
The sequence of diagnosis of tumor type was
recorded. The status at the end of the study was recorded as alive,
breast cancer-related mortality, endometrial cancer-related
mortality or mortality due to other causes. The histological grades
were recorded as well-differentiated (grade I),
moderately-differentiated (grade II), poorly-differentiated (grades
III–IV) or unknown. The pathological lymph node status was recorded
as negative, positive or unknown. The breast cancer receptor status
for the estrogen receptor (ER) and the progesterone receptor (PR)
was recorded as positive, negative or unknown. The age at the time
of the second tumor diagnosis was recorded in years and the time
between the first and second tumor diagnoses was recorded in
months.
The endpoints for this study were breast
cancer-specific mortality and endometrial cancer-specific
mortality. These endpoints were recorded using the cause of
mortality and the total completed months of follow-up noted in the
SEER database.
A comparative risk regression analysis was used to
analyze the risk of mortality secondary to breast cancer or
endometrial cancer with regard to the tumor type at first
diagnosis, the lymph node status, the histological differentiation
of the two tumor types and the hormone receptor status. This
analysis was dichotomized into an early follow-up period (<2.5
years) and a late follow-up period (2.5–5 years) to account for a
survival crossover observed in the cumulative risk analysis. A
cause-specific cumulative risk analysis was performed in the
analysis of the risk of mortality with regard to the order of the
tumor type diagnosis. All analyses utilized a null hypothesis
rejection with a P-value of <0.05. All statistical analyses were
performed using R version 2.13.0 of the cmprsk package (4).
Results
Patients and demographics
Using the SEER database, a total of 2,027 women who
had a primary diagnosis of invasive breast cancer plus a primary
diagnosis of endometrioid-type endometrial cancer were identified
during the period of 1998–2007. Table
I provides a summary of the patient and tumor characteristics
that were utilized for the present study. During the study period,
1,296 women (63.9%) were identified with an initial cancer
diagnosis of invasive breast cancer. The remaining 731 (36.1%) were
women with an initial cancer diagnosis of endometrial cancer or
those who had endometrial cancer diagnosed synchronously with their
