Introduction
Inappropriate activation of survival signaling
pathways causes uncontrolled proliferation, resistance to apoptosis
and an increased motility of cells and is important for cancer
development, progression and resistance to treatment (1). In nasopharyngeal carcinoma (NPC),
standard treatments with systemic agents, including adjuvant
cisplatin chemotherapy, has been unrewarding in general (2,3), which
is primarily due to the failure in the induction of cell death and
the development of the resistance mechanism(s) to chemotherapeutic
reagents (4,5) and radioactive rays (6).
Apoptosis is the principal mechanism by which cells
are physiologically eliminated in metazoan organisms (7). During apoptotic death, cells are
digested by caspases and packaged into apoptotic bodies as a
mechanism to avoid immune activation. Necrosis, previously
hypothesized as a passive, unorganized method of cell death, has
emerged as an alternate form of programmed cell death whose
activation may have important biological consequences, including
the induction of an inflammatory response (7). Autophagy has also been reported as a
possible mechanism for non-apoptotic death despite evidence from a
number of species showing that autophagy represents a survival
strategy in times of stress. Recent advances have been important
for the definition of the function and mechanism of programmed
necrosis and the role of autophagy in cell survival and suicide
(7).
Autophagy is a highly-conserved pathway in
eukaryotic cells that has evolved to degrade bulk cytoplasmic
material (8). Autophagy, a process
of ‘self-eating’, has been classically studied in response to
energy deprivation, including that which results from nutritional
starvation of amino acids or fatty acids (9). Autophagy also provides an important
function in the clearance of aggregated or misfolded proteins
(10). However, it has also been
reported to play a dual role in cancer and the induction of
autophagy has been demonstrated to inhibit tumor cell growth and
result in autophagic cell death (11). By contrast, it has been shown that
autophagy may function as a cytoprotective mechanism by responding
to stress situations, including hypoxia, low energy, oxidative
stress and damaged mitochondria (12).
In the present study, we aimed to investigate the
role of 3-methyladenine (3-MA) in NPC therapies, including
chemotherapy and radiotherapy, as well as determine whether
cisplatin (DDP), ionizing radiation (IR), 2-deoxy-D-glucose (2-DG)
or tunicamycin (TM) induce autophagy via endoplasmic reticulum (ER)
stress.
Materials and methods
Cell lines
Human NPC cell lines, CNE-1 and CNE-2, were obtained
from the American Type Culture Collection (Manassas, VA, USA) and
maintained at the Anhui Engineering Technology Research Center of
Biochemical Pharmaceuticals (Anhui, China). Cells were cultured in
DMEM medium containing 10% NBCS (both Gibco-BRL, Carlsbad, CA,
USA), 2 mmol/l L-glutamine, 100 U/ml penicillin and 100 μg/ml
streptomycin and cultured at 37°C in a humidified atmosphere of 95%
air and 5% CO2.
Drugs and antibodies
DDP was purchased from Qilu Pharmaceutical Co., Ltd.
(Jinan, China). 2-DG and TM were purchased from Sigma-Aldrich (St.
Louis, MO, USA). 3-MA was purchased from an affiliate of Merck KgaA
(Darmstadt, Germany). Horseradish peroxidase (HRP)-conjugated
anti-rabbit IgG, anti-rabbit IgG (H+L chains)-fluorescein
isothiocyanate (FITC), anti-LC3 and anti-beclin 1 were purchased
from MBL Biotech Co. (Beijing, China). β-actin was obtained from
Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell proliferation assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-di-
phenyltetrazolium bromide (MTT)was performed as described
previously (13). Briefly, cells
were plated in 96-well culture clusters (Costar, Cambridge, MA,
USA) at a density of 1×105 cells/well in triplicate. MTT
(5.0 mg/l; 15 μl) was added 4 h later, followed by the addition of
150 μl DMSO into each well. The absorbance (A) of the formazan
product was determined at 570 nm using a plate microreader and
calculated using the following formula: Cell viability (%) =
(Asample − Ablank)/(Acontrol −
Ablank) × 100.
Annexin V/propidium iodide (PI)-FITC
double staining
Cell apoptosis was detected using the annexin V-FITC
apoptosis detection kit (BD Pharmingen, San Diago, CA, USA)
according to the manufacturer’s instructions. Briefly, the cells
were seeded in 24-well plates and then subjected to various
experimental conditions for 24 h. Following incubation, the cells
were trypsinized and suspended in 1 ml phosphate buffered saline
(PBS). In each cell suspension, 2×105 cells were
centrifuged and re-suspended in 500 μl 1X binding buffer. In each
sample, 5 μl annexin V-FITC and 5 μl PI were added. The mixture was
incubated for 5 min in the dark and immediately analyzed using a
FACSCalibur flow cytometer and Cell Quest software (BD Biosciences,
San Jose, CA, USA).
Colony formation assay
A colony formation assay was performed as described
previously (14). In brief, the
cells were seeded at 1×103 cells/well in 6-well culture
plates, allowed to grow for 24 h and then treated as indicated. The
cells were then washed twice with ice-cold PBS and fixed with
ice-cold methanol for 10 min. Methanol was aspirated off from the
plates, 0.5% crystal violet solution (Sciencelab.com, Inc.,
Houston, TX, USA) was added and the plates were incubated at room
temperature for 10 min. Distilled water was used to rinse the
plate. Images were captured with the Bio-Rad VersaDoc™ imaging
system (Hercules, CA, USA).
