Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is an
aggressive malignant disease induced by the malignant
transformation of T-cell precursors. T-ALL accounts for 10–15% of
all leukemias in children and adolescents (1). The molecular mechanisms underpinning
T-ALL are likely to be complex (2).
A series of studies have demonstrated that the abnormal activation
of the Notch1 signaling pathway plays a significant role in the
pathogenesis of T-ALL (3,4).
The Notch1 gene encodes a single-pass heterodimeric
transmembrane receptor, which has a fundamental function in the
development of normal T cells (5).
Normally, the activation of Notch signaling is triggered by Notch
receptor-ligand interactions. The direct binding of a ligand from a
signaling cell to a Notch receptor on the membrane of a receiving
cell initiates two successive proteolytic cleavages by the
TNF-α-converting enzyme (TACE) and the γ-secretase/presenilin
complex. The proteolytic cleavage ultimately results in the release
of the Notch intracellular domain (NICD), which translocates into
the cell nucleus and interacts with the recombination signal
binding protein Jκ (RBP-J). The NICD/RBP-J complex transactivates
downstream target genes, including the hairy/enhancer-of-split 1
(Hes-1) gene (6). However, in T-ALL
patients, mutations in the Notch1 gene are common and may lead to
aberrant activation of Notch signaling that is independent of
ligand binding (3). By contrast,
the Notch1 proteins in the T-ALL cells also serve as surface
receptors that may be triggered by Notch ligands that are expressed
by specific cell types, including bone marrow stromal cells.
Increasing evidence has suggested that the interaction between
tumor cells and the stromal microenvironment results in the
resistance to chemotherapy in leukemia and myeloma (7,8). Notch
signaling has been shown to be one of the molecular mechanisms
involved. It has been shown that the signaling driven by Notch1 may
inhibit apoptosis in developing thymocytes, mature T cells and
T-ALL cells (9–11).
In contrast to the roles of the Notch1 receptor, the
roles for Notch ligands in T-ALL biology are less clear. The known
Notch ligands in mammals include Jagged1 and 2, and Delta-like
(DLL)-1, 3 and 4 (6). The actions
of these ligands differ in the initiation of Notch signaling and
may result in a diverse or opposed biological outcome (12). The present study assessed the role
of Jagged1 in the survival of Jurkat T-ALL cells when exposed to a
cytotoxic drug, with or without stromal support.
Stromal cells derive from their mesodermal
precursors, mesenchymal stem cells (MSCs), which are
non-hematopoietic progenitor cells that are located in the bone
marrow and a number of other tissues (13,14).
Currently, bone marrow is the main source of MSCs. However, the
aspiration of bone marrow involves invasive procedures and the
yield of bone marrow-derived MSCs (BM-MSCs) decreases significantly
with the age of the donor (15).
The umbilical cord is an excellent alternative to bone marrow as a
source of MSCs for experimental and clinical needs (16). However, data on the application of
umbilical cord-derived MSCs is limited. In the present study, human
umbilical cord-derived MSCs (hUC-MSCs) were used as stromal cells
to evaluate their function in the drug resistance of T-ALL
cells.
Materials and methods
Cell culture
The human T-ALL cell line, Jurkat, was cultured in
suspension in RPMI 1640 medium (Gibco BRL, Grand Island, NY, USA)
supplemented with 10% fetal bovine serum (FBS; Sijiqing, Hangzhou,
China) and 1% penicillin/streptomycin (Gibco BRL). The cells were
maintained at 37°C in a humidified chamber with 5% CO2
and routinely subcultured every 2–3 days, ensuring that the cell
density in the culture did not exceed 1×106
cells/ml.
hUC-MSC cultures were established from the umbilical
cords of healthy donors using the direct plastic adherence method
after informed consent had been obtained. The study was approved by
the ethics committee of the School of Life Science and
Biopharmaceutics of Guangdong Pharmaceutical University (Guangzhou,
China). Briefly, the umbilical cord samples were sheared into
2–3-cm long segments and washed thoroughly to remove the residual
cord blood. Each cord segment was dissected along its length to
expose the blood vessels (two arteries and one vein), which were
pulled away and discarded. The remaining cord tissue pieces were
collected, minced into 1–2-mm3 fragments, plated
separately in 6-cm polystyrene tissue culture dishes and maintained
in DMEM/F12 medium (Gibco BRL) at 37°C in a humidified atmosphere
with 5% CO2. The non-adherent tissues were removed on
day seven and the culture medium was changed every 3–4 days
thereafter. Approximately three weeks later, when well-developed
colonies of fibroblast-like cells had appeared (80–90% confluent),
the cultures were washed, harvested with 0.25% trypsin (Gibco BRL)
and passed through a 100-μm sterile mesh to remove any residual
tissue pieces. The filtered cells were then seeded in larger flasks
for further expansion. The hUC-MSCs at passages 3–8, displaying a
homogeneous mesenchymal immunophenotype and multipotent
differentiation potential into adipocytic, osteoblastic and
chondrocytic lineages, were used for the experiments.
Polymerase chain reaction (PCR)
Total RNA was extracted from the hUC-MSCs and Jurkat
cells using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer’s instructions. Reverse transcription
was carried out using the PrimeScript II 1st strand cDNA synthesis
kit (Takara, Otsu, Japan) with 1 μg total RNA as a template and
oligo dT as a primer. All semiquantitative PCR experiments were
performed using the same serially-diluted cDNA batches as
templates. Amplification was performed at 95°C for 5 min followed
by 38 cycles of 94°C for 30 sec, 56°C for 30 sec and 72°C for 30
sec, then a final extension at 72°C for 7 min. The amplified
fragments were analyzed using electrophoresis on a 2% agarose gel.