breast cancer. The median age at the time of the diagnosis of the
second cancer was 68 years old. The median time measured between
the initial diagnosis of cancer and the diagnosis of the second
cancer type was 45 months. At the end of the study period, 1,703
women (84.0%) were still living, while 324 women (16%) had
succumbed to various causes. The cause of mortality recorded in the
SEER database was attributed to breast cancer in 83 women (4.1%),
to endometrial cancer in 63 women (3.1%) and to other causes not
associated with breast or endometrial cancer in 178 women
(8.8%).
 | Table ISummary of characteristics of interest
for 2,027 women diagnosed with breast and endometrial
carcinoma. |
Table I
Summary of characteristics of interest
for 2,027 women diagnosed with breast and endometrial
carcinoma.
| Characteristic | Value |
|---|
| First tumor
diagnosis, n (%) |
| Breast cancer | 1296 (63.9) |
| Endometrial
cancer/synchronous | 731 (36.1) |
| Status at end of
study, n (%) |
| Alive | 1703 (84.0) |
| Breast
mortality | 83 (4.1) |
| Endometrial
mortality | 63 (3.1) |
| Other mortality | 178 (8.8) |
| Endometrium
histological grade, n (%) |
| Well-differentiated
(SEER grade I) | 913 (45.0) |
|
Moderately-differentiated (SEER grade
II) | 643 (31.7) |
|
Poorly-differentiated (SEER grades
III–IV) | 316 (15.6) |
| Unknown | 155 (7.6) |
| Breast histological
grade, n (%) |
| Well-differentiated
(SEER grade I) | 394 (19.4) |
|
Moderately-differentiated (SEER grade
II) | 813 (40.1) |
|
Poorly-differentiated (SEER grades
III–IV) | 641 (31.6) |
| Unknown | 179 (8.8) |
| Endometrium lymph
node status, n (%) |
| Negative | 980 (48.3) |
| Positive | 87 (4.3) |
| Unknown | 960 (47.4) |
| Breast lymph node
status, n (%) |
| Negative | 1263 (62.3) |
| Positive | 522 (25.8) |
| Unknown | 242 (11.9) |
| Breast ER status, n
(%) |
| Negative | 323 (15.9) |
| Positive | 1364 (67.3) |
| Unknown | 340 (16.8) |
| Breast PR status, n
(%) |
| Negative | 466 (23.0) |
| Positive | 1178 (58.1) |
| Unknown | 383 (18.9) |
| Median age at second
tumor diagnosis, years (interquartile range) | 68 (60–76) |
| Median time between
first and second tumors, months (interquartile range) | 45 (17–81) |
The tumor characteristics shown in Table I demonstrate that cancers of the
endometrium were more likely to be of a lower histological grade at
the time of diagnosis. Endometrial cancers were observed to be