Western blot analysis
A western blot analysis was performed as described
previously (15). Labeled bands
were detected by the Immun-Star™ HRP Chemiluminescence kit
(Bio-Rad) and images were captured using the Bio-Rad VersaDoc image
system.
Immunocytochemistry
The cells were seeded in 12-well plates at a density
of 1.2×105 cells/well. Following 24 h of drug or IR
exposure, the cells were washed with PBS twice and fixed with PBS
containing 4% paraformaldehyde for 15 min at room temperature.
Subsequent to being blocked with 5% bovine serum albumin for 2 h,
the cells were washed with PBS and incubated with anti-beclin 1
antibody (1:100) overnight at 4°C. The cells were then washed with
PBS and incubated in the dark with 100 μl FITC-conjugated
anti-rabbit IgG (1:100) for 2 h. The cells were washed with PBS and
the fluorescence signal was detected by an inverted fluorescent
microscope (Olympus Corporation, Tokyo, Japan).
DAPI staining
The cells were seeded in 12-well plates at a density
of 1.2×105 cells/well. Following 24 h of drug or IR
exposure, the cells were washed with PBS twice and fixed with PBS
containing 4% PFA for 15 min at room temperature. The cells were
washed further with PBS and then stained with 10 μg/ml DAPI
(Beyotime Biotech, Jiangsu, China) for 5 min in the dark at room
temperature. The solution was then removed and the cells were
washed twice with PBS and analyzed using an inverted fluorescence
microscope.
Mitochondrial membrane potential
(Δψm)
The cells were seeded at 1×105 cells/well
in 24-well plates and allowed to culture to reach exponential
growth for 24 h prior to treatment. Changes in Δψm were studied by
staining the cells with the cationic dye, JC-1, according to the
manufacturer’s instructions (Molecular Probes, Eugene, OR, USA), as
described previously (16). CCCP,
which induces apoptosis, was used as the positive control.
Statistical analysis
All experiments described were performed at least in
triplicate. Data are expressed as mean ± SD. All statistical
analyses were performed using two-tailed paired Student’s t-tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Sensitivity of human NPC cells to DDP-
and IR-induced apoptosis is not significant
The proliferation of the NPC cells was inhibited by
various concentrations of DDP and IR (Fig. 1A). This inhibition was enhanced with
increasing concentrations of DDP and IR and prolonged with
increasing duration of exposure of the cells to DDP and IR.
Following treatment with 6.00 μmol/l DDP for 24, 36 and 48 h, the
survival rate of the CNE-1 and CNE-2 cells reached 80.74, 79.95 and
78.92% and 82.15, 82.05 and 79.13%, respectively. Treatment of the
CNE-1 and CNE-2 cells with 4 Gy IR for 24, 36 and 48 h resulted in
a survival rate of 2.29, 73.51 and 62.35% and 92.35, 83.58 and
72.37%, respectively. Based on these results, 6.00 μmol/l DDP and 4