The gene-specific primers that were used for PCR are listed in
Table I. The PCR of human β-actin
was performed as a control.
 | Table IPrimers for the PCR analysis. |
Table I
Primers for the PCR analysis.
| Genes | Primers, 5′-3′ | Size of targets,
bp |
|---|
| Notch1 |
| F |
CTACCTGTCAGACGTGGCCT | 357 |
| R |
CGCAGAGGGTTGTATTGGTT | |
| Jagged1 |
| F |
CTCATCAGCCGTGTCTCAAC | 297 |
| R |
GGCACACACACTTAAATCCG | |
| DLL1 |
| F |
TATCCGCTATCCAGGCTGTC | 297 |
| R |
GGTGGGCAGGTACAGGAGTA | |
| DLL4 |
| F |
AAGGCTGCGCTACTCTTACC | 538 |
| R |
ATCCTCCTGGTCCTTACAGC | |
| Hes-1 |
| F |
ATCACACAGGATCCGGAGCT | 300 |
| R |
TGACACTGGCTGGGGTAGC | |
| β-actin |
| F |
CTACAATGAGCTGCGTGTGG | 314 |
| R |
CGGTGAGGATCTTCATGAGG | |
Detection of signaling molecules using
flow cytometry
The Jurkat cells were harvested and prepared for
flow cytometry following co-culture with hUC-MSCs. In brief, the
hUC-MSCs were plated into 6-well plates at 2×105 cells
per well to form a confluent monolayer. Following this,
2×106 Jurkat cells were added to each well of the
adherent hUC-MSCs or cultured alone for 72 h. The co-cultured
Jurkat cells were then separated from the hUC-MSCs by careful
pipetting with ice-cold PBS. For the flow cytometry, the cells from
the various cultures were washed and adjusted to a concentration of
5×106 cells/ml in PBS. Aliquots of 100 μl cell
suspension were then added into separate tubes. Fc receptors were
blocked using the Fc Receptor Blocking reagent (Miltenyi Biotec,
Bergisch Gladbach, Germany) for 15 min at 4°C. Surface antibodies
were added and incubated for 30 min at 4°C in the dark. The unbound
antibodies were removed by washing the cells twice in PBS and the
cells were resuspended in 500 μl PBS for the final flow cytometric
analysis on a Gallios cytometer (Beckman Coulter, Brea, CA, USA).
The antibodies that were used were allophycocyanin (APC)-conjugated
anti-CD45 (eBioscience, San Diego, CA, USA), carboxyfluorescein
(CFS)-conjugated anti-Jagged1 (R&D systems, Minneapolis, MN,
USA), phycoerythrin (PE)-conjugated anti-Notch1 (R&D systems),
PE-conjugated anti-CD28 (eBioscience) and non-specific
isotype-matched antibodies.
Apoptosis analysis
To induce apoptosis, the Jurkat cells were cultured
alone or co-cultured with hUC-MSCs for 72 h as described previously
and then exposed to dexamethasone (Sigma, St Louis, MO, USA; final
concentration 1 μM) for an additional 24 h. The blocking
experiments were performed by incubating the hUC-MSCs and Jurkat
cells with neutralizing monoclonal antibodies against human Jagged1
(R&D Systems; 1 μg/ml) prior to their inoculation into culture
plates. Recombinant human Jagged1 proteins (R&D systems; 1
μg/ml) were used to stimulate the Jurkat cells directly. Apoptotic
cell death was detected by Annexin V/propidium iodide (PI) staining
using the MEBCYTO apoptosis kit (MBL, Nagoya, Japan). Briefly, the
Jurkat cells from the various cultures were harvested, washed and
immunolabeled with APC-conjugated anti-CD45. The cells were then
washed and resuspended in 85 μl binding buffer, followed by
incubation with 10 μl Annexin V-FITC and 5 μl PI at room
temperature for 15 min in the dark. Following incubation, 400 μl
binding buffer was added and the cell samples were measured using
flow cytometry.
Statistical analysis
All statistical calculations were performed using
the GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA,
USA). The data are presented as the mean ± SD. When applicable,
Student’s unpaired t-test, a one-way ANOVA and Holm-Sidak tests
were used to determine significance. P<0.05 was considered to
indicate a statistically significant difference.
Results
Characterization of hUC-MSCs
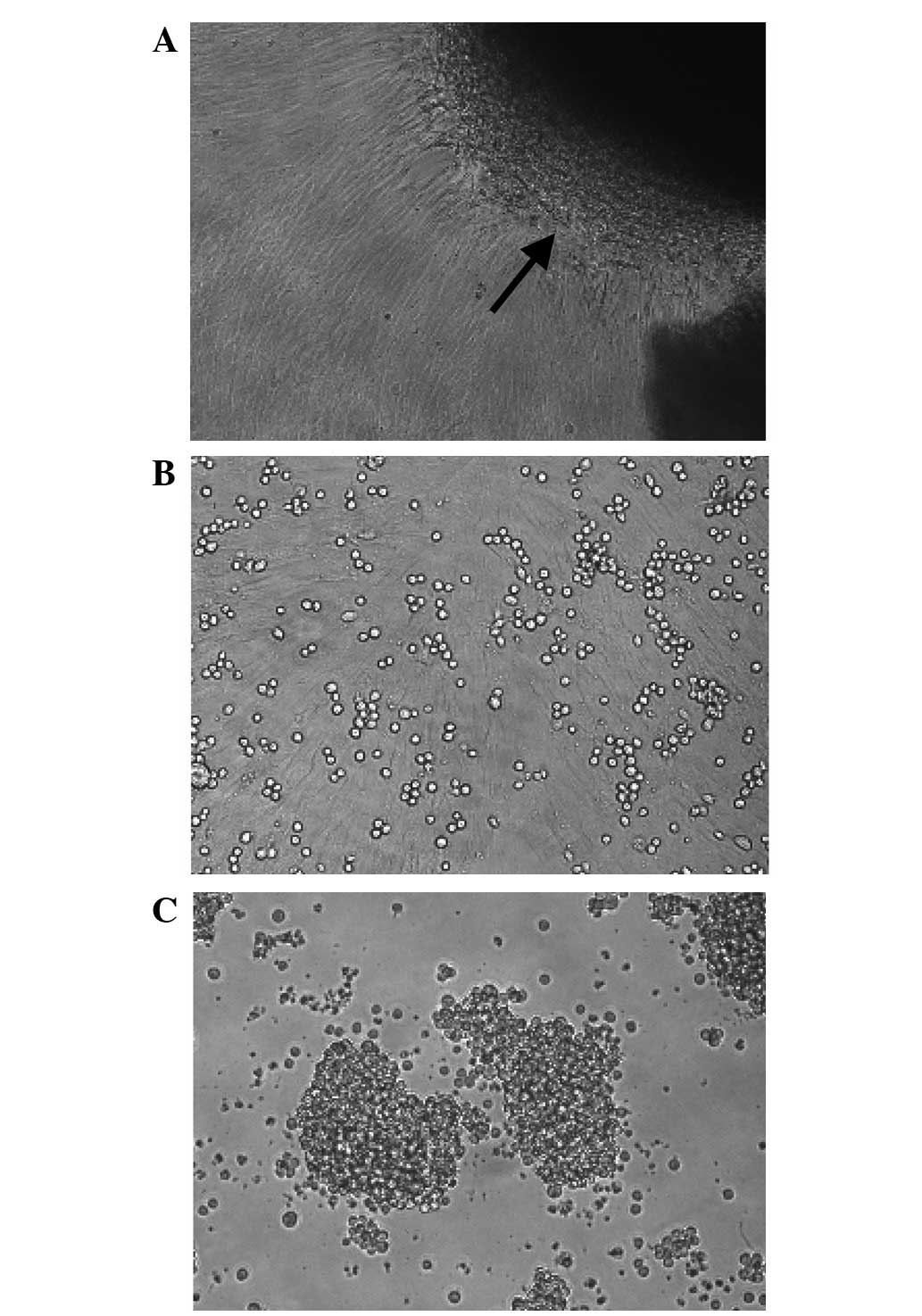
Fibroblast-like cells were successfully isolated
from hUC tissues using the direct plastic adherence method in the
present study (Fig. 1A). The cells
formed whirlpool-like arrays when a confluent monolayer had
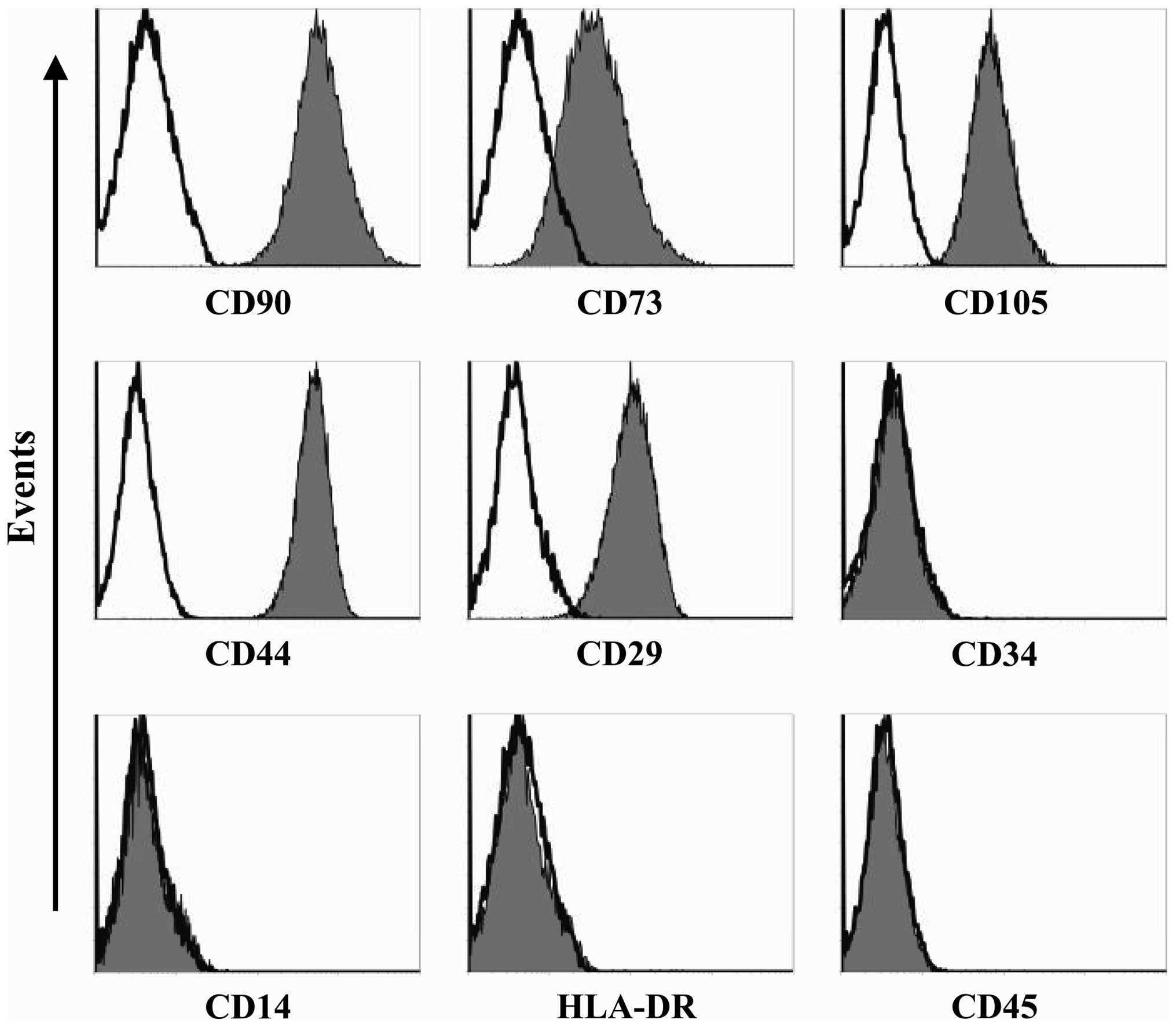
developed (Fig. 1A and B). The flow
cytometry analysis demonstrated that the hUC-MSCs showed good
homogeneity and expressed MSC markers CD73, CD90, CD105, CD44 and
CD29, but were negative for CD34, CD45, human leukocyte antigen
(HLA)-DR and CD14 (Fig. 2). The
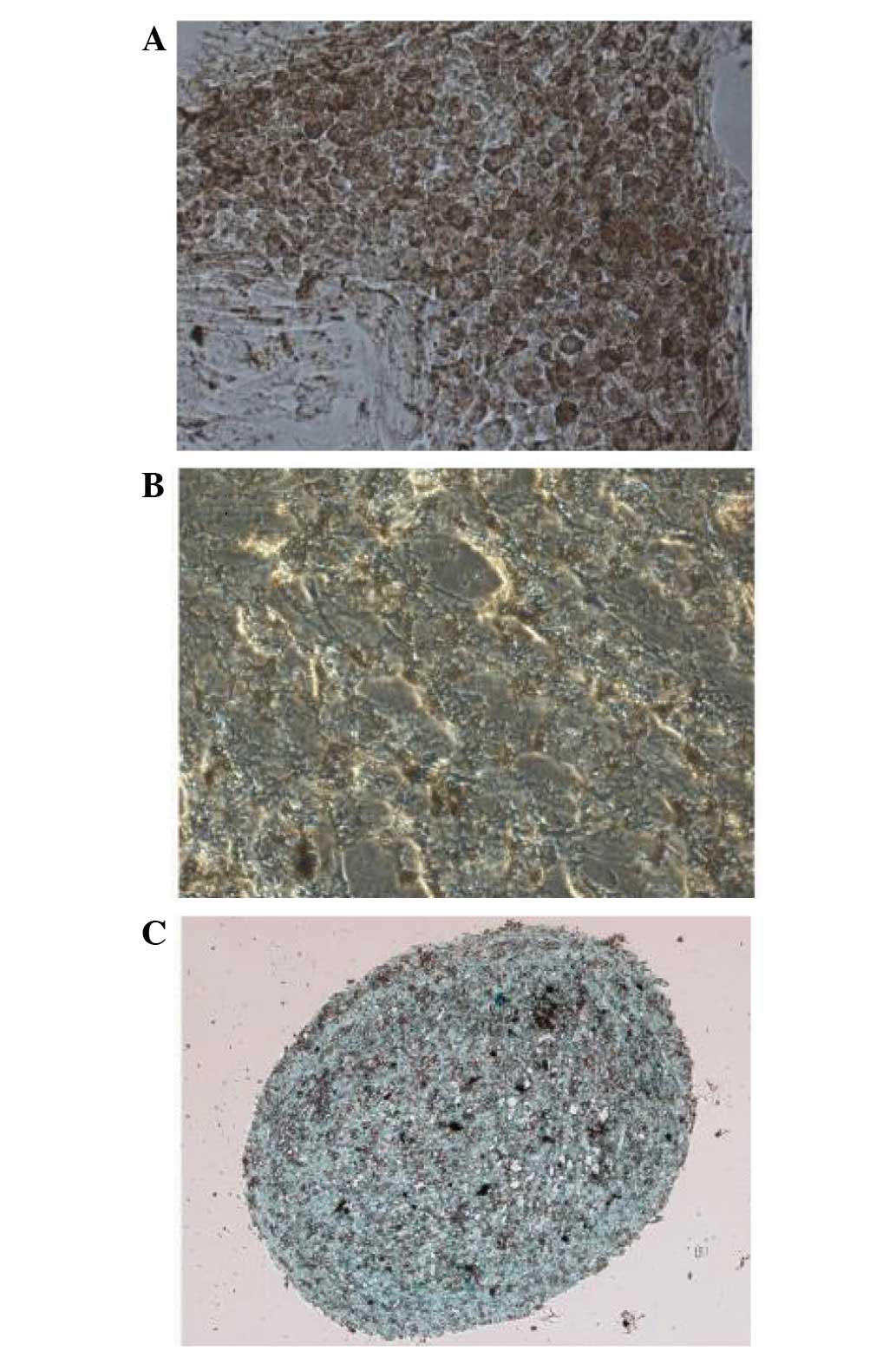
same cells showed multilineage differentiation potential, as
assessed by culturing in adipogenic, osteogenic or chondrogenic
medium (Fig. 3).
Expression of Notch ligands by
hUC-MSCs
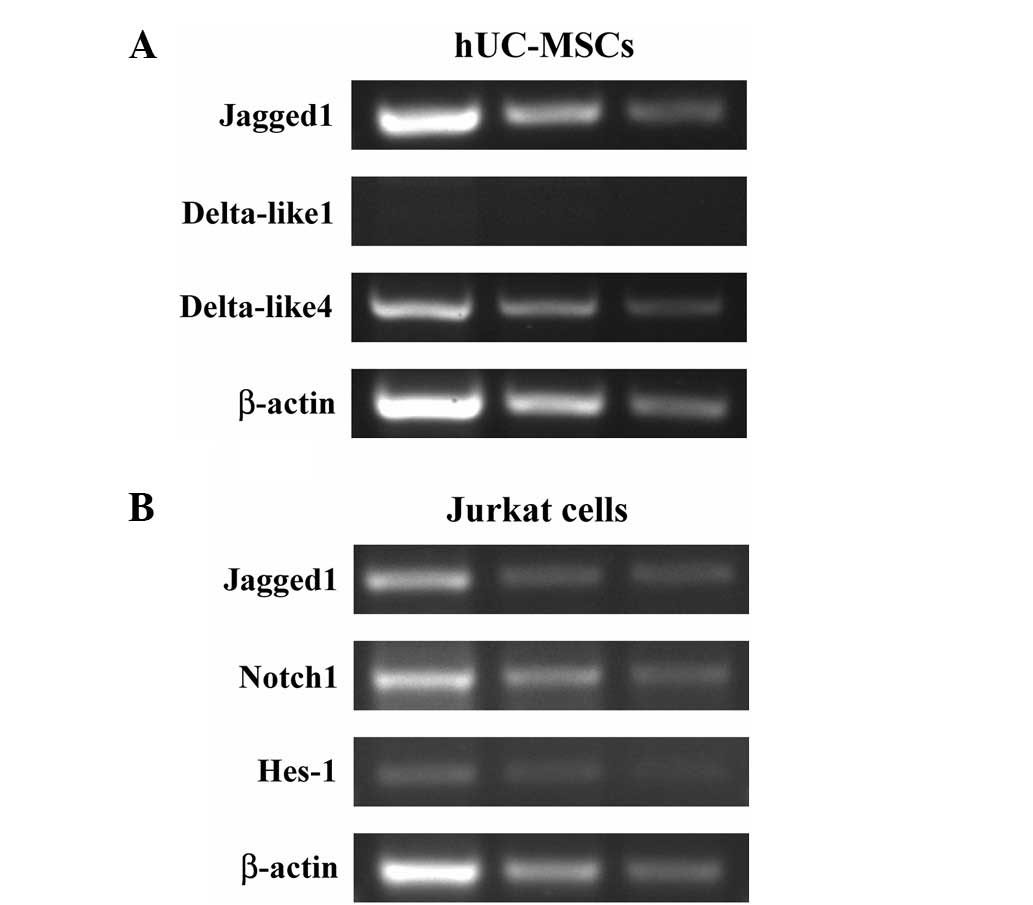
To assess a possible role for the hUC-MSCs in
inducing Notch signaling in the Jurkat T-ALL cells, the expression
of Notch ligands Jagged1, DLL1 and DLL4 were examined in the
hUC-MSCs by PCR using gene-specific primers, with β-actin as an
internal control (Table I). This
analysis revealed that transcripts for Jagged1 and DLL4 were
detected in the hUC-MSCs, while the transcript for DLL1 was
undetectable (Fig. 4A). In
addition, Jagged1 was relatively highly expressed by the hUC-MSCs
at the mRNA level.
Upregulation of Notch1, Jagged1 and CD28
in Jurkat cells following contact with hUC-MSCs
The expression of the Notch-related genes in the
Jurkat cells was further analyzed. PCR analysis showed that the
Jurkat cells expressed the Notch1 receptor and its ligand, Jagged1
(Fig. 4B), suggesting that the
receptor and ligand pair may play a role in T-ALL cells. Hes-1, one
of the main downstream molecules of the Notch pathway, was also
expressed in the normally-cultured Jurkat cells (Fig. 4B), suggesting that Notch signaling
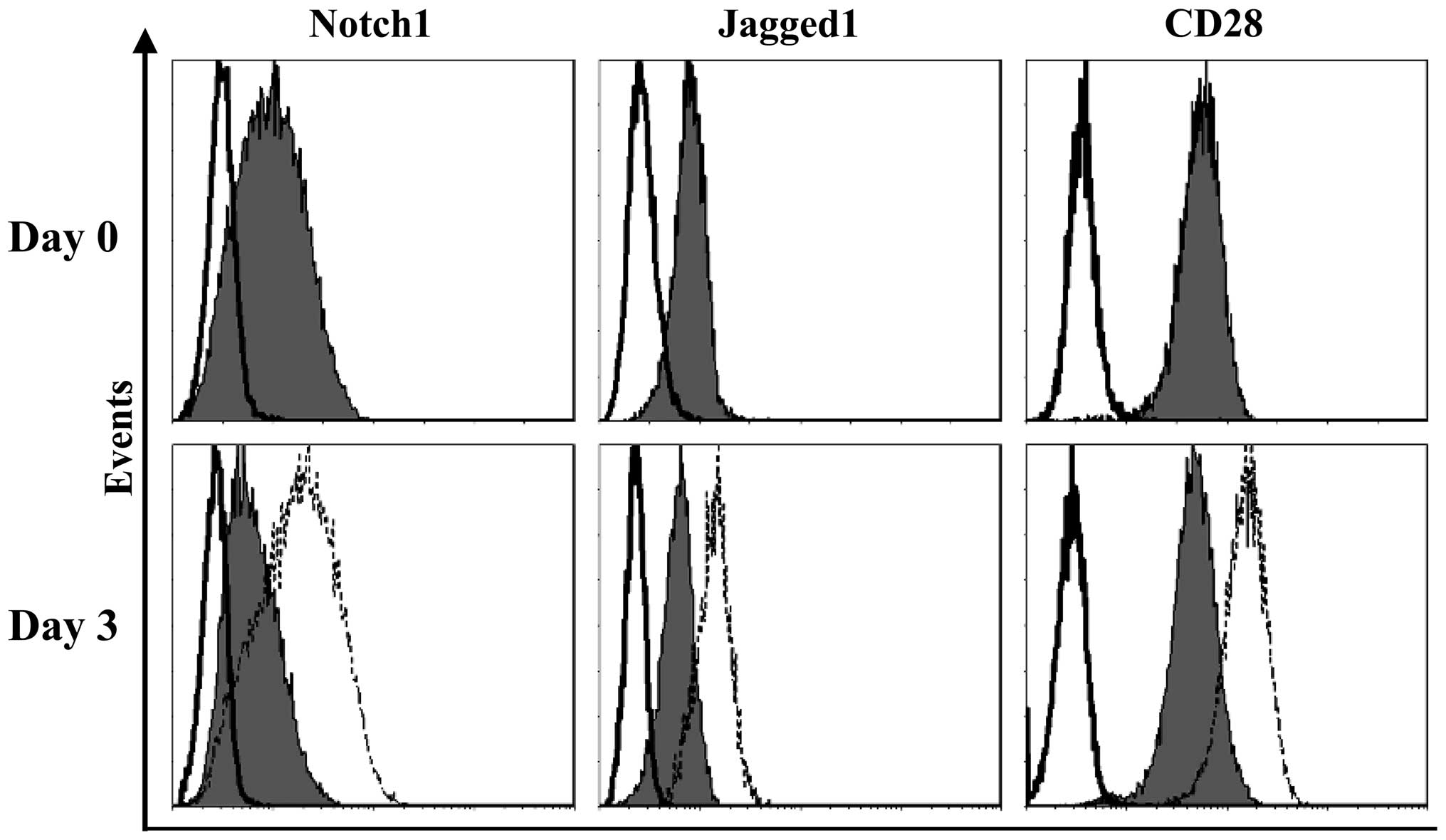
is constitutively active in these cells. Flow cytometry was then
used to assess the expression of Notch1, Jagged1 and CD28 in the
Jurkat cells. As shown in Fig. 5,
at basal conditions, the Jurkat cells expressed CD28 and moderate
levels of Jagged1 and Notch1. Notably, following contact with the
hUC-MSCs, an upregulation in the expression of all the molecules
was observed in the Jurkat cells (Fig.
5; Table II), indicating their
involvement in the functional interaction between the hUC-MSCs and
the Jurkat T-ALL cell line.
 | Table IIExpression of Notch-related molecules
by Jurkat cells cultured alone or co-cultured with hUC-MSCs. |
Table II
Expression of Notch-related molecules
by Jurkat cells cultured alone or co-cultured with hUC-MSCs.
| Jurkat cells |
|---|
|
|
|---|
| Jagged1 | Notch1 | CD28 |
|---|
| Alone | 0.6±0.2 | 1.2±0.5 | 5.7±2.0 |
| Co-culture | 1.6±0.4 | 2.6±0.7 | 17.6±3.5 |
| Student’s
t-test | P<0.05 | P<0.05 | P<0.01 |
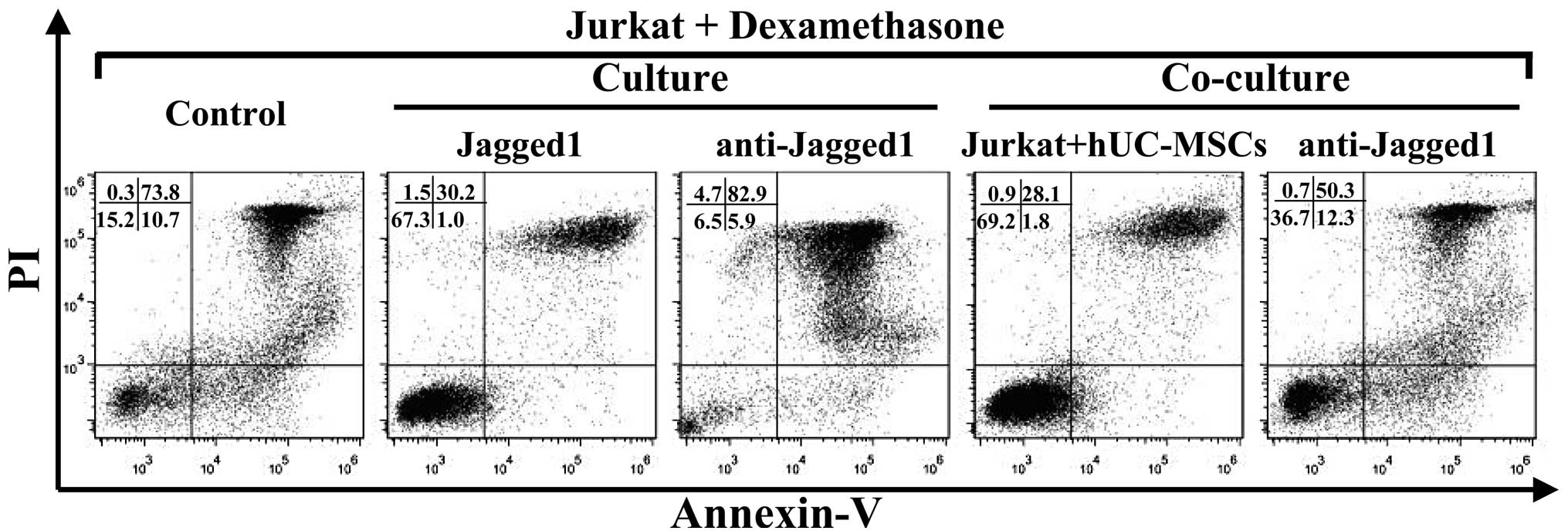
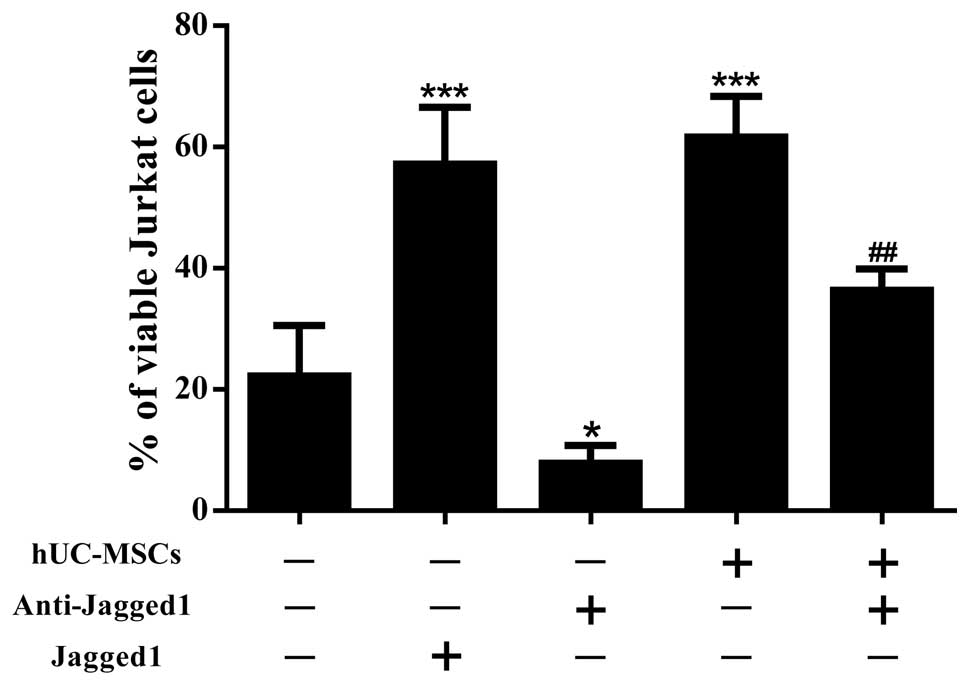
hUC-MSCs inhibit drug-induced apoptosis
in Jurkat cells
To study the capability of the hUC-MSCs to support
leukemia cell survival, the Jurkat cells were cultured alone or
co-cultured with the hUC-MSCs at a 10:1 ratio for 72 h and then
exposed to dexamethasone for an additional 24 h. When observed
using light microscopy, the Jurkat cells in the co-culture system
showed an improved cell morphology compared with those that were
cultured alone (Fig. 1B and C). As
assessed by Annexin V/PI staining (Figs. 6 and 7), the Jurkat cells that were in contact
with the hUC-MSCs underwent far less apoptosis induced by
dexamethasone than those that were cultured alone. These data
suggested that the hUC-MSCs were able to maintain the viability of
the Jurkat T-ALL cells by preventing apoptosis.
Jagged1 contributes to the drug
resistance of Jurkat cells
To gain an improved understanding of the role of
Jagged1 in the survival of the Jurkat cells, blocking experiments
were performed using anti-Jagged1 neutralizing antibodies. By
blocking Jagged1, a significant reduction in the percentage of live
cells was achieved in the Jurkat cells that were exposed to
dexamethasone, in the presence or absence of hUC-MSCs (Figs. 6 and 7). To further confirm the involvement of
Jagged1 in the maintenance of Jurkat cell viability, recombinant
Jagged1 was added to the Jurkat cell cultures. The
exogenously-added Jagged1 significantly enhanced Jurkat cell
survival in the presence of dexamethasone (Figs. 6 and 7). Overall, these results indicate that
Jagged1 favored Jurkat cell survival under the pressure of drug
treatment.
Discussion
The interactions between hematological malignant
cells and the elements of the stromal microenvironment play a key
role in patient survival and the response to chemotherapy. BM-MSCs
are commonly used as stromal cells for in vitro studies on
hematological malignancies. Apart from being used as stromal cells
for experimental requirements, MSCs also represent a homogeneous
stem cell population with multilineage differentiation capabilities
and immune regulatory properties (17), which make them an attractive tool
for the cell-based therapy of numerous human disorders, including
graft-versus-host disease (GvHD), in hematological malignancy
patients undergoing hematopoietic stem cell transplantation (HSCT)
(18,19). In the present study, hUC-MSCs were
used as stromal cells. Compared with BM-MSCs, hUC-MSCs have several
advantages, including an improved ability to expand, painless
collection procedures, a lower risk of viral contamination and the
fact that they are a possible source for autologous cell therapy
(20). Despite these attractive
features, the efficacy and safety of hUC-MSCs have to be evaluated
in preclinical models prior to using them in clinical trials. In
the co-culture experiments of the present study, the hUC-MSCs
dramatically enhanced the ex vivo survival of the Jurkat
T-ALL cells that were exposed to dexamethasone. This observation
indicates a side-effect of the hUC-MSCs, which may maintain
residual leukemia cells and lead to the recurrence of the disease.
The same anti-apoptotic effects have also been observed on
malignant cells in BM-MSCs (8,21),
which constitutes a significant limitation for their clinical
application and may explain to a certain extent the emerging
evidence indicating that the co-transplantation of MSCs may
increase the risk of hematological malignancy relapse following
HSCT (22).
To explore the underlying mechanism, the present
study focused on Notch signaling due to its involvement in the
pathogenesis of T-ALL and its potential role in regulating cell
apoptosis. The interaction between Notch receptors and the
membrane-bound ligands of the Delta and Jagged families is critical
for the activation of Notch signaling (6). Mammals have four Notch receptors
(Notch1–4) that bind to five various transmembrane ligands, DLL1, 3
and 4 and Jagged1 and 2 (6). The
actions of the ligands differ in the initiation of Notch signaling.
Jagged1 and 2 and DLL1, commonly known as Delta/Serrate/LAG-2
(DSL), are ligands for Notch receptors 1–4 (6,23).
DLL4 is able to bind and activate the Notch1 and 4 receptors
(6,23, 24),
whereas DLL3 is able to bind and activate Notch1 or similar Notch
receptors (6,23,25).
Furthermore, Notch signaling that is triggered by various ligands
may result in a diverse or opposed biological outcome (12).
In the present study, one of the Notch ligands,
Jagged1, was observed to be expressed by the hUC-MSCs and the
Jurkat T-ALL cell line. Jagged1 is a membrane-spanning protein with
a large extracellular domain that is important for Notch receptor
binding (26). This ligand has been
indicated to be expressed at a significant level in BM-MSCs
(27) and is associated with
certain BM-MSC functions, including the regulation of the
hematopoietic stem cell (HSC) niche (28), suppressive effects on immune cells
(29) and cellular differentiation
(30). The expression of Jagged1 by
hUC-MSCs may initiate the stimulation of Notch signaling in the
Jurkat cells by binding to the Notch1 receptor and thus, may
contribute to the hUC-MSC-induced survival of the T-ALL cells. By
contrast, Jagged1 was also expressed by the Jurkat T-ALL cell line,
in addition to the constitutive expression of the Notch1 receptor.
The contemporary expression of the Notch1 receptor and its ligand
on the cell surface may lead to auto- or reciprocal activation of
Notch signaling among the T-ALL cells and thus, favor their own
survival. As expected, in the present study, the blockade of
Jagged1 significantly abrogated the drug resistance of the Jurkat
cells that were in contact with the hUC-MSCs, and also increased
Jurkat cell sensitivity to dexamethasone in the absence of the
hUC-MSCs. By contrast, the addition of recombinant Jagged1 protein
enhanced the survival of the Jurkat cells that were treated with
dexamethasone. The results of the blocking and stimulating
experiments implied that Jagged1 contributed to hUC-MSC-induced
drug resistance and to the self-maintenance of the Jurkat T-ALL
cells.
In order to identify certain targets that are
involved in the prevention of apoptosis mediated by hUC-MSCs, CD28
expression was assessed in the Jurkat cells in the present study.
CD28 is one of the co-stimulatory molecules that are expressed by T
cells (31). In the present study,
the high expression level of CD28 in the Jurkat T-ALL cell line was
more apparent following contact with the hUC-MSCs. CD28 has been
identified as a direct target of Notch signaling (32), and has also been shown to be
associated with the enhanced survival of immature (33) and activated (34) T cells. Therefore, the role of CD28
in the drug resistance of T-ALL warrants further investigation.
In conclusion, the present data indicate that the
hUC-MSCs induced the drug resistance of the Jurkat T-ALL cell line.
Jagged1, one of the Notch ligands, contributes to this phenomenon,
which may also play a role in the self-maintenance of T-ALL cells
and thus be a potential target for the treatment of human T-ALL.
The evaluation of additional T-ALL cell lines, as well as primary
T-ALL cells, using this co-culture system is necessary to expand
these observations and to lay a theoretical basis for the
development of new therapeutic strategies for T-ALL in the
future.
Acknowledgements
The authors would like to thank Dr Danliang Chen,
Department of Gynecology and Obstetrics, First Affiliated Hospital
of Jinan University, for assisting with the umbilical cord sample
collection. This study was funded by the National Natural Science
Foundation of China (grant no. 31100664).
References
|
1
|
Pui CH, Relling MV and Downing JR: Acute
lymphoblastic leukemia. N Engl J Med. 350:1535–1548. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Keersmaecker K, Marynen P and Cools J:
Genetic insights in the pathogenesis of T-cell acute lymphoblastic
leukemia. Haematologica. 90:1116–1127. 2005.
|
|
3
|
Weng AP, Ferrando AA, Lee W, et al:
Activating mutations of NOTCH1 in human T cell acute lymphoblastic
leukemia. Science. 306:269–271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu YM, Zhao WL, Fu JF, et al: NOTCH1
mutations in T-cell acute lymphoblastic leukemia: prognostic
significance and implication in multifactorial leukemogenesis. Clin
Cancer Res. 12:3043–3049. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Allman D, Punt JA, Izon DJ, Aster JC and
Pear WS: An invitation to T and more: notch signaling in
lymphopoiesis. Cell. 109:S1–S11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Radtke F, Fasnacht N and MacDonald HR:
Notch signaling in the immune system. Immunity. 32:14–27. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang R, Huang GS, Wang Z, et al: Effects
of human bone marrow stromal cell line (HFCL) on the proliferation,
differentiation and apoptosis of acute myeloid leukemia cell lines
U937, HL-60 and HL-60/VCR. Int J Hematol. 87:152–166. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nefedova Y, Landowski TH and Dalton WS:
Bone marrow stromal-derived soluble factors and direct cell contact
contribute to de novo drug resistance of myeloma cells by distinct
mechanisms. Leukemia. 17:1175–1182. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nefedova Y, Cheng P, Alsina M, Dalton WS
and Gabrilovic DI: Involvement of Notch-1 signaling in bone marrow
stroma-mediated de novo drug resistance of myeloma and other
malignant lymphoid cell lines. Blood. 103:3503–3510. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carlesso N, Aster JC, Sklar J and Scadden
DT: Notch1-induced delay of human hematopoietic progenitor cell
differentiation is associated with altered cell cycle kinetics.
Blood. 93:838–848. 1999.
|
|
11
|
Sade H, Krishna S and Sarin A: The
anti-apoptotic effect of Notch-1 requires p56lck-dependent,
Akt/PKB-mediated signaling in T cells. J Biol Chem. 279:2937–2944.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rutz S, Mordmüller B, Sakano S and
Scheffold A: Notch ligands Delta-like1, Delta-like4 and Jagged1
differentially regulate activation of peripheral T helper cells.
Eur J Immunol. 35:2443–2451. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Friedenstein AJ, Petrakova KV, Kurolesova
AI and Frolova GP: Heterotopic of bone marrow. Analysis of
precursor cells for osteogenic and hematopoietic tissues.
Transplantation. 6:230–247. 1968.PubMed/NCBI
|
|
14
|
Rebelatto CK, Aguiar AM, Moretão MP, et
al: Dissimilar differentiation of mesenchymal stem cells from bone
marrow, umbilical cord blood, and adipose tissue. Exp Biol Med
(Maywood). 233:901–913. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rao MS and Mattson MP: Stem cells and
aging: expanding the possibilities. Mech Ageing Dev. 122:713–734.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Romanov YA, Svintsitskaya VA and Smirnov
VN: Searching for alternative sources of postnatal human
mesenchymal stem cells: candidate MSC-like cells from umbilical
cord. Stem Cells. 21:105–110. 2003.PubMed/NCBI
|
|
17
|
Phinney DG and Prockop DJ: Concise review:
mesenchymal stem/multipotent stromal cells: the state of
transdifferentiation and modes of tissue repair - current views.
Stem Cells. 25:2896–2902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ball LM, Bernardo ME, Roelofs H, et al:
Cotransplantation of ex vivo expanded mesenchymal stem cells
accelerates lymphocyte recovery and may reduce the risk of graft
failure in haploidentical hematopoietic stem-cell transplantation.
Blood. 110:2764–2767. 2007. View Article : Google Scholar
|
|
19
|
Le Blanc K, Rasmusson I, Sundberg B, et
al: Treatment of severe acute graft-versus-host disease with third
party haploidentical mesenchymal stem cells. Lancet. 363:1439–1441.
2004.PubMed/NCBI
|
|
20
|
Baksh D, Yao R and Tuan RS: Comparison of
proliferative and multilineage differentiation potential of human
mesenchymal stem cells derived from umbilical cord and bone marrow.
Stem Cells. 25:1384–1392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scupoli MT, Perbellini O, Krampera M,
Vinante F, Cioffi F and Pizzolo G: Interleukin 7 requirement for
survival of T-cell acute lymphoblastic leukemia and human
thymocytes on bone marrow stroma. Haematologica. 92:264–266. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ning H, Yang F, Jiang M, et al: The
correlation between cotransplantation of mesenchymal stem cells and
higher recurrence rate in hematologic malignancy patients: outcome
of a pilot clinical study. Leukemia. 22:593–599. 2008. View Article : Google Scholar
|
|
23
|
Apelqvist A, Li H, Sommer L, et al: Notch
signalling controls pancreatic cell differentiation. Nature.
400:877–881. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lobov I, Renard R, Papadopoulos N, et al:
Delta-like ligand 4 (Dll4) is induced by VEGF as a negative
regulator of angiogenic sprouting. Proc Natl Acad Sci USA.
104:3219–3224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Loomes KM, Stevens SA, O’Brien ML, et al:
Dll3 and Notch1 genetic interactions model axial segmental and
craniofacial malformations of human birth defects. Dev Dyn.
236:2943–2951. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ascano JM, Beverly LJ and Capobianco AJ:
The C-terminal PDZ-ligand of JAGGED1 is essential for cellular
transformation. J Biol Chem. 278:8771–8779. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Docheva D, Haasters F and Schieker M:
Mesenchymal stem cells and their cell surface receptors. Curr
Rheumatol Rev. 4:155–160. 2008. View Article : Google Scholar
|
|
28
|
Calvi LM, Adams GB, Weibrecht KW, et al:
Osteoblastic cells regulate the haematopoietic stem cell niche.
Nature. 425:841–846. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liotta F, Angeli R, Cosmi L, et al:
Toll-like receptors 3 and 4 are expressed by human bone
marrow-derived mesenchymal stem cells and can inhibit their T-cell
modulatory activity by impairing Notch signaling. Stem Cells.
26:279–289. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kurpinski K, Lam H, Chu J, et al:
Transforming growth factor-beta and notch signaling mediate stem
cell differentiation into smooth muscle cells. Stem Cells.
28:734–742. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen L: Co-inhibitory molecules of the
B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol.
4:336–347. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chadwick N, Zeef L, Portillo V, et al:
Identification of novel Notch target genes in T cell leukaemia. Mol
Cancer. 8:352009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van den Brandt J, Wang D and Reichardt HM:
Resistance of single-positive thymocytes to glucocorticoid-induced
apoptosis is mediated by CD28 signaling. Mol Endocrinol.
18:687–695. 2004.PubMed/NCBI
|
|
34
|
Boise LH, Minn AJ, Noel PJ, et al: CD28
costimulation can promote T cell survival by enhancing the
expression of Bcl-XL. Immunity. 3:87–98. 1995. View Article : Google Scholar
|