Introduction
Serous effusions are complications that are observed
in lymphoma patients (1–4). Statistics show that in patients with
non-Hodgkin's lymphoma (NHL) and Hodgkin's disease (HD), 20–30%
will develop a pleural effusion (3,5).
However, effusions in the peritoneal and pericardial cavities are
uncommon (5). Of all the various
subtypes, T-cell originated lymphomas, particularly lymphoblastic
lymphoma, usually involve serous effusions (5–7,9). The
main reasons for pleural effusions in HD patients are an
obstruction of the thoracic duct and impaired lymphatic drainage
(8). In NHL, the primary mechanism
for effusions is direct pleural infiltration (9). Cytological examinations of the
effusions may occasionally be of use. The percentage of positive
cytological findings may vary widely between NHL pleural effusions
(22.2–94.1%) (8). To attain a more
precise diagnosis, immunocytochemistry (ICC), morphometry, flow
cytometry (FCM) and cytogenetics have been applied in the clinic.
Effusions are generally associated with a poor outcome for patients
with lymphoma (5,9). The present study describes the case of
a 28-year-old male patient with aggressive NHL involving pleural
and abdominal chylous effusions. Furthermore, a detailed review of
the literature on the pathogenesis of serous effusions in lymphoma
and possible treatment plans is discussed. Written informed consent
was obtained from the patient.
Case report
A 28-year-old male patient was admitted to The First
Affiliated Hospital of Zhejiang University School of Medicine
(Hangzhou, Zhejiang, China) in January 2012 for continuous
treatment for NHL. The patient had suffered abdominal pain 5 months
prior to admittance, for which he was treated at a local hospital.
Abdominal computed tomography (CT) revealed a mass in the bowels
and retroperitoneal lymphadenopathy. The patient was sent for
abdominal exploratory surgery and the excised mass was identified
to be a lymphoma with positive results for leukocyte common antigen
(LCA), CD20 and CD79a. The cytogenetic analysis showed a
46,XY,t(8,14)(q24,q32) translocation. The
hematologist administered a cycle of DOLP (40 mg/m2
daunorubicin on days 1–3; 1.4 mg/m2 vincristine on days
1, 8, 15 and 22; 6,000 U/m2 L-asparaginase on days
11–20; and 45 mg/m2 prednisone on days 1–28) plus CTX
(750 mg/m2 cyclophosphamide on day 1), and then a cycle
of HDAra-C (3 g/m2 high-dose cytarabine, every 12 h on
days 1–3) plus 6,000U/m2 L-asparaginase on days 4–13 and
a cycle of VDCP (1.4 mg/m2 vincristine on days 1, 8, 15
and 22; 40 mg/m2 daunorubicin on days 1–3; 750
mg/m2 cyclophosphamide on days 1 and 8; and 45
mg/m2 prednisone on days 1–28). The patient was stable
following the treatment. Following this, the patient suffered mild
abdominal pain and a cough and was admitted to The First Affiliated
Hospital of Zhejiang University School of Medicine hospital for
further treatment of the lymphoma.