histologically well-differentiated in 913 of patients (45.0%),
moderately-differentiated in 643 of patients (31.7%) and
poorly-differentiated in 316 of patients (15.6%). This was compared
with the findings in the breast tumors, which were histologically
well-differentiated in 394 of patients (19.4%),
moderately-differentiated in 813 of patients (40.1%) and
poorly-differentiated in 641 of patients (31.6%). The histological
grade could not be determined from the SEER database in 155 (7.6%)
of patients with endometrial tumors and in 179 (8.8%) of patients
with breast tumors. The lymph node status at the time of diagnosis
was less likely to be known for the endometrial tumors, although
when it was known, it was positive for disease in only 87 (4.3%) of
patients and negative in 980 (48.3%) of patients. The lymph node
disease burden in breast cancer was noted to be negative in 1,263
(62.3%) of patients and positive in 522 (25.8%) of patients. The
hormone receptor status of the breast tumors revealed that the
tumors were more likely to be positive rather than negative for ER
and PR. The ER status was negative in 323 (15.9%) of tumors,
positive in 1,364 (67.3%) of tumors and unidentifiable in 340
(16.8%) of tumors. The PR status was negative in 466 (23.0%) of
tumors, positive in 1,178 (58.1%) of tumors and unidentifiable in
383 (18.9%) of tumors.
Mortality risk analyses
The results of the analysis of the cause-specific
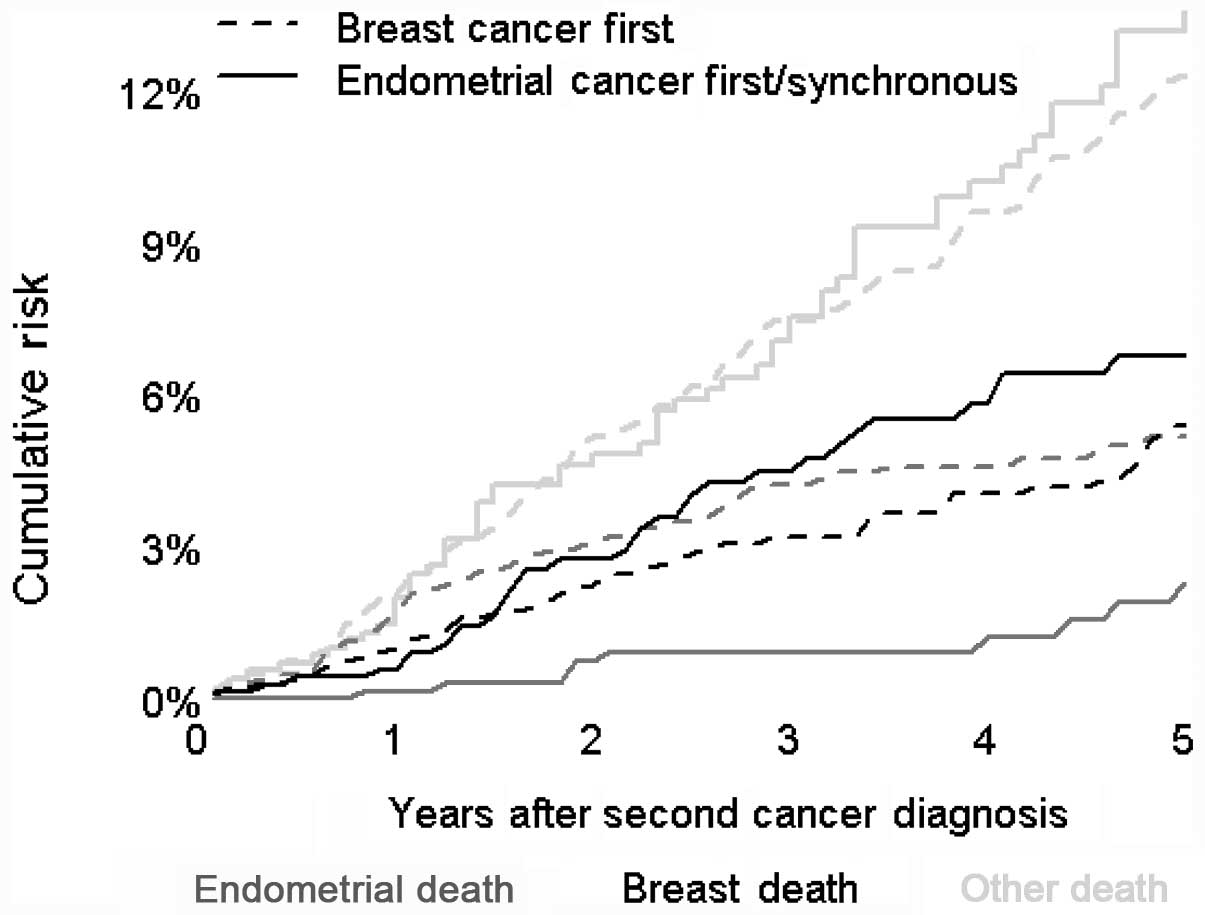
cumulative risks of mortality are shown in Fig. 1. The greatest risk of mortality,
independent of which tumor type was identified at the primary
diagnosis, was attributed to factors other than breast or
endometrial cancer. The risk of breast cancer being the cause of
mortality was similar regardless of whether the patients were
initially diagnosed with breast or endometrial cancer. The risk of
mortality attributed to endometrial cancer was also similar to the
risk of succumbing to breast cancer at the five-year time-point, if
the tumor at the initial diagnosis was breast cancer. The risk of
mortality attributed to endometrial cancer, if the tumor at the
initial diagnosis was endometrial cancer, was lowest at the
five-year time-point when compared with other causes.
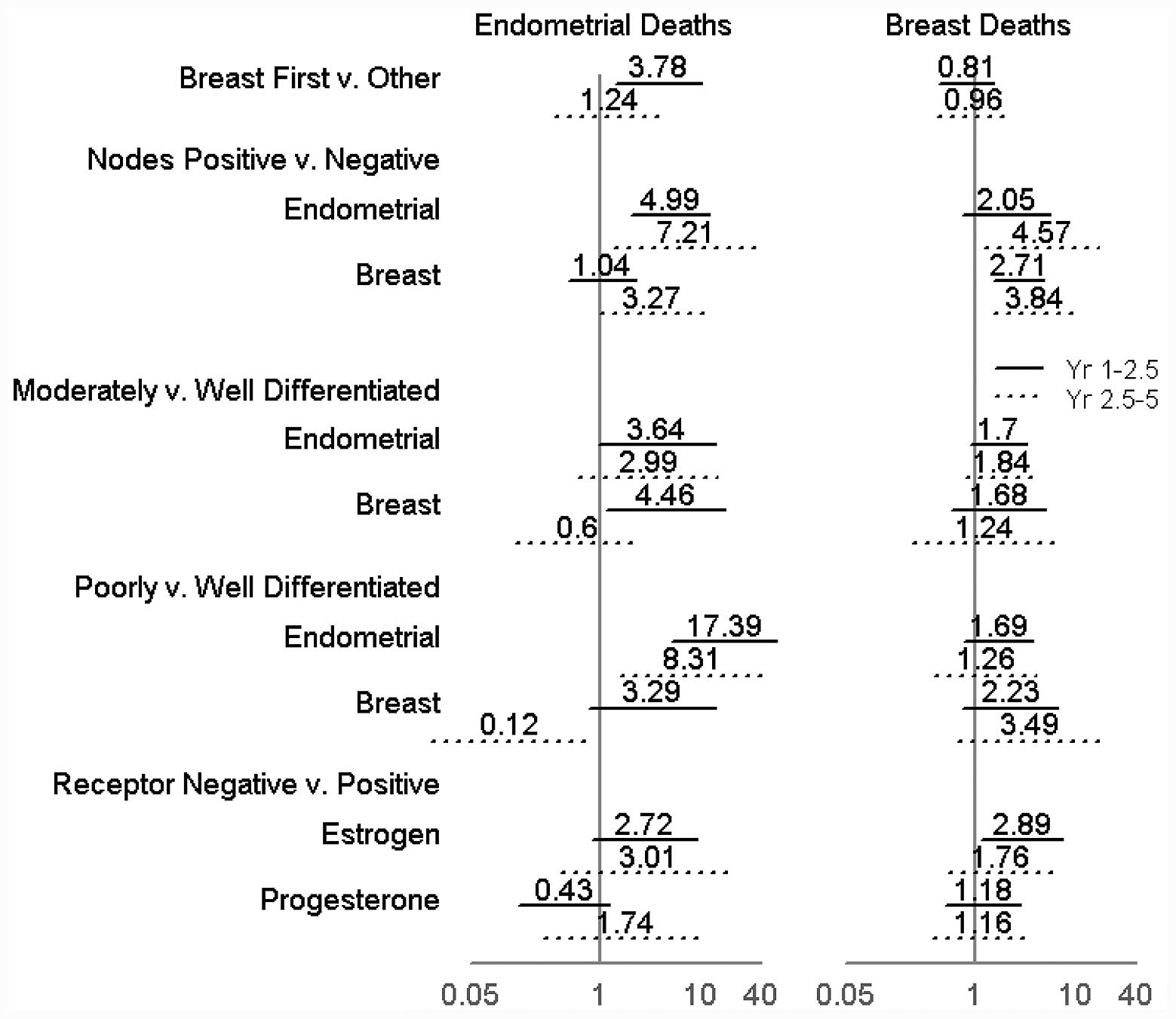
The regression analyses of the comparative risk of
endometrial cancer or breast cancer mortalities as associated with
various factors are summarized in Fig.
2. The analysis was performed using two time periods in the
study; the study time was divided at the 2.5-year mark. This was
established due to the dichotomy of the endometrial cancer
mortality cumulative risk lines observed in Fig. 1. The lines deviated from each other
in the first half of the study, but became parallel in the second
half.
Prognostic factors
As expected, in the two halves of the study,
positive lymph node disease was associated with an increased risk
of mortality of the respective cancer type. The positive burden of
breast cancer in the lymph nodes increased the risk of mortality
from breast cancer in the first [Hazard ratio (HR), 2.71) and
second half (HR, 3.84) of the study. The positive burden of
endometrial cancer in the lymph nodes increased the risk of
mortality from endometrial cancer in the first (HR, 4.99) and
second half (HR, 7.21) of the study. The presence of lymph nodes
with an endometrial cancer burden was also associated with a
significant increase in the risk of mortality due to breast cancer
in the second half of the study compared with the first half of the
study (HR, 2.05 and HR, 4.57, respectively). The histological grade
of breast cancer, when adjusted for other factors, did not have a
significant association with breast cancer mortalities. Endometrial
cancer mortalities did show an increased association with a
poorly-differentiated tumor status upon histological examination
when compared with the well-differentiated tumors. This effect was
more significant in the first half of the study period compared
with the second half (HR, 17.39 and 8.31, respectively). The risk
of mortality from endometrial cancer was also observed to have a
significant association with the differentiation level of the
breast tumor. In the first half of the study, breast tumors with
moderate or poor differentiation were associated with an increased
risk of mortality from endometrial cancer (HR, 4.46 and 3.29,
respectively). Conversely, in the second half of the study breast
tumors with poor differentiation were associated with a decreased
risk of mortality from endometrial cancer (HR, 0.12). The only
association of significance with regard to hormone receptor status
was identified in the first half of the study, whereby a negative
ER status in a breast tumor was associated with an increased risk
of mortality due to breast cancer (HR, 2.89).
Discussion
Breast cancer is the most commonly diagnosed cancer
in women in the US and endometrial cancer is the fourth most common
cancer diagnosis (1). The present
study investigated the impact of a synchronous or metachronous
diagnosis of invasive breast and endometrial cancer on survival
outcomes. This appears to be the first study of survival outcomes
as impacted by these two types of cancer. The present study was an
observational study of 2,027 women identified from the SEER
database as having a diagnosis of both types of cancer. The results
of this study may aid clinicians in treating patients diagnosed
with both types of cancer.
The association of endometrial cancer following the
treatment of a previously diagnosed breast cancer has been
established in the literature, specifically with regard to the use
of tamoxifen in the adjuvant treatment of breast cancer (5,6). The
various types of endometrial cancer that develop in women during
and after tamoxifen therapy have been previously studied in the
literature (5,6). Women were observed to be more likely
to develop a high-grade or high-risk type of endometrial cancer
(type II) when the diagnosis was established following the
cessation of tamoxifen. Bland et al(7) noted this difference after a six-month
time frame from the completion of therapy and Ferguson et
al(8) noted it after a 12-month
period between tamoxifen therapy discontinuation and endometrial
cancer diagnosis. It has also been observed that there is an
increased risk of these high-risk subtypes of endometrial cancer in
women who complete the standard five-year course of tamoxifen
therapy compared with those are administered it for <5 years
(7). The present study specifically
investigated the endometrioid variant of endometrial cancer, which
is classified as a type I endometrial tumor in the majority of
cases, although if it is of a high histological grade it may be
classified as a type II tumor. We were unable to discern with
certainty whether any cases of endometrial cancer in the present
patients were due to tamoxifen therapy, as this variable was not
recorded in the SEER dataset. There were 1,296 (63.9%) women who
were diagnosed with breast cancer first and 67.3% of the breast
tumors in the study were ER-positive. There was a median of 45
months and an interquartile range of 17–81 months between the
diagnoses of the first and second tumors. We would infer from this
data that a significant percentage of these patients were likely
offered endocrine therapy as adjuvant treatment for their breast
cancer, but we are unable to determine whether tamoxifen was
utilized, versus an aromatase inhibitor, or whether there was a
causal relationship with the patients’ subsequent endometrial
cancer.
In the present study, the risk of mortality due to
other causes was greater than the risk of mortality from either
breast or endometrial cancer. This finding of ‘other cause’
mortality has been documented in previous literature for breast
cancer, but the data is unclear on this matter for endometrial
cancer (9). In the present
analysis, it was shown that the risk of mortality from breast
cancer was similar regardless of which tumor type was diagnosed
initially. By contrast, the risk of mortality from endometrial
cancer was markedly lower if the initial diagnosis was endometrial
cancer. This may also be related to the fact that patients whose
first diagnosis was breast cancer would likely have received
tamoxifen and subsequently were at risk of developing a higher
grade of endometrial cancer, as opposed to those whose initial
diagnosis of endometrial cancer was more likely to be of a lower
tumor grade (10,11).
There were a number of unexpected outcomes from the
present analysis. As expected, the patients with the higher
histological grades of endometrial cancer were more likely to
succumb to endometrial cancer. However, this effect was not
observed in the analyses of histological grades and breast
cancer-specific survival. Although the histological grade of breast
tumors has been shown to be correlated with a poorer prognosis in
previous studies (12–14), the present study did not observe any
significant effect on the risk of mortality from breast cancer
based on the increasing histological grade. There was an unexpected
effect of the breast tumor histological grade on the risk of
mortality from endometrial cancer. In the present study, patients
who had high-grade breast tumors were at an increased risk of
mortality due to endometrial cancers in the first half of the
study. This effect was not observed in the second half of the
study. The clinical significance of this finding is unclear. It may
be a reflection of the shorter interval between the two cancer
diagnoses and the more aggressive biology of the endometrial
cancer.
As expected, as lymph node burden increases for a
specific cancer, there is a concomitantly increased risk of
mortality from that specific disease. An unexpected finding was
observed in the later stage of the study, where the lymph node
burden of endometrial cancer showed significance in an increased
association with mortality due to breast cancer. There was also an
increased risk of mortality due to endometrial cancer with a
positive lymph node burden of breast cancer in the second half of
the study, although this association was not at a statistically
significant level. Although these correlations between lymph node
disease and mortality due to the opposing cancer type were of
statistical interest in the present analyses, it is unclear whether
there is a clinical link between the two histologies that would
result in this finding.
There are a number of limitations to the present
study that are derived from its nature as a retrospective cohort
study. The primary outcome that was assessed was cancer-specific
mortality, but the comparison only included patients with a
diagnosis of both cancer types. A helpful addition would be the
comparison of this group with patients with a diagnosis of breast
or endometrial cancer only. There are also limitations associated
with the use of the SEER database and the information available for
analysis. The addition of information with regard to the
comorbidities, the margin status of tumor resections and the
adjuvant treatments are important variables that were not available
in the present analysis of this specific group of patients. The
majority of patients did not have lymph node disease and there was
a large volume of patients with unknown lymph node status in the
endometrial cancer group. The results of the analysis may be
different if a group of patients with a larger burden of disease at
diagnosis was examined. Despite these limitations, the SEER
database is a large population database that is used frequently in
epidemiological studies (15).
The present study provides the first mortality
analysis of patients with either synchronous or metachronous breast
and endometrial cancer, two commonly diagnosed cancers among women
in the US. These findings should be considered when clinicians
enter discussions concerning prognoses with patients of similar
standing. It is important to encourage patients to continue
surveillance for a second type of cancer even after they have been
diagnosed with a primary type of cancer. It is equally important
for clinicians to continue screening practices for other cancers
for patients who have been treated for another cancer diagnosis.
For example, women who have been diagnosed with endometrial cancer
should be encouraged to continue to undergo annual screening
mammography. Furthermore, it is of particular importance for
clinicians to educate patients who have been treated with adjuvant
tamoxifen for breast cancer on the signs and symptoms of
endometrial cancer and the necessity of reporting these signs and
symptoms to their physician in a timely manner so that diagnostic
interventions may be utilized.
References
|
1
|
American Cancer Society. Cancer Facts and
Figures. 2012, http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf.
Accessed January 23, 2012
|
|
2
|
National Cancer Institute. SEER Stat Fact
Sheets: Breast Cancer Statistics. http://seer.cancer.gov/statfacts/html/breast.html.
Accessed March 6, 2012
|
|
3
|
National Cancer Institute. SEER Stat Fact
Sheets: Corpus and Uterus, NOS. Cancer Statistics. http://seer.cancer.gov/statfacts/html/corp.html.
Accessed March 6, 2012
|
|
4
|
R Development Core Team. R: A language and
environment for statistical computing. R Foundation for Statistical
Computing; Vienna, Austria: 2011, http://www.r-project.org.
Accessed March 16, 2012
|
|
5
|
Fisher B, Costantino JP, Redmond CK,
Fisher ER, Wickerham DL and Cronin WM: Endometrial cancer in
tamoxifen-treated breast cancer patients: findings from the
National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J
Natl Cancer Inst. 86:527–537. 1994. View Article : Google Scholar
|
|
6
|
Fisher B, Costantino JP, Wickerham DL,
Cecchini RS, Cronin WM, Robidoux A, Bevers TB, Kavanah MT, et al:
Tamoxifen for the prevention of breast cancer: current status of
the National Surgical Adjuvant Breast and Bowel Project P-1 study.
J Natl Cancer Inst. 97:1652–1662. 2005. View Article : Google Scholar
|
|
7
|
Bland AE, Calingaert B, Secord AA, Lee PS,
Valea FA, Berchuck A, Soper JT and Havrilesky L: Relationship
between tamoxifen use and high risk endometrial cancer histologic
types. Gynecol Oncol. 112:150–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferguson SE, Soslow RA, Amsterdam A and
Barakat RR: Comparison of uterine malignancies that develop during
and following tamoxifen therapy. Gynecol Oncol. 101:322–326. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chapman JA, Meng D, Shepherd L, Parulekar
W, Ingle JN, Muss HB, Palmer M, Yu C and Goss PE: Competing causes
of death from a randomized trial of extended adjuvant endocrine
therapy for breast cancer. J Natl Cancer Inst. 100:252–260. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bergman L, Beelen ML, Gallee MP, Hollema
H, Benraadt J and van Leeuwen FE: Risk and prognosis of endometrial
cancer after tamoxifen for breast cancer. Comprehensive Cancer
Centres’ ALERT Group Assessment of Liver and Endometrial cancer
Risk following Tamoxifen. Lancet. 356:881–887. 2000.PubMed/NCBI
|
|
11
|
Saadat M, Truong PT, Kader HA, Speers CH,
Berthelet E, McMurtrie E and Olivotto IA: Outcomes in patients with
primary breast cancer and a subsequent diagnosis of endometrial
cancer: comparison of cohorts treated with and without tamoxifen.
Cancer. 110:31–37. 2007. View Article : Google Scholar
|
|
12
|
Arriagada R, Le MG, Dunant A, Tubiana M
and Contesso G: Twenty-five years of follow-up in patients with
operable breast carcinoma: correlation between clinicopathologic
factors and the risk of death in each 5-year period. Cancer.
106:743–750. 2006.PubMed/NCBI
|
|
13
|
Soerjomataram I, Louwman MW, Ribot JG,
Roukema JA and Coebergh JW: An overview of prognostic factors for
long-term survivors of breast cancer. Breast Cancer Res Treat.
107:309–330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Russo J, Frederick J, Ownby HE, Fine G,
Hussain M, Krickstein HI, Robbins TO and Rosenberg B: Predictors of
recurrence and survival of patients with breast cancer. Am J Clin
Pathol. 88:123–131. 1987.PubMed/NCBI
|
|
15
|
Merrill RM and Dearden KA: How
representative are the surveilance, epidemiology, and end results
(SEER) program cancer data of the United States. Cancer Causes
Control. 15:1027–1034. 2004. View Article : Google Scholar : PubMed/NCBI
|