Gy IR was used, each for 24 h, for further experiments.
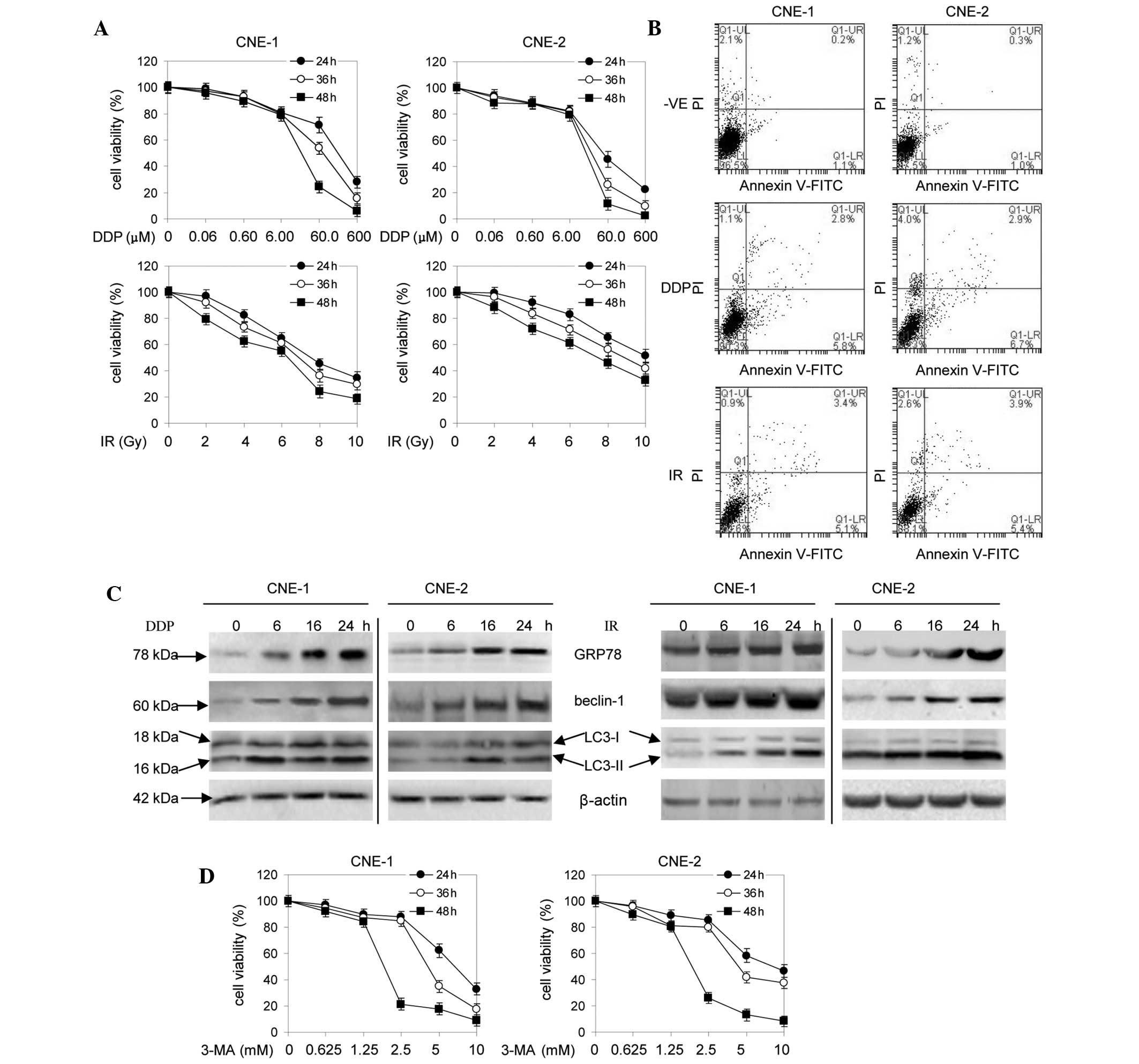 | Figure 1Effects of DDP, IR or 3-MA on the
viability and apoptosis of NPC cells. (A) Cells were treated with
0–600 μmol/l DDP or 0–10 Gy of IR for 24, 36 or 48 h and viability
was measured by MTT assay. (B) Cells were treated with 6 μmol/l DDP
or 4 Gy IR for 24 h and cell death was detected by Annexin
V-FITC/PI. (C) Cells were treated with 6 μmol/l DDP or 4 Gy IR for
6, 16 and 24 h. (D) Cells were treated with 0–10 mmol/l of 3-MA for
24, 36 or 48 h and viability was measured by MTT assay (n=3). DDP,
cisplatin; IR, ionizing radiation, 3-MA, 3-methyladenine; NPC,
nasopharyngeal carcinoma; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromide;
FITC, fluorescein isothiocyanate; PI, propidium iodide; LC3,
microtubule-associated protein 1 light chain 3. |
Following treatment with 6.00 μmol/l DDP for 24 h,
the induction of apoptosis in the CNE-1 and CNE-2 cells was only
5.8 and 6.7%, respectively, which was not statistically significant
when compared with the negative control group (Fig. 1B). Subsequent to treatment with 4 Gy
IR for 24 h, the induction of apoptosis in the CNE-1 and CNE-2
cells was only 5.1 and 5.4%, respectively, which was not
statistically significant when compared with the negative control
group (Fig. 1B).
DDP and IR induce ER stress and autophagy
in human NPC cells
It has been previously demonstrated that DDP and IR
activate autophagy via ER stress (17). Therefore, the expression levels of
GRP78, LC-3 and beclin 1 were determined. The levels of GRP78 and
beclin 1 were increased in the cells treated with 6.00 μmol/l DDP
or 4 Gy IR at various times (Fig.
1C). Western blot analysis revealed that microtubule-associated
protein 1 light chain 3 (LC3) was converted from the free form
(LC3-I) to a lipid-conjugated membrane-bound form (LC3-II) in the
cells treated with 6.00 μmol/l DDP or 4 Gy IR for 24 h (Fig. 1C). As hypothesized, these
observations indicate that DDP or IR induces ER stress and
autophagy in human NPC cells.
3-MA enhances the sensitivity of human
NPC cells to DDP and IR
The proliferation of the NPC cells was inhibited by
various concentrations of 3-MA (P<0.05; Fig. 1D). Inhibition was enhanced with
increasing concentrations of 3-MA and prolonged with increasing
duration of cell exposure to 3-MA. Combining 1 mmol/l 3-MA with
6.00 μmol/l DDP or 4 Gy IR reduced cell viability compared with the
individual use of each agent, as revealed by MTT assay (Fig. 2A). Colony formation assays were used
to further confirm that 3-MA enhanced the sensitivity of the NPC
cells to DDP or IR. The two cell lines formed fewer colonies when
treated with 3-MA (0.1 mmol/l) in the presence of DDP (0.60 μmol/l)
or IR (0.4 Gy) compared with the individual agent (Fig. 2B).
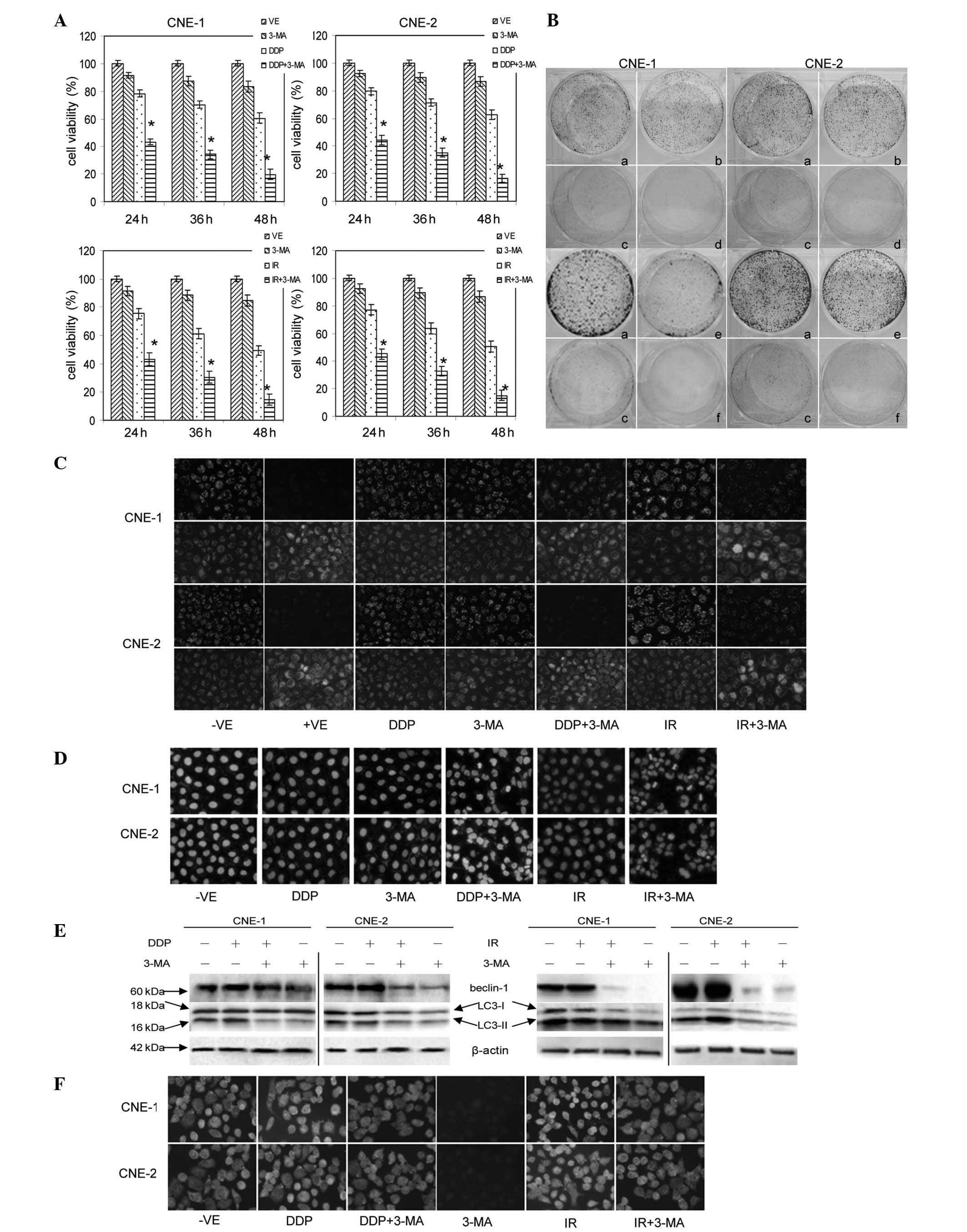 | Figure 2Effects of 3-MA with DDP or IR on the
viability, apoptosis and autophagy of cultured NPC cells. (A) Cells
were treated with 1 mmol/l 3-MA combined with 6 μmol/l DDP or 4 Gy
IR for 24, 36 or 48 h and viability was measured by MTT assay.
*P<0.05 vs. control group. (B) Cells were treated
with (a) fresh culture medium, (b) 0.6 μmol/1 DDP or (e) 0.4 Gy IR,
(c) 0.1 mmol/l 3-MA and (d) 0.6 μmol/l DDP or (f) 0.4 Gy IR
combined with 0.1 mmol/l 3-MA for 5 days. Cells were treated with 6
μmol/l DDP or 4 Gy IR in the presence or absence of 1 mmol/l 3-MA
for 24 h. (C) Cells were collected for staining with JC-1; (D)
cells were collected for staining with DAPI; (E) cells were
subjected to western blot analysis; and (F) cells were collected
for staining with anti-beclin 1 antibody (n=3). DDP, cisplatin; IR,
ionizing radiation, 3-MA, 3-methyladenine; NPC, nasopharyngeal
carcinoma; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromide; LC3,
microtubule-associated protein 1 light chain 3. |
JC-1 staining assay results reveal the conversion of
fluorescence from red to green in the cells treated with 50 μmol/l
CCCP for 20 min. Following treatment with 1 mmol/l 3-MA combined
with 6.00 μmol/l DDP or 4 Gy IR for 24 h, the extent of the
conversion of fluorescence from red to green in the cells was
significant (Fig. 2C). These
observations indicate that 3-MA promotes apoptosis in DDP- or
IR-treated NPC cells. Consistent with this, the DAPI staining
results in Fig. 2D revealed typical
morphological changes, including chromatin condensation and
apoptotic body formation, in the NPC cells treated with 3-MA
combined with DDP or IR. By contrast, the cells in the control
group did not reveal any abnormal morphologies. These results
indicate that 3-MA promotes apoptosis in IR- or DDP-treated NPC
cells.
Effects of 3-MA with DDP or IR on
autophagy in human NPC cells
The GRP78 and beclin 1 protein levels were increased
in the cells treated with 6.00 μmol/l DDP or 4 Gy IR for 24 h.
Western blot analysis revealed the conversion of LC3 from LC3-I to
LC3-II in the cells treated with 6.00 μmol/l DDP or 4 Gy IR for 24
h (Fig. 2E). Notably, the treatment
with 1 mmol/l 3-MA reversed these effects. The results from the
immunocytochemistry analysis of the expression of beclin 1 further
confirm these observations (Fig.
2F).
Effects of 3-MA with 2-DG or TM on the
proliferation and apoptosis of human NPC cells
The proliferation of the NPC cells was inhibited by
various concentrations of 2-DG or TM (Fig. 3A). This inhibition was enhanced with
increasing concentrations of 2-DG or TM, and prolonged with the
increasing duration of cell exposure to 2-DG or TM. Following
treatment with 5 mmol/l 2-DG for 24, 36 and 48 h, the survival rate
of the CNE-1 and CNE-2 cells reached 72.13, 53.14 and 47.99% and
69.32, 68.02 and 67.02%, respectively. When the cells were treated
with 2 μmol/l TM for 24, 48 and 72 h, the survival rate of the
CNE-1 and CNE-2 cells reached 72.43, 69.05 and 65.13% and 93.28,
91.58 and 79.21%, respectively. Based on these results, 5 mmol/l
2-DG and 1 μmol/l TM were each used for 24 h for further
experiments.
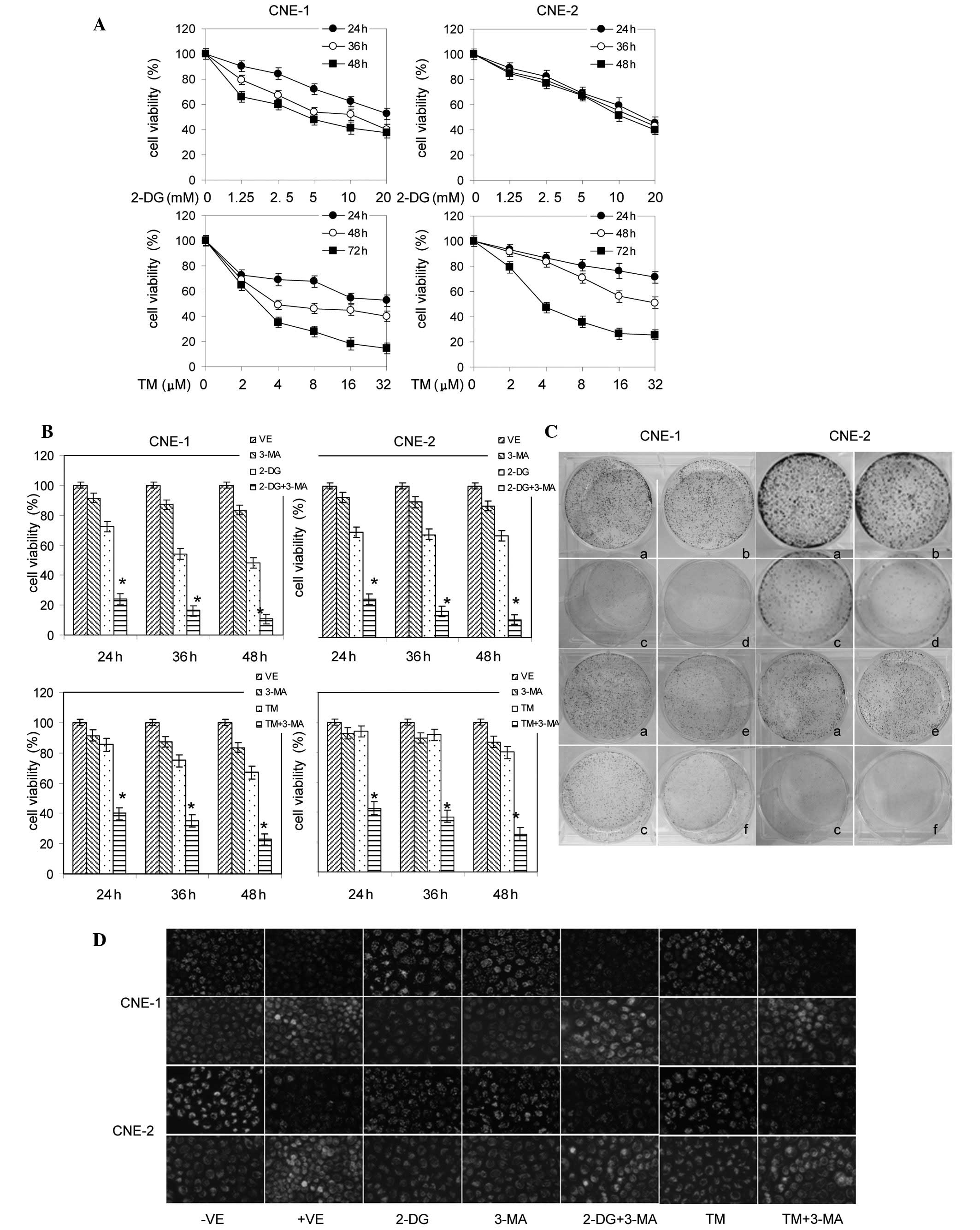 | Figure 3Effects of 3-MA with 2-DG or TM on the
viability and apoptosis of cultured NPC cells. (A) Cells were
treated with various concentrations (0–20 mmol/l) of 2-DG or (0–32
μmol/l) of TM for 24, 36 and 48 h and cell viability was measured
by MTT assay. (B) Cells were treated with 1mmol/l 3-MA combined
with 5 mmol/l 2-DG or 1 μmol/l TM for 24, 36 and 48 h and cell
viability was measured by MTT assay. *P<0.05 vs.
control group. (C) Cells were treated with (a) fresh culture
medium, (b) 0.5 mmol/l 2-DG or (e) 0.1 μmol/l TM, (c) 0.1 mmol/l
3-MA and (d) 0.5 mmol/l 2-DG or (f) 0.1 μmol/l TM combined with 0.1
mmol/l 3-MA for 5 days. (D) Cells were treated with 5 mmol/l 2-DG
or 1 μmol/l TM in the presence or absence of 1 mmol/l 3-MA for 24
h. Cells were collected for staining with JC-1 (n=3). 3-MA,
3-methyladenine; 2-DG, 2-deoxy-D-glucose; TM, tunicamycin; NPC,
nasopharyngeal carcinoma; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromide. |
The treatment of the cells with 1 mmol/l 3-MA
combined with 5 mmol/l 2-DG or 1 μmol/l TM reduced the cell
viability compared with the individual use of each agent, as
revealed by MTT assay (P<0.05; Fig.
3B). These results were further supported by the colony
formation assays (Fig. 3C).
Colony formation assays were used to further confirm
the fact that 3-MA enhances the sensitivity of human NPC cells to
2-DG and TM. The two cell lines formed fewer colonies when treated
with 3-MA in the presence of 2-DG or TM compared with with 2-DG or
TM alone.
The treatment of the cells with 1 mmol/l 3-MA
combined with 5 mmol/l 2-DG or 1 μmol/l TM for 24 h resulted in the
significant conversion of fluorescence from red to green in the
cells (Fig. 3D). This indicates
that 3-MA promotes apoptosis in 2-DG- or TM-treated NPC cells.
These data were further supported by the DAPI staining results.
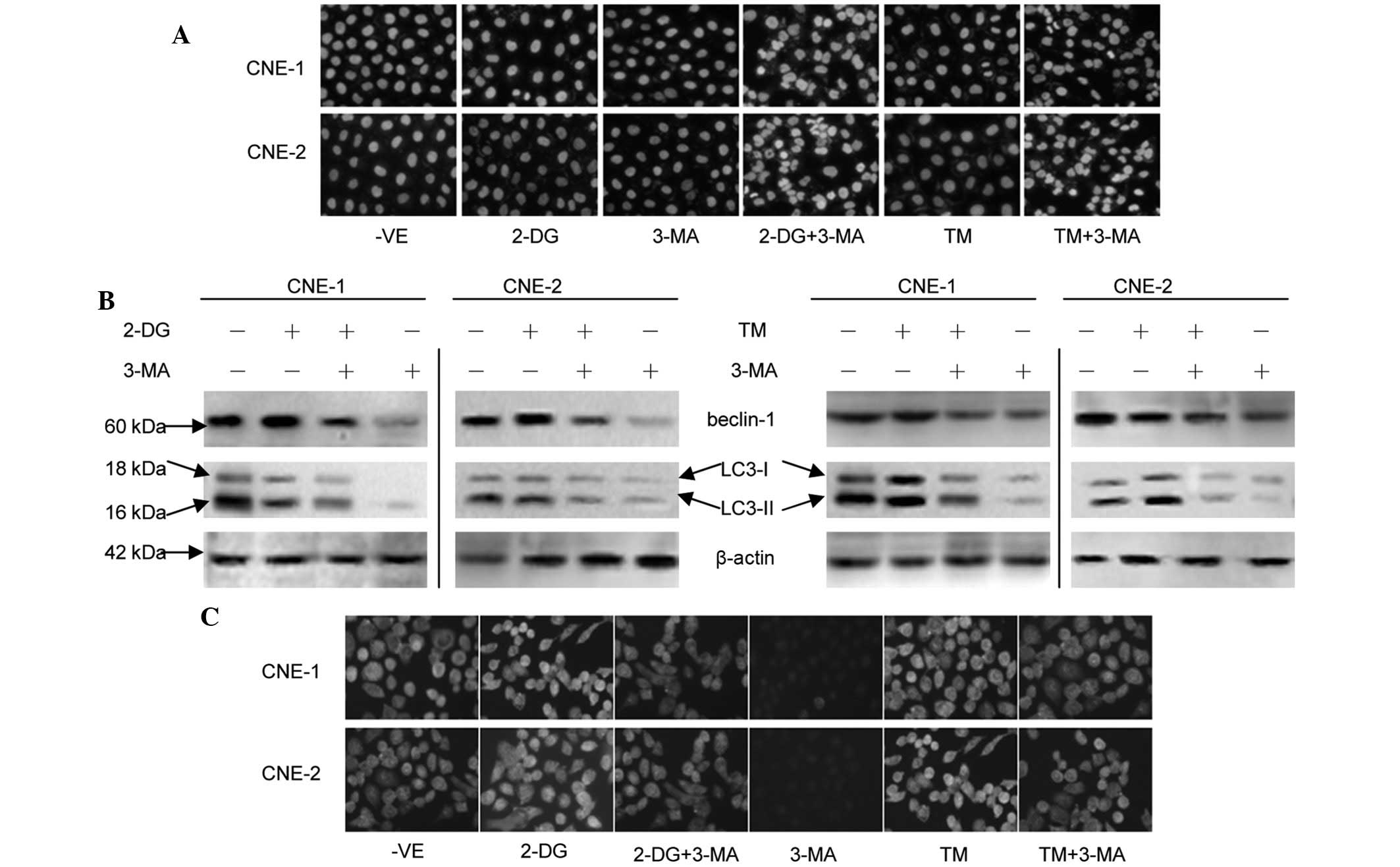
Fig. 4A demonstrates that the
treatment of the cells with 2-DG or TM did not appreciably induce
apoptosis in the cells, but typical morphological changes
associated with apoptosis, including chromatin condensation,
apoptotic body formation and DNA fragmentation, were evident in the
cells treated with 3-MA combined with 2-DG or TM. By contrast, the
cells in the control group did not exhibit any abnormal
morphology.
The beclin 1 protein levels were increased in the
cells treated with 5 mmol/l 2-DG or 1 μmol/l TM for 24 h (Fig. 4B). Western blot analysis revealed
the conversion of LC3 from LC3-I to LC3-II in the cells treated
with 5 mmol/l 2-DG or 1 μmol/l TM for 24 h. Notably, 1 mmol/l 3-MA
was able to reverse this effect. The results from the
immunocytochemistry analysis of the expression of beclin 1 further
confirmed these observations (Fig.
4C).
Discussion
The 5-year survival rate following the combination
of radiotherapy and adjuvant DDP chemotherapy is only 50–60% and
the rates of 5-year cumulative local relapse and distant metastasis
are 20–30 and 20–25%, respectively (17). Etiological factors that have been
identified for NPC include Epstein-Barr virus infection,
environmental risk factors and genetic susceptibility (18).
In the present study, DDP or IR was shown to induce
cell death. However, the sensitivity of human NPC cells to DDP and
IR-induced apoptosis was not significant.
Several studies have reported that ER stress induces
autophagy in mammalian cancer cell lines and mouse embryonic
fibroblasts (19,20). ER stress is caused by the
accumulation of misfolded or premature proteins in the ER lumen or
the cytosol. Changes in the environment of the ER lumen, including
changes in calcium levels, redox status and ER function, all affect
correct protein folding. The major mitigating mechanism for ER
stress is the unfolded protein response (UPR), which is mediated by
several signaling mechanisms that alleviate ER stress. In mammalian
cells, UPR is mediated by the PERK, ATF6 and IRE1 pathways. Current
studies support the hypothesis that an ER chaperone protein, BiP
(also known as GRP78), serves as a master UPR regulator and plays
essential roles in activating IRE1, PERK and ATF6 in response to ER
stress (7,13). As demonstrated in yeast and
mammalian cells (9,11), ER stress activates other major
cellular degradation mechanisms, including autophagy, which, in
turn, affects ER stress and, consequently, cell death.
3-MA is a popular inhibitor of the autophagic
pathway (20). 3-MA has been
reported to inhibit the activity of PI3-kinase and to block the
formation of preautophagosomes, autophagosomes and autophagic
vacuoles. Autophagy has been reported to increase as a result of
chemotherapy, resulting in the autophagic cell death of cancer
cells (programmed cell death) or the adaptation of cancer cells to
cytotoxicity induced by drugs, including in apoptosis (20). However, although autophagy has been
hypothesized to represent a potential therapeutic target in
adjuvant chemotherapy, the exact role and relevance of autophagy,
autophagic cell death and apoptosis in cancer remains poorly
understood and appears to be more complex than previously
considered.
In the present study, 3-MA in combination with DDP
or IR was shown to increase cell death more markedly than using DDP
or IR alone. As demonstrated in Fig.
2C, 3-MA promoted apoptosis in the DDP- or IR-treated NPC
cells. These data were further supported by the results of DAPI
staining. Taken together, these results show that 3-MA enhances the
sensitivity of NPC cells to DDP or IR. The western blot analysis
revealed an increase in the GRP78 and beclin 1 protein levels, and
the conversion of LC3 from LC3-I to LC3-II in the cells treated
with DDP or IR. Notably, 3-MA was able to reverse this effect.
These data were further supported by the immunocytochemistry
analysis of beclin 1 expression. These observations indicate the
important role of autophagy in ER stress-induced apoptosis.
In the current study, other ER stress inducers were
used to demonstrate the broad applicability of our observations.
The cells were exposed to the classic ER stress inducers, 2-DG and
TM. 2-DG, a synthetic glucose analog that acts as a glycolytic
inhibitor, is currently under clinical evaluation for targeting
tumor cells. The glucosamine-containing nucleoside antibiotic, TM,
is an inhibitor of N-linked glycosylation and of the formation of
N-glycosidic protein-carbohydrate linkages.
The results from the MTT and colony formation assays
demonstrated that the proliferation of the cells was inhibited by
2-DG and TM. 3-MA combined with 2-DG or TM reduced the cell
viability compared with the individual use of each agent. These
data were further supported by the results from the colony
formation assay. The JC-1 staining assay revealed that 3-MA
promotes apoptosis in the 2-DG- or TM-induced NPC cells. These data
were further supported by the DAPI staining results.
Next, the molecular changes that occur following
2-DG or TM treatment were explored by immunoblotting assay. The
beclin 1 protein levels increased following treatment with 2-DG or
TM. Western blot analysis revealed the conversion of LC3 from LC3-I
to LC3-II following treatment with 2-DG or TM. Notably, treatment
with 3-MA reversed these effects. Finally, results from the
immunocytochemistry analysis of the expression of beclin 1 further
confirm these observations
The results of the present study indicate that
autophagy is a protective mechanism in response to the apoptosis
induced by DDP, IR, 2-DG or TM. These observations are likely to
serve as a foundation for further investigations into autophagy in
NPC. The extension of this study into in vivo studies and
clinical trials is important.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China (grant nos. 81000992 and 81072207), the
Key Program of the Natural Science Foundation of Anhui province,
China (grant no. KJ2012A202) and the Natural Science Foundation of
Anhui Province, China (grant no. 090413135).
Abbreviations:
|
NPC
|
nasopharyngeal carcinoma
|
|
DDP
|
cisplatin
|
|
IR
|
ionizing radiation
|
|
2-DG
|
2-deoxy-D-glucose
|
|
TM
|
tunicamycin
|
|
3-MA
|
3-methyladenine
|
|
ER
|
endoplasmic reticulum
|
|
GRP78
|
glucose-regulated protein 78
|
|
UPR
|
unfolded protein response
|
|
PERK
|
protein kinase-like ER kinase
|
|
ATF6
|
activation of transcription factor
6
|
|
IRE1
|
inositol-requiring transmembrane
kinase and endonuclease 1
|
References
|
1
|
Simons MJ: Nasopharyngeal carcinoma as a
paradigm of cancer genetics. Chin J Cancer. 30:79–84. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee N, Harris J, Garden AS, Straube W,
Glisson B, Xia P, Bosch W, Morrison WH, Quivey J, Thorstad W, Jones
C and Ang KK: Intensity-modulated radiation therapy with or without
chemotherapy for nasopharyngeal carcinoma: radiation therapy
oncology group phase II trial 0225. J Clin Oncol. 27:3684–3690.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hassan KA, Ang KK, El-Naggar AK, Story MD,
Lee JI, Liu D, Hong WK and Mao L: Cyclin B1 overexpression and
resistance to radiotherapy in head and neck squamous cell
carcinoma. Cancer Res. 62:6414–6417. 2002.PubMed/NCBI
|
|
4
|
Sasaki N, Kudo N, Nakamura K, Lim SY,
Murakami M, Kumara WR, Tamura Y, Ohta H, Yamasaki M and Takiguchi
M: Activation of microbubbles by short-pulsed ultrasound enhances
the cytotoxic effect of cis-diamminedichloroplatinum (II) in a
canine thyroid adenocarcinoma cell line in vitro. Ultrasound Med
Biol. 38:109–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oberoi HS, Laquer FC, Marky LA, Kabanov AV
and Bronich TK: Core cross-linked block ionomer micelles as
pH-responsive carriers for cis-diamminedichloroplatinum(II). J
Control Release. 153:64–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shrivastav M, De Haro LP and Nickoloff JA:
Regulation of DNA double-strand break repair pathway choice. Cell
Res. 18:134–147. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Witko-Sarsat V: Apoptosis, cell death and
inflammation. J Innate Immun. 2:201–203. 2010. View Article : Google Scholar
|
|
8
|
Bhogal RH, Weston CJ, Curbishley SM, Adams
DH and Afford SC: Autophagy: A cyto-protective mechanism which
prevents primary human hepatocyte apoptosis during oxidative
stress. Autophagy. 8:545–558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kobayashi S, Xu X, Chen K and Liang Q:
Suppression of autophagy is protective in high glucose-induced
cardiomyocyte injury. Autophagy. 8:577–592. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Graziotto JJ, Cao K, Collins FS and Krainc
D: Rapamycin activates autophagy in Hutchinson-Gilford progeria
syndrome: implications for normal aging and age-dependent
neurodegenerative disorders. Autophagy. 8:147–151. 2012. View Article : Google Scholar
|
|
11
|
Gui YX, Fan XN, Wang HM, Wang G and Chen
SD: Glyphosate induced cell death through apoptotic and autophagic
mechanisms. Neurotoxicol Teratol. 34:344–349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kovaleva V, Mora R, Park YJ, Plass C,
Chiramel AI, Bartenschlager R, Dohner H, Stilgenbauer S, Pscherer
A, Lichter P and Seiffert M: miRNA-130a targets ATG2B and DICER1 to
inhibit autophagy and trigger killing of chronic lymphocytic
leukemia cells. Cancer Res. 72:1763–1772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu ZY, Zhu XF, Zhong ZD, Sun J, Wang J,
Yang D and Zeng YX: ApoG2, a novel inhibitor of antiapoptotic Bcl-2
family proteins, induces apoptosis and suppresses tumor growth in
nasopharyngeal carcinoma xenografts. Int J Cancer. 123:2418–2429.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang T, Gao Y, Mao Y, Zhang Q, Lin C, Lin
P, Zhang J and Wang X: Growth inhibition and apoptotic effect of
alpha-eleostearic acid on human breast cancer cells. J Nat Med.
66:77–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen LH, Jiang CC, Kiejda KA, Wang WF,
Thorne RF, Zhang XD and Hersey P: Thapsigargin sensitizes human
melanoma cells to TRAIL-induced apoptosis by up-regulation of
TRAIL-R2 through the unfolded protein response. Carcinogenesis.
28:2328–2336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang XD, Wu JJ, Gillespie S, Borrow J and
Hersey P: Human melanoma cells selected for resistance to apoptosis
by prolonged exposure to tumor necrosis factor-related
apoptosis-inducing ligand are more vulnerable to necrotic cell
death induced by cisplatin. Clin Cancer Res. 12:1355–1364. 2006.
View Article : Google Scholar
|
|
17
|
Siddique MA, Sabur MA, Kundu SC, Mostafa
MG, Khan JA, Ahmed S, Karim MA and Hanif MA: Difficulty in
diagnosis of nasopharyngeal carcinoma. Mymensingh Med J.
21:158–161. 2012.PubMed/NCBI
|
|
18
|
Carle LN, Ko CC and Castle JT:
Nasopharyngeal carcinoma. Head Neck Pathol. 6:364–368. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang JH, Chang YC and Maurizi MR:
4-O-carboxymethyl ascochlorin causes er stress and induced
autophagy in human hepatocellular carcinoma cells. J Biol Chem.
287:15661–15671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu D, Yang Y, Liu Q and Wang J:
Inhibition of autophagy by 3-MA potentiates cisplatin-induced
apoptosis in esophageal squamous cell carcinoma cells. Med Oncol.
28:105–111. 2001. View Article : Google Scholar : PubMed/NCBI
|