Upon admission, the patient experienced mild
abdominal pain and a temperature of 38ºC. Small lymph nodes around
the neck region could be palpated. The sternal pressing sign was
negative. The liver and spleen were not enlarged upon palpation. An
abdominal shifting dullness test was positive. The patient produced
a white, non-sticky sputum as a result of coughing. Although the
patient was able to breathe freely, a small amount of chest
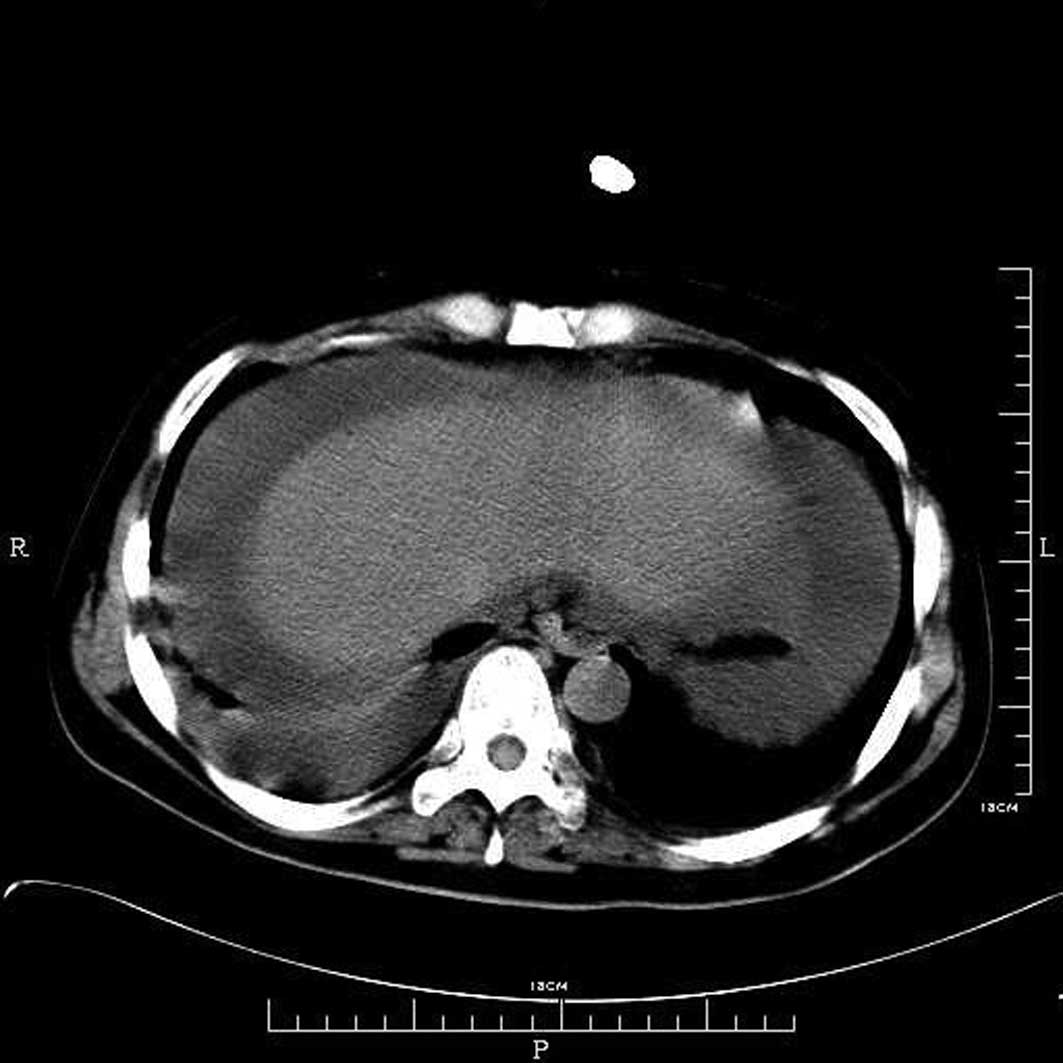
distress was observed. The patient was sent for a chest and
abdominal CT and the results revealed pleural and abdominal
effusions (Fig. 1).
The patient was prescribed antibiotics and a whole
body evaluation was performed prior to another round of
chemotherapy. However, the patient's condition worsened at a speed
greater than expected. Initially, the pleural effusion caused
difficulty in breathing, then the patient's temperature increased
further. The antibiotic dose was adjusted based on the sputum
culture results, and a pleurocentesis and abdominocentesis were
performed to remove the pressure. The effusions were milk-colored,
indicating chylous effusions. The biochemistry results confirmed
that the effusions were chylous (Table
I). The cytological studies identified 89.62% aberrant B
lymphocytes, of which, 20.93% were CD79a-positive, 35.10% were
CD5-positive, 99.50% were HLA-DR-positive, 27.24% were
CD10-positive, 88.38% had λ chain expression, 96.58% had κ chain
expression, 63.78% were cIgM-positive and 65.80% were
sIgM-positive, as determined by FCM.
 | Table IBiochemistry laboratory test results
for the pleural and abdominal effusions. |
Table I
Biochemistry laboratory test results
for the pleural and abdominal effusions.
| Biochemistry
exams | Pleural effusion | Ascites |
|---|
| Appearance | Milk-like | Milk-like |
| WBCs, μl | 25000 | 28000 |
| Lymphocytes, μl | 5000 | 4200 |
| Neutrophils, μl | 20000 | 23800 |
| RBCs, μl | 1020 | 200 |
| LDH, U/μl | 4051 | 4730 |
| ADA, U/μl | 34 | 40 |
| Rivatal test | Positive | Positive |
| Chylous test | Positive | Positive |
As the infection was believed to be under control,
the patient was administered VMCP chemotherapy (1.4
mg/m2 vincristine on days 1, 8, 15 and 22; 10
mg/m2 mitoxatrone on days 1–3; 750 mg/m2
cyclophosphamide on days 1 and 8; and 45 mg/m2
prednisone on days 1–28). At the end of this round of chemotherapy,
the patient was again febrile and had a severe cough. The patient
eventually succumbed due to septic shock.
Discussion
Serous effusions occur in a number of malignancies
(1). Johnston et al studied
584 patients with serous effusions and identified that 15% were due
to lymphoma (3). Other causes
include various types of cancers, tuberculosis and renal disease
(1,4). Among the total lymphoma patients with
serous effusions recorded by Johnston et al, 72% were males
and 29% were females, suggesting a predominance in males (3). Serous effusions also have another
preference in the various subtypes of lymphoma. T-cell originated
lymphomas are more commonly observed with serous effusions than
B-cell originated neoplasms. Pleural effusions have been identified
in 26.2% of T-cell lymphomas, while, in contrast, only rare B-cell
lymphomas develop pleural effusions (10,11).
Among all the NHL subtypes, 41.6% of lymphoblastic lymphomas have
been recorded with pleural effusions compared with only 3.8% of the
other subtypes (12).
Various causes may lead to serous effusions in
lymphoma patients, including impaired lymphatic drainage due to
obstruction in the mediastinal lymph nodes or the thoracic duct,
venous obstruction, pulmonary infection, radiation therapy or
pleural involvement of the tumor (13). The main cause of pleural effusion in
HD has been identified as thoracic duct obstruction. However in
NHL, the primary consideration was shown to be direct pleural
infiltration (4). Chylous effusions
are always caused by an obstruction of the lymphatic trunks. In the
present case, the pleural and abdominal effusions were identified
to be chylous, strongly indicating that the effusions originated in
the lymph trunks. Possible reasons for the effusions may be that
the metastatic lymphoma cells blocked the lymph tunnels, leading to
obstruction and further impairment of these tunnels.
Removal of the fluids from the thorax by
thoracocentesis, from the peritoneal cavity by abdominal
paracentesis and from the pericardial cavity by pericardiocentesis,
are diagnostic methods and treatments to relieve pressure. The
fluids that are extracted from the body cavities may be used to
study the disease qualities and to look for malignant cells. Das
et al compared fine-needle aspiration cytology with pleural
effusion cytological studies and identified that in 93.7% of the
cases, the cytological findings matched the diagnosis (8). Following the development of new
techniques and our improved understanding of biomarkers,
immunochemistry, FCM and cytogenetics are being widely used to aid
in obtaining a fast and precise diagnosis (14,15).
In the present study, a thoracocentesis and a paracentesis were
performed. An evaluation of the fluids extracted identified the
presence of malignant lymphoma cells, indicating that the serous
effusions were actually due to the malignancy. Removal of the
fluids aided in the relief of the symptoms and improved our
understanding of the etiology.
Patients with lymphoma seldom have any symptoms
other than serous effusions. There is a type of lymphoma called
primary effusion lymphoma (PEL), which only presents with serous
effusions, but no detectable solid masses. Numerous molecular
markers have been used to diagnose this lymphoma in the early stage
(16–18). PEL has been associated with advanced
AIDS. Although PEL is considered to have no detectable solid
masses, studies have shown the involvement of certain lymph nodes,
tongue-based lesions and the secondary bowel (17–20).
The mechanism for PEL has not been well characterized. However,
studies have shown that an increased level of vascular endothelial
growth factor (VEGF) may induce capillary growth in PEL effusions
(21). The diagnosis of PEL mainly
depends on the clinical presentation, imaging results and
cytological analysis.
Serous effusions in lymphoma are generally
associated with a poor outcome (22–24).
Relieving symptoms and increasing the quality of life of the
patient are the primary treatment targets. In high-grade malignant
lymphomas, including Burkitt's lymphoma and lymphoblastic lymphoma,
serous effusions are commonly observed and associated with a poor
outcome (25–27). If the affected patients are treated
with chemotherapy, there is a possibility that a condition called
acute tumor lysis syndrome may develop. Studying serous effusions
may also aid in the detection of tumor lysis syndrome, therefore,
allowing the patient to undergo anti-uric treatment.
In summary, the present study described the case of
a young male with NHL involving chylous pleural and abdominal
effusions. Serous effusions are common in lymphomas, particularly
those of a high grade, including Burkitt's and lymphoblastic
lymphomas. However, it is rare for a lymphoma patient to have
serous effusions in the pleural and abdominal cavities. The serous
effusions of the present case were identified to be chylous. The
present case further indicates that serous effusions are formed due
to the mechanism of lymphatic trunk obstruction, and that the
appearance of serous effusions is associated with a poor outcome,
particularly in patients with malignant lymphoma.
Acknowledgements
This study was supported in part by the Research
Plan of Medical Science of Health Administration of Zhejiang (No.
2013KYB102), the Research Plan of the Education Administration of
Zhejiang Province (No. Y201327033) and the Research Plan of the
Science Technology Department of Zhejiang Province, China (No.
2013C33125).
References
|
1
|
DeCamp MM Jr, Metzer SJ, Swanson SJ and
Sugarbaker DJ: Malignant effusive disease of the pleura and
pericardium. Chest. 112(4 Suppl): S291–S295. 1997. View Article : Google Scholar
|
|
2
|
Naylor B: Pleural, peritoneal, and
pericardial fluids. Comprehensive cytopathology. Bibbo M: 2nd
edition. WB Saunders; Philadelphia: pp. 551–621. 1997
|
|
3
|
Johnston WW: The malignant pleural
effusion. A review of cytopathologic diagnosis of 584 specimens
from 472 consecutive patients. Cancer. 56:905–909. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sahn SA: Malignant pleural effusions.
Fishman's Pulmonary Diseases and Disorders. Fishman AP, Elias JA,
Fishman JA, Grippe MA, Kaiser LR and Senior RM: 3rd edition.
McGraw-Hill; New York, NY: pp. 1429–1438. 1998
|
|
5
|
Das DK: Serous effusions in malignant
lymphoma: a review. Diagn Cytopathol. 34:335–347. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weick JK, Kieley JM, Harrison EG Jr, Carr
DT and Scanlon PW: Pleural effusion in lymphoma. Cancer.
31:848–853. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berkman N, Breuer R, Kramer MR and
Polliack A: Pulmonary involvement in lymphoma. Leuk Lymphoma.
20:229–237. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Das DK, Gupta SK, Ayyagari S, Bambery PK,
Datta BN and Datta U: Pleural effusions in non-Hodgkin's lymphoma.
A cytomorphologic, cytochemical and immunologic study. Acta Cytol.
31:119–124. 1987.
|
|
9
|
Alexandrakis MG, Passam FH, Kyriakou DS
and Bouros D: Pleural effusions in hematologic malignancies. Chest.
125:1546–1555. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Uchiyama K, Kobayashi Y, Tanaka R, et al:
Primary malignant lymphoma of the central nervous system presenting
with ascites and pleural effusion. Hematologica (Budap).
30:143–148. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Watanabe N, Sugimoto N, Matsushita A, et
al: Association of intestinal lymphoma and ulcerative colitis.
Intern Med. 42:1183–1187. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Das DK, Gupta SK, Datta U, Sharma SC and
Datta BN: Malignant lymphoma of convoluted lymphocytes: diagnosis
by fine needle aspiration cytology and cytochemistry. Diagn
Cytopathol. 2:307–311. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fraser RS, Muller NL, Colman N and Pare
PD: Pleural Effusion. Fraser and Pare's Diagnosis of Diseases of
the Chest. 4th edition. WB Saunders; Philadelphia: pp. 2759–2760.
1999
|
|
14
|
Johnson EJ, Scott CS, Parapia LA and Stark
AN: Diagnostic differentiation between reactive and malignant
lymphoid cells in serous effusions. Eur J Cancer Clin Oncol.
23:245–250. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dunphy CH: Combined cytomorphologic and
immunophenocytic approach to evaluation of effusion for
lymphomatous involvement. Diagn Cytopathol. 15:427–430. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paner GP, Jensen J, Foreman KE and Reyes
CV: HIV and HHV-8 negative primary effusion lymphoma in a patient
with hepatitis C virus-related liver cirrhosis. Leuk Lymphoma.
44:1811–1814. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang Q, Chang KL, Gaal K and Arber DA:
Primary effusion lymphoma with subsequent development of a small
bowel mass in an HIV-seropositive patient: a case report and
literature review. Am J Surg Pathol. 26:1363–1367. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Michai M, Goto H, Hattori S, et al:
Soluble CD30: a possible serum tumor marker for primary effusion
lymphoma. Asian Pac J Cancer Prev. 13:4939–4941. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ariad S, Benharroch D, Lupu L, Davidovici
B, Dupin N and Boshoff C: Early peripheral lymph node involvement
of human herpesvirus 8-associated body cavity-based lymphoma in a
human immunodieficiency virus-negative patient. Arch Pathol Lab
Med. 124:753–755. 2000.PubMed/NCBI
|
|
20
|
Mate JL, Navarro JT, Ariza A, et al: Oral
solid form of primary effusion lymphoma mimicking plasmablastic
lymphoma. Hum Pathol. 35:632–635. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aoki Y and Tosato G: Vascular endothelial
growth factor/vascular permeability factor in the pathogenesis of
primary effusion lymphomas. Leuk Lymphoma. 41:229–237. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kirn D, Mauch P, Shaffer K, et al:
Large-cell and immunoblastic lymphoma of the mediastinum:
prognostic features and treatment outcome in 57 patients. J Clin
Oncol. 11:1336–1343. 1993.PubMed/NCBI
|
|
23
|
Morel P, Dupriez B, Plantier-Colcher I, et
al: Long-term outcome of follicular low-grade lymphoma. A report of
91 patients. Ann Hematol. 66:303–308. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sandlund JT, Crist WM, Abromowitch M, et
al: Pleural effusion is associated with poor treatment outcome in
stage III small non-cleaved cell lymphoma. Leukemia. 5:71–74.
1991.PubMed/NCBI
|
|
25
|
Kabat-Koperska J, Kutrzeba J and Chosia M:
Acute kidney failure and ascites in Burkitt's lymphoma of the
stomach. Pol Arch Med Wewn. 105:67–70. 2001.(In Polish).
|
|
26
|
Klumb CE, de Resende LM, Stefanoff CG,
Vicuña CH, Renault IZ and Maia RC: Burkitt-like lymphoma in an
infant: a case report. Rev Hosp Clin Fac Med Sao Paulo. 58:33–36.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lerza R, Botta M, Barsotti B, et al:
Dexamethazone-induced acute tumor lysis syndrome in a T-cell
malignant lymphoma. Leuk Lymphoma. 43:1129–1132. 2002. View Article : Google Scholar : PubMed/NCBI